Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Evaluating the Efficacy of Autologous Blood Cell Derivative Growth Factor Concentrate-(Abcd- Endosera) in Enhancing Endometrial Thickness and Live Birth Rates in Patients with Refractory Thin Endometrium
*Corresponding author: Vasanthi Palanivel, Seragen Biotherapeutics Private Limited, WFF4, Bangalore Bioinnovation Centre, Helix Biotech Park, Electronics City Phase 1, Bangalore, Karnataka 560100, India.
Received: June 08, 2024; Published: June 14, 2024
DOI: 10.34297/AJBSR.2024.22.003026
Abstract
Objective: To evaluate the efficacy of ABCD-Endosera derived from platelets in enhancing endometrial thickness and live birth rates among patients with thin endometrium.
Methods: This self-controlled pilot study included 50 patients with a history of refractory thin endometrium and minimum three cycle cancellations due to this condition. Participants underwent intrauterine infusions of autologous growth factors ABCD-Endosera in their current cycle and were compared to their previous cycle without ABCD-Endosera treatment.
Results: The endometrial thickness before the treatment was 6.6 mm (±1) significantly increased to 8.2 mm (±0.9) on the day of embryo transfer with a mean increase of 1.6 mm. Secondary outcomes indicated that embryo transfer was carried out in 94% (47/50) of the patients, with 4% (2/50) experiencing cycle cancellation, and 2% (1/50) lost to follow up. The clinical pregnancy rate stood at 50% (25/50), while 44% (22/50) did not result in pregnancy. One patient (2%, 1/50) had a biochemical pregnancy, and three patients (6%, 3/50) experienced a miscarriage. The live birth rate was 42% (21/50). The procedure was well tolerated without severe adverse events.
Conclusions: Our findings suggest that ABCD-Endosera significantly improves endometrial thickness and shows promising live birth rates in patients with refractory thin endometrium. This pilot study provides a foundation for future larger trials, suggesting ABCD- Endosera could offer a viable treatment strategy for patients with thin endometrium seeking successful pregnancies and live births.
Plain Language Summary
This study evaluated the effectiveness of a new treatment called ABCD-Endosera in women with thin endometrium. Thin endometrium is a condition in which the lining of the uterus is too thin, which can make it difficult to get pregnant. Fifty women with thin endometrium were included in the study. They received intrauterine infusions of ABCD-Endosera, which is a mixture of growth factors derived from platelets. The researchers compared the women's endometrial thickness and pregnancy outcomes to their previous cycles without ABCD-Endosera treatment. The results showed that ABCD-Endosera significantly increased endometrial thickness. Of the 50 women, 47 were able to have embryo transfers. The clinical pregnancy rate was 50%, and the live birth rate was 42%. The researchers concluded that ABCD-Endosera is a safe and effective treatment for women with thin endometrium. It significantly improves endometrial thickness and shows promising live birth rates.
Introduction
The human endometrium, which undergoes numerous cycles of growth, differentiation, and detachment throughout a woman's life, plays a crucial role in providing the site for embryo implantation and sustaining the normal development and survival of the embryo. Assisted Reproductive Technologies (ART) heavily rely on the successful implantation of a genetically normal embryo within a receptive endometrium, the innermost epithelial layer lining the uterus. Thin endometrium is a significant cause of implantation failure and recurrent pregnancy loss in Assisted Reproductive Technology (ART) treatments [1,2]. A morphologically normal endometrium is a critical factor for the success of ART programs. Current data suggests that 50% to 66% of implantation failures are linked to inadequate endometrial receptivity. An optimal endometrial thickness for embryo transfer is considered to be 7mm or more [3,4]. A thickness less than 7mm, known as thin endometrium, often results in reduced implantation, early-stage miscarriages, premature births, low birth weight infants, and failures in ART programs. Over the years, numerous methods, such as hormonal manipulation through extended estrogen dosing or enhancement of endometrial perfusion via low dose aspirin, pentoxifylline, vitamin E, sildenafil, and Granulocyte colony-stim-ulating factor (G-CSF), have been employed to achieve optimal endometrial thickness. Nevertheless, not all thin endometrium cases have demonstrated improvements with these methods. Some studies have reported improved Endometrial Thickness (ET) following intrauterine Platelet-Rich Plasma (PRP) administration [5,6].
In recent years, Platelet-Rich Plasma (PRP) has been investigated as a potential therapy to improve endometrial thickness and reproductive outcomes in women with thin endometrium and the concept of using PRP for in vivo treatment of human thin endometrium was first introduced in 2015 [7]. PRP represents a promising and emerging trend in the field of regenerative and reproductive medicine, particularly for thin endometrium treatment, thereby garnering the interest of IVF specialists. The method involves the concentration of platelets in plasma at a level exceeding that found in whole blood which release various growth factors and cytokines upon activation, promoting tissue repair and regeneration [8]. The efficacy of this method is attributed to the biologically active growth factors secreted by platelets, including pro-regenerative, proliferative, angiogenic, chemotactic, anti-inflammatory, and anti-apoptotic activities. PRP is rich in growth factors such as vascular endothelial Growth Factor (VEGF), Epidermal Growth Factor (EGF), Platelet-Derived Growth Factor (PDGF), Insulin-Like Growth Factor (IGF), Transforming Growth Factor (TGF), and other cytokines that stimulate proliferation and growth [9,10]. The use of growth factors has shown promise in various medical fields, such as wound healing, orthopedics, and dentistry, where they have been used to promote tissue regeneration and repair [11]. This evidence supports the potential therapeutic application of growth factors in the treatment of thin endometrium. The focus has shifted toward the use of Growth Factor Concentrate (GFC) derived from platelets, which has demonstrated promising results in preclinical studies [12]. ABCD-Endosera contains a higher concentration of growth factors and cytokines, which may contribute to its enhanced regenerative effects on the endometrium compared to PRP [13]. Additionally, ABCD-Endosera has been shown to promote angiogenesis, cell proliferation, and migration, