Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Epidemiology of RSV in Young Children in Asia: A Systematic Review
*Corresponding author: Heinz J Schmitt, Scientific advisor, Vaccelerate, Cologne University, Cologne, Germany.
Received: April 05, 2024; Published: April 22, 2024
DOI: 10.34297/AJBSR.2024.22.002936
Abstract
Respiratory Syncytial Virus (RSV) is the most prominent cause of acute lower respiratory tract infections in infants and young children, and it is responsible for almost all childhood deaths in low- and middle-income countries. This study summarizes the available information on the epidemiology of RSV in children < 5 years of age in selected Asian countries and investigates whether there is any data gap in these countries. In this systematic review, the PubMed database was used on February 25, 2024, to identify relevant articles. The inclusion criteria were original studies within 51 countries in Asia containing detailed information on the study population (children < 5 years), RSV-associated associated hospitalization rates, and case fatality rates after 2012. Non-human studies, studies on the adult population, reviews, studies in languages other than English, German, and Persian, and studies with a lack of necessary data for calculating RSV-related hospitalization or case fatality rates were excluded. RSV-associated hospitalization and case fatality rates were extracted based on predefined criteria. Among 1,924 publications, 16 studies in 19 countries met the predefined criteria. RSV-associated hospitalizations for children < 1 year of age were in the range between 0.85 and 4.07 per 100 person-years. Case fatality rates were only provided from Hong Kong and India. RSV-associated hospitalization rates were in line with data from Europe and the USA. The availability of new means to prevent RSV infections in infancy warrants conducting local studies to fill the data gap identified as the basis for future public health recommendations.
Keywords: Respiratory syncytial virus, RSV, Epidemiology
Abbreviations: ACIP: Advisory Committee on Immunization Practices; ALRI: Acute Lower Respiratory Infection; ELISA: Enzyme- Linked Immunosorbent Assay; EMA: European Medicines Agency; FDA: Food and Drug Administration; GISRS: Global Influenza Surveillance and Response System; IFA: Immunofluorescence Assay; LMIC: Low- and Middle-Income Countries; LRTI: Lower Respiratory Tract Infection; mAb: Monoclonal Antibody; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta- Analyses; RSV: Respiratory Syncytial Virus; SARI: Severe Acute Respiratory Infections; WHO: World Health Organization
Introduction
Respiratory Syncytial Virus (RSV) is a single‐stranded negative‐sense enveloped RNA pneumovirus in the Pneumoviridae family with two distinct serotypes (A and B) that cause annual respiratory disease epidemics [1-4]. The virus was first isolated from chimpanzees in 1955 and then from humans in 1956 [5,6]. Transmission can occur through airborne droplets or infection from direct contact with contaminated surfaces [7,8]. RSV can infect the respiratory tract causing a range of symptoms from asymptomatic upper respiratory tract infection to severe lower respiratory tract infection (LRTI) that may result in hospitalization and even death [8]. The virus leads to outbreaks from late fall to early spring in temperate climates and may be more prolonged or even occur throughout the year in warmer climates [9].
The highest burden of RSV infection is in children under 5 years of age, high-risk adults (those with chronic diseases or those who are immunocompromised), and older adults [10-12]. RSV infection has a major impact on virtually all children by the age of two years, many of them even twice [13]. In a pooled data analysis, the RSV-associated hospitalization rate for US infants was 1.94% (95%CI 1.79-2.09) [14]. Similar numbers have been published for the UK, where in the first year of life the estimated hospitalization rate for bronchiolitis (2011) was 4.61% with an annually increasing rate of 1.8% since 2004[15]. In a study, RSV-associated acute lower respiratory infection (ALRI) hospitalization rates were investigated in 2019. They found hospitalization rates in children younger than 5 years at the global level to be around 5.3 (4.2-6.8) per 1,000 children per year [16]. Another study on the burden of RSV in children under 5 years of age in Europe from 2006 to 2018 showed that across the EU, about 10 children per 1,000 were hospitalized due to RSV each year with the highest numbers in France, UK, and Germany [17]. A study on the RSV burden of disease in children under 5 years of age in 72 GAVI-eligible countries based on DALYs per 1,000 person-years, showed the lowest burden of RSV in Mongolia and Vietnam and the highest burden in Senegal and Pakistan. They estimated the disease burden to be around 20.8 million cases which led to 1.8 million hospitalizations and 40 thousand deaths in all 72 Gavi-eligible countries [18]. Additional studies showed that RSV was the main reason for hospitalization during infancy [19,20]. Thus, RSV was the most common pathogen in ALRI in children and it was responsible for about 99% of childhood deaths in low- and middle-income countries (LMICs) [21]. In a study performed in Mali, 13.4% of all recurrent wheeze/asthma cases at 6 years were attributable to RSV LRTI [22].
From countries with the scientifically valid burden of disease data, RSV disease has a huge economic burden on health systems, patients, and governments. The direct medical cost in LMICs is more than three billion US dollars with additional direct non-medical costs and indirect costs [21]. Another study showed each episode of RSV disease costs on average 3,400 Euros and 300 Euros for inpatient and outpatient disease management, respectively at the global level. If a medical follow-up is needed, these numbers may increase to 8,600 and 2,200 Euros for a 2-year period [23]. Another study in children under 5 years of age in 72 GAVI-eligible countries, estimated the inpatient and outpatient costs of RSV treatment around 611 million dollars [18].
Palivizumab, a monoclonal antibody (mAb) for high-risk infants (preterm, immunocompromised, or infants with congenital or pulmonary heart disease), was licensed in 1998 in many countries worldwide, but the high cost as well as the necessity for monthly infusions are barriers to using this mAb in LMICs. Even high-income countries do not generally recommend the product for all infants but restrict its use to a few groups with the highest risk [24-27]. In 2023, two innovative preventive interventions against RSV were licensed for the protection of infants, nirsevimab (Beyfortus™) for passive immunization and a bivalent recombinant pre-fusion RSV-F protein for vaccination during pregnancy (Abrysvo™). The first is a long-acting mAb was approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for use in children up to 2 years of age based on documentation of high efficacy and a good safety profile. The product is recommended by the Advisory Committee on Immunization Practices (ACIP) to protect infants under 8 months and older high-risk children [28,29]. Abrysvo™ is the first vaccine ever specifically licensed for use in pregnancy to prevent RSV-LRTI and severe RSV-LRTI in infants by US FDA [30] and by EMA [31].
The impact of RSV infections on the health of children outside medical settings as well as on the burden to hospitals and public health in general is still underestimated, as there is hardly any systematic testing for the pathogen in daily medical practice, resulting in relevant data gaps. While there is a huge number of studies on the epidemiology of RSV from the European Union and North America, there is a lack of such studies in Asian countries. Here we aimed to identify such studies from Asian countries, investigate whether there is any lack of data in these target countries, and provide some suggestions to fill the data gap and therefore, to improve public health. A list of Asian countries, their population, and economy are shown in Table 1.
Materials and Methods
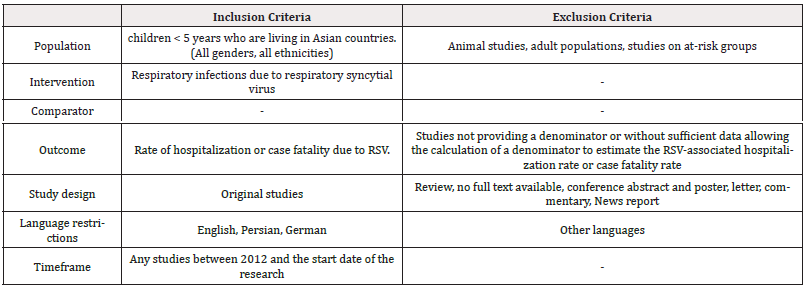
This study is a systematic review and is reported according to the guidelines in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [34]. The PubMed database was used to identify relevant studies. After the initial search, screening for additional studies in the reference list of included studies will be performed to ensure capturing all relevant studies through the search strategy. Search terms were “respiratory syncytial virus” [MeSH Terms], “respiratory syncytial virus infections” [MeSH Terms], and “Asia” as well as the names of target countries. The literature search was performed on October 16, 2023, and the search process was re-run on February 25, 2024, before the final analysis to capture new studies. Original studies within the target countries were included if they mentioned in detail the study population (children < 5 years), the rate of RSV hospitalization or case fatality, and if they were published after 2012. Non-human studies, studies on adult populations, review articles, studies in languages other than English, German, and Persian, and studies not providing a denominator or without sufficient data allowing the calculation of a denominator to estimate the RSV-associated hospitalization or case fatality rate were excluded. The criteria for selecting studies will be based on Table 2.
All the identified studies were entered into the reference management software (Mendeley) to exclude duplicates. The title and abstract were screened by two members of the review team in parallel, blinded to each other based on the eligibility criteria. Afterward, for the titles and abstracts that met the eligibility criteria or in case of hesitancy, the full text was screened in the same way. In case of disagreement between the reviewers, it was solved by discussion.
Data was extracted by two members of the review team in parallel, blinded to each other and disagreement was solved through discussion between the reviewers. Data were extracted from the selected articles including author, year of publication, study population (country, age), RSV detection method, study period, study type, and the rate of RSV-associated hospitalization or case fatality.
Based on the data in selected studies, we extracted the RSV-associated hospitalization rate for children < 6 months, 6-11 months, 12-23 months, < 1 year, < 2 years, and < 5 years, and the RSV case fatality rate for children < 6 months, < 1 year, < 2 years, and < 5 years as far as possible. The annual incidence of hospitalization of infants in the publication by Li, et al. [35] was calculated by using the country’s published birth cohort information [32]. Studies were summarized by the method of identification of RSV cases (Immunofluorescence assay (IFA), RT-PCR, enzyme-linked immunosorbent assay (ELISA), immunochromatography, or ICD-9 Codes) and by the method of microbiological confirmation of the infection (Active surveillance, retrospective, and model-based), similar to the methods described in McLaughlin, et al. [14]. There was no funding source for this study.
Results
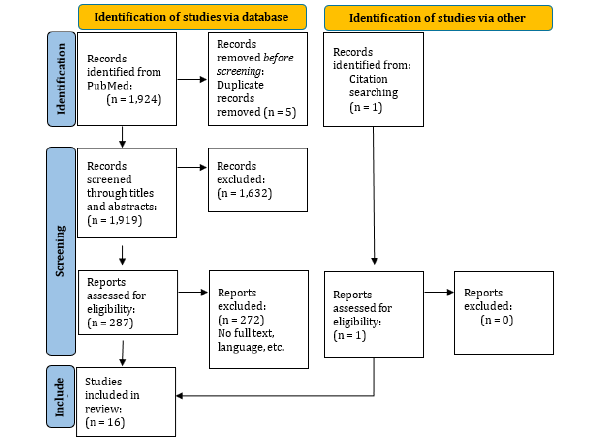
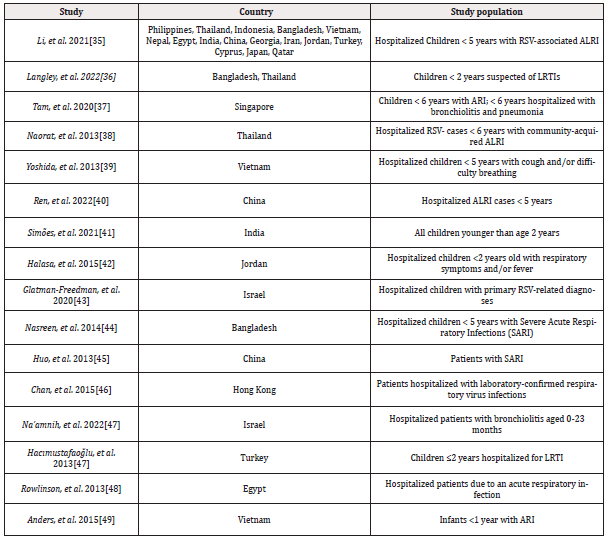
1,924 publications were found in PubMed until February 25, 2024, and one additional article was found through references in relevant articles. Five duplicate publications were found by the reference manager software and excluded from the study. After screening titles and abstracts, 1,632 articles were excluded. The full text of the 288 remaining articles was screened and 16 original studies were included for data extraction according to the eligibility criteria. The PRISMA flow diagram for the study selection process is shown in Figure 1 and a general overview of the selected studies is shown in Table 3.
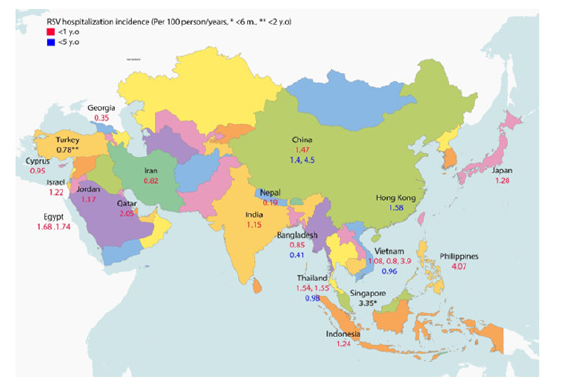
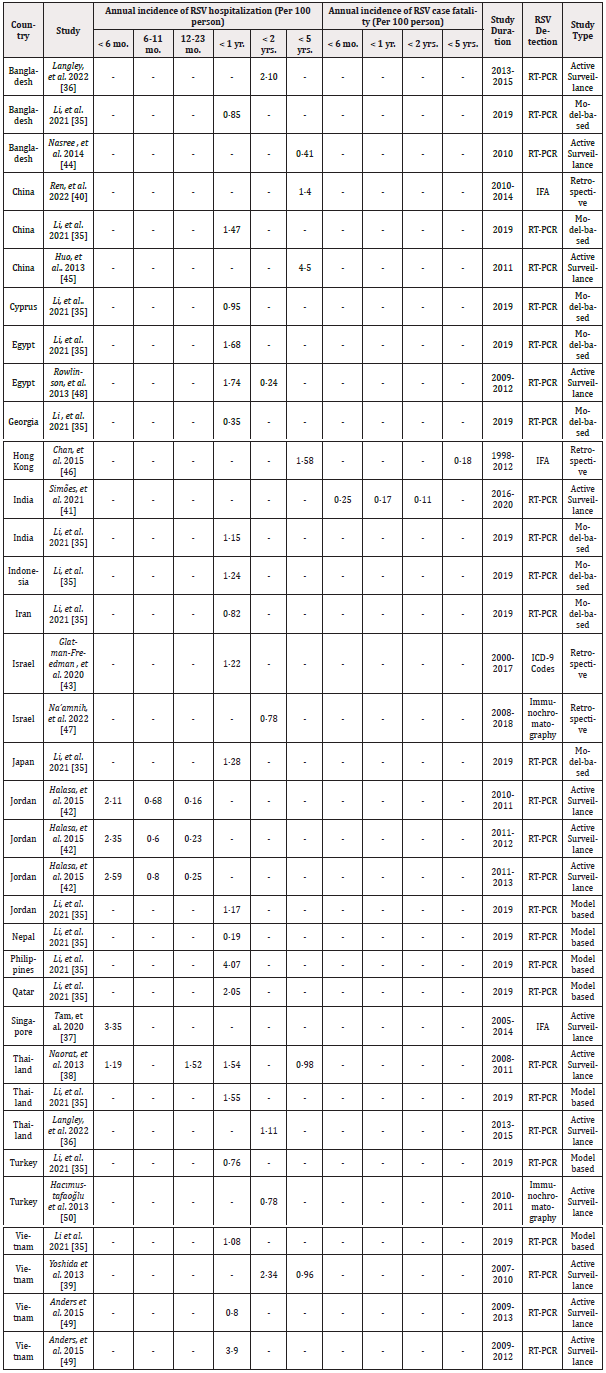
A general overview of the extracted data on the annual incidence of RSV-related hospitalizations in children <1 and <5 years of age is shown in Figure 2, and the detailed extracted data including the annual incidence of RSV-associated hospitalization and case fatality in the target countries is shown in Table 4. Out of all the 51 countries in Asia, the selected publications investigated the incidence rate for RSV hospitalization and case fatality in 19 and two countries, respectively. No studies with our predefined criteria on RSV-associated hospitalization or case fatality rates were found for the other countries. Between the Asian countries, the majority of identified studies with an RSV hospitalization rate belonged to Bangladesh, China, Thailand, and Vietnam with three articles for each country.
Li, et al. [35] investigated the RSV hospitalization rates in 16 different countries. Most of the RSV-associated hospitalization rates were reported for children up to one year of age. The range of hospitalization incidences in this age range was from 0.35 to 4.07 per 100 person-years, with the highest rate in the Philippines (4.07 per 100 person-years) and the lowest rate in Georgia (0.35 per 100 person-years). Based on two studies that reported the RSV case fatality rate in India and Hong Kong, the rate was reported 0.25, 0.17, 0.11, 0.18 per 100 person-years for children < 6 months, < 1 year, < 2 years, and < 5 years of age, respectively.
Detection of RSV infection was performed mostly by RT-PCR laboratory technique in ten studies and immunofluorescence assay, Immunochromatography, and ICD-9 Codes were used in the other studies. The microbiological confirmation method of the infection was active surveillance for 11 studies and retrospective and model-based methods were conducted in four and one study, respectively. Figure 2, Table 4.
Discussion and Conclusion
For 32 of the 51 countries in Asia, we found no information on the RSV-associated hospitalization rate in children < 5 years of age since 2012. As a comparison, in a systematic review and meta-analysis performed among US infants, McLaughlin, et al. [14] found a total of 31 estimates of RSV hospitalization rates within the United States alone, published between 2000 and 2020. Moreover, since 2012, there has been no information on the RSV case fatality rate in children < 5 years of age for 49 countries of the 51 Asian countries. In comparison, in the US, Hansen, et al. [51] in a cross-sectional study assessed the RSV case fatality rate in children < 5 years of age between 1999 to 2018. As RSV is the most common and severe infectious disease early in life, this lack of data in Asian countries is puzzling.
Most published data were available for children < 1 year of age. As shown in Table 3, available information shows annual RSV-associated hospitalization rates between 0.35 and 4.07 per 100 person-years, and these numbers are in the range of the data published for the US [14] and the UK [15]. Another study showed this estimate for children < 1 year of age to be 5.06 per 100 person-years for Denmark, 2.72 per 100 person-years for Germany, 2.37 per 100 person-years for Argentina, and 1.91 per 100 person-years at the global level [52]. Thus, the little information published from the selected Asian countries is in the range of data published from the other countries, suggesting that RSV-associated hospitalization patterns may be similar around the globe.
While the little data we found on Asian countries’ RSV case fatality rates is not sufficient to make a comparison, in additional studies RSV-associated case fatality rates appeared to be higher in low- and middle-income countries as compared to the US or Europe [21]. In their meta-analysis, Nair, et al. estimated the RSV case fatality rate in children < 5 years of age in low- and middle-income countries and high-income countries to be 2.1% and 0.3%, respectively [53]. In the US, Hansen, et al. [51] showed RSV case fatality rate between 1999 to 2018 as 0.0027 per 100 person-years in children <1 year and 0.0011 per 100 person-years in children <5 years of age. In Argentina, Caballero, et al. [54] estimated the community case fatality rate due to RSV in infants <6 months of age to be 0.027 per 100 person-years in 2019.
The reason for the lack of data on the epidemiology of RSV in the target countries could be a lack of well-established surveillance systems. At the global level, The World Health Organization (WHO) is working on leveraging the Global Influenza Surveillance and Response System (GISRS) for RSV surveillance to improve understanding of RSV disease burden and RSV hospitalization rates [52]. On the other hand, focusing on RSV in the current local surveillance systems for influenza might result in relevant costs and also require the development of a separate infrastructure which is more feasible in high-income countries. Some Western countries investigated RSV surveillance through their influenza surveillance system [55].
The data sought here on the epidemiology of RSV in infants in the countries targeted is much needed for several reasons. First, some of these countries are located in the tropics and the (year-round) seasonality of respiratory viruses like RSV may result in disease characteristics that are different from those in regions in the northern or southern hemispheres [56, 57]. With that in mind, medical practices for controlling the infection might require different interventions and different types of awareness in the target countries, e.g., year-round RSV testing versus seasonal testing only. Second, most of the countries of interest here were low and lower-middle-income countries (LMIC). Hospitalization rates, ICU admissions, and death rates due to RSV infection in LMICs may differ [53] and the estimates from high-income regions may not apply when predicting the RSV burden in LMICs. Third, evaluating the public health burden of RSV including direct and indirect costs of the infection may vary with differences in available medical care resources. This needs to be studied separately for each country. Fourth, and most important of all, long-acting monoclonal antibodies for use in neonates and also vaccines licensed for use during pregnancy, both based on RSV-pre-fusion-F-proteins as antigens, are now available [58]. They are the optimal strategy for RSV prevention, however, require local data for decision-making. It is fair to predict that these interventions will incur huge costs to any public health system in Asia and the resources needed will only be made available if there is convincing evidence to predict that the benefits on child health and mortality will be sufficiently high to justify the cost of each local RSV vaccination program. Currently, there is no official recommendation for the use of mAbs in neonates or RSV vaccines during pregnancy in Asian countries.
To provide better-qualified data at the global level, global and local initiatives would address some surveillance systems to improve the case definition of RSV and investigate the seasonality of RSV and the burden of disease and as a result, provide evidence for policy-making, especially on vaccines and other preventives. On the other hand, training, funding, and improving technical infrastructure should be addressed to have a qualified well-established surveillance system.
This study has limitations. First, we only searched for papers in the recent 11-year period; more studies might have been published before this time period. Second, we only reviewed original articles in English, German, and Persian language, and this may have led to not finding some articles specifically in Chinese language.
In conclusion, the incidence of RSV-associated hospitalizations identified here for Asian countries is in the range of what was published for the US and Europe, and with the few data identified for RSV case fatality rate, comparison is not possible. With respect to the tropical climate, possible different rates of hospitalization, ICU admission, death rates, different costs of infection, and the need for local data for immunization programs in Asian countries, running local studies might have a big impact on improving the knowledge of preventing RSV infection, hospitalization, and mortality.
The availability of new means to prevent RSV in infancy warrants conducting local studies to fill the data gap identified as the basis for future public health recommendations - which may have huge public health benefits.
Acknowledgments
This research received no external funding.
Conflict of interest
The authors declare no conflict of interest.
Author Contributions
Conceptualization, D.M.N., R.H.A and H.-J.S.; methodology, D.M.N., R.H.A, and H.-J.S.; investigation, D.M.N., R.H.A, and H.-J.S.; writing-original draft preparation, D.M.N., R.H.A, and H.-J.S.; writing-review and editing, D.M.N., R.H.A, and H.-J.S.; supervision, H.-J.S. All authors have read and agreed to the published version of the manuscript.
Data availability
All data are available in the manuscript.
References
- Graham BS, Rutigliano JA, Johnson TR (2002) Respiratory Syncytial Virus Immunobiology and Pathogenesis. Virology 297(1): 1-7.
- Kachikis A, Cho H, Englund JA (2023) Respiratory Syncytial Virus-An Update for Prenatal and Primary Health Providers. Obstetrics and Gynecology Clinics of North America 50(2): 421-437.
- Duan Y, Jiang M, Huang Q, Jia M, Yang W, et al. (2023) Incidence, hospitalization, and mortality in children aged 5 years and younger with respiratory syncytial virus-related diseases: A systematic review and meta-analysis. 17(5): 13145.
- Falsey AR, Mcelhaney JE, Beran J, Van Essen GA, Duval X, et al. (2014) Respiratory Syncytial Virus and Other Respiratory Viral Infections in Older Adults With Moderate to Severe Influenza-like Illness. 209(12): 1873-1881.
- CHANOCK R, ROIZMAN B, MYERS R (1957) Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA). I. Isolation, properties and characterization. American journal of hygiene 66(3): 281-290.
- BLOUNT REJ, MORRIS JA, SAVAGE R E (1956) Recovery of cytopathogenic agent from chimpanzees with coryza. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine (New York, N.Y.) 92(3): 544-549.
- Hall CB (1982) Respiratory Syncytial Virus: Its Transmission in the Hospital Environment. THE YALE JOURNAL OF BIOLOGY AND MEDICINE 55(3-4): 219-223.
- Verwey C, Madhi SA (2023) Review and Update of Active and Passive Immunization Against Respiratory Syncytial Virus. BioDrugs 37(3): 295-309.
- Bennett JE, Dolin R, Blaser M J (2014) Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases (Vol. 1-2). Elsevier Inc.
- Nguyen Van Tam JS, O leary M, Martin ET, Heijnen E, Callendret B, et al. (2022) Burden of respiratory syncytial virus infection in older and high-risk adults: a systematic review and meta-analysis of the evidence from developed countries. Eur Respir Rev 31(166): 220105.
- Troeger C, Blacker B, Khalil IA, Rao PC, Cao J, et al. (2018) Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Infectious Diseases 18(11): 1191-1210.
- Jackson M L, Scott E, Kuypers J, Nalla A K, Roychoudury P, et al. (2021) Epidemiology of Respiratory Syncytial Virus Across Five Influenza Seasons Among Adults and Children One Year of Age and Older-Washington State, 2011/2012-2015/2016. The Journal of infectious diseases 223(1): 147-156.
- Heppe Montero M, Walter S, Hernández Barrera V, Gil Prieto R, Gilde Miguel Á (2022) Burden of respiratory syncytial virus-associated lower respiratory infections in children in Spain from 2012 to 2018. BMC Infectious Diseases 22(1): 1-11.
- Mclaughlin JM, Khan F, Schmitt HJ, Agosti Y, Jodar L, et al. (2022) The Journal of Infectious Diseases Respiratory Syncytial Virus-Associated Hospitalization Rates among US Infants: A Systematic Review and Meta-Analysis. The Journal of Infectious Diseases ® 225(6): 1100-1111.
- Green CA, Yeates D, Goldacre A, Sande C, Parslow RC, et al. (2016) Admission to hospital for bronchiolitis in England: Trends over five decades, geographical variation and association with perinatal characteristics and subsequent asthma. Archives of Disease in Childhood 101(2): 140-146.
- Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, et al. (2022) Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet (London, England) 399(10340): 2047-2064.
- Riccio M Del, Spreeuwenberg P, Osei Yeboah R, Johannesen CK, Vazquez Fernandez L, et al. (2023) Burden of Respiratory Syncytial Virus in the European Union: estimation of RSV-associated hospitalizations in children under 5 years. J Infect Dis 228(11): 1528-1538.
- Li X, Willem L, Antillon M, Bilcke J, Jit M, et al. (2020) Health and economic burden of respiratory syncytial virus (RSV) disease and the cost-effectiveness of potential interventions against RSV among children under 5 years in 72 Gavi-eligible countries. BMC Medicine 18(1): 1-16.
- Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, et al. (2009) The Burden of Respiratory Syncytial Virus Infection in Young Children. New England Journal of Medicine 360(6): 588-598.
- Leader S, Kohlhase K (2002) Respiratory syncytial virus-coded pediatric hospitalizations, 1997 to 1999. Pediatric Infectious Disease Journal 21(7): 629-632.
- Mazur NI, Löwensteyn Y, Terstappen J, Wildenbeest J, Bont L, et al. (2023) Respiratory syncytial virus prevention within reach: the vaccine and monoclonal antibody landscape. The Lancet Infectious Diseases 23(1): e2-e21.
- Ortiz JR, Laufer RS, Brunwasser SM, Coulibaly F, Diallo F, et al. (2023) Model-estimated impacts of pediatric respiratory syncytial virus prevention programs in Mali on asthma prevalence. Journal of Allergy and Clinical Immunology Global 2(2): 100092.
- Zhang S, Akmar LZ, Bailey F, Rath B A, Alchikh M, et al. (2020) Cost of Respiratory Syncytial Virus-Associated Acute Lower Respiratory Infection Management in Young Children at the Regional and Global Level: A Systematic Review and Meta-Analysis. The Journal of Infectious Diseases 222(S7): 680-687.
- Ananworanich, J, Heaton PM (2021) Bringing Preventive RSV Monoclonal Antibodies to Infants in Low-and Middle-Income Countries: Challenges and Opportunities. Vaccines (Basel) 28: 9(9): 961.
- Mahmud S, Baral R, Sanderson C, Pecenka C, Jit M, et al. (2023) Cost-effectiveness of pharmaceutical strategies to prevent respiratory syncytial virus disease in young children: a decision-support model for use in low-income and middle-income countries. BMC Med 21(1): 138.
- (2023) Medicines. (n.d.).
- Updated Guidance for Palivizumab Prophylaxis Among Infants and Young Children at Increased Risk of Hospitalization for Respiratory Syncytial Virus Infection. (n.d.).
- (2024) FDA Approves New Drug to Prevent RSV in Babies and Toddlers | FDA. (n.d.).
- (2023) CDC Recommends a Powerful New Tool to Protect Infants from the Leading Cause of Hospitalization | CDC Online Newsroom | CDC. (n.d.).
- (2024) FDA Approves First Vaccine for Pregnant Individuals to Prevent RSV in Infants | FDA. (n.d.).
- (2024) First RSV vaccine to protect infants up to 6 months of age and older adults | European Medicines Agency. (n.d.).
- (2024) Birth Rate by Country | Macro Trends. (n.d.).
- (2024) World Bank Country and Lending Groups - World Bank Data Help Desk. (n.d.).
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, et al. (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372: n71.
- Li Y, Johnson EK, Shi T, Campbell H, Chaves SS, et al. (2021) National burden estimates of hospitalizations for acute lower respiratory infections due to respiratory syncytial virus in young children in 2019 among 58 countries: a modelling study. The Lancet Respiratory Medicine 9(2): 175-185.
- Langley J M, Bianco V, Domachowske J B, Madhi SA, Stoszek SK, et al. (2022). Incidence of Respiratory Syncytial Virus Lower Respiratory Tract Infections During the First 2 Years of Life: A Prospective Study Across Diverse Global Settings. The Journal of infectious diseases 226(3): 374-385.
- Tam CC, Yeo K T, Yeo KT, Tee N, Lin R, et al. (2020) Burden and Cost of Hospitalization for Respiratory Syncytial Virus in Young Children, Singapore. Emerging Infectious Diseases 26(7): 1489-1496.
- Naorat S, Chittaganpitch M, Thamthitiwat S, Henchaichon S, Sawatwong P, et al. (2013) Hospitalizations for acute lower respiratory tract infection due to respiratory syncytial virus in Thailand, 2008-2011. Journal of Infectious Diseases 208(SUPPL. 3): 2008-2011.
- Yoshida LM, Suzuki M, Nguyen HA, Le MN, Dinh Vu T, et al. 2013) Respiratory syncytial virus: co-infection and paediatric lower respiratory tract infections. The European respiratory journal 42(2): 461-469.
- Ren S, Shi T, Shan W, Shen S, Chen Q, et al. (2022). Hospitalization rate of respiratory syncytial virus-associated acute lower respiratory infection among young children in Suzhou, China, 2010-2014. Influenza and other respiratory viruses 16(4): 789-799.
- Simões EAF, Dani V, Potdar V, Crow R, Satav S, et al. (2021) Mortality From Respiratory Syncytial Virus in Children Under 2 Years of Age: A Prospective Community Cohort Study in Rural Maharashtra, India. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 73(Suppl_3): S193-S202.
- Halasa N, Williams J, Faouri S, Shehabi A, Vermund S H, et al. (2015) Natural history and epidemiology of respiratory syncytial virus infection in the Middle East: Hospital surveillance for children under age two in Jordan Vaccine 33(47): 6479-6487.
- Glatman Freedman A, Kaufman Z, Applbaum Y, Dichtiar R, Steiman A, et al. (2020) Respiratory Syncytial Virus hospitalization burden: a nation-wide population-based analysis, 2000-2017. The Journal of infection, 81(2): 297-303.
- Nasreen S, Luby SP, Brooks WA, Homaira Nal, Mamun A, et al. (2014) Population-based incidence of severe acute respiratory virus infections among children aged <5 years in rural Bangladesh, June-October 2010. PloS one 9(2): e89978.
- Huo X, Fang B, Liu L, Yu H, Chen H, et al. (2013) Clinical and epidemiologic characteristics of respiratory syncytial virus infection among children aged <5 years, Jingzhou City, China, 2011. The Journal of infectious diseases 208 Suppl S184-8.
- Chan PKS, Tam WWS, Lee TC, Hon KL, Lee N, et al. (2015) Hospitalization Incidence, Mortality, and Seasonality of Common Respiratory Viruses Over a Period of 15 Years in a Developed Subtropical City. Medicine 94(46): e2024.
- Na’amnih W, Kassem E, Tannous S, Kagan V, Jbali A, et al. (2022) Incidence and risk factors of hospitalizations for respiratory syncytial virus among children aged less than 2 years. Epidemiology and infection 150: e45.
- Rowlinson E, Dueger E, Taylor T, Mansour A, Van Beneden C, et al. (2013) Incidence and clinical features of respiratory syncytial virus infections in a population-based surveillance site in the Nile Delta Region. The Journal of infectious diseases, 208 Suppl: S189-96.
- Anders KL, Nguyen HL, Nguyen NM, Van Thuy NT, Hong Van NT, et al. (2015) Epidemiology and virology of acute respiratory infections during the first year of life: a birth cohort study in Vietnam. The Pediatric infectious disease journal 34(4): 361-370.
- Hacımustafaoğlu M, Celebi S, Bozdemir SE, Ozgür T, Ozcan I, et al. (2013). RSV frequency in children below 2 years hospitalized for lower respiratory tract infections. The Turkish journal of pediatrics 55(2): 130-139.
- Hansen CL, Chaves SS, Demont C, Viboud C (2022) Mortality Associated With Influenza and Respiratory Syncytial Virus in the US, 1999-2018. JAMA network open 5(2): e220527.
- (2023) Global Influenza Programme. (n.d.).
- Nair DNBH, Theodoratou E, Rudan I, Nokes DJ, Ngama HNDM et al. (2010) Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. The Lancet 375(9725): 1545-1555.
- Caballero MT, Bianchi AM, Grigaites SD, De PX, Niveyro I et al. (2021) Community Mortality Due to Respiratory Syncytial Virus in Argentina : Population-based Surveillance Study 73(Suppl 3): 210-217.
- Obando Pacheco P, Justicia Grande AJ, Rivero Calle I, Rodríguez Tenreiro C, Sly P et al. (2018) Respiratory syncytial virus seasonality: A global overview. Journal of Infectious Diseases 217(9): 1356-1364.
- Janet S, Broad J, Snape MD (2018) Respiratory syncytial virus seasonality and its implications on prevention strategies. Human Vaccines and Immunotherapeutic 14(1): 234-244.
- Tan KWJ, Yung CF, Maiwald M, Saffari SE, Thoon KC et al. (2021) Respiratory viral infections in hospitalized pediatric patients in the tropics. Journal of Pediatrics and Child Health 57(4): 559-565.
- (2024) RSV ACIP Vaccine Recommendations | CDC. (n.d.).




 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.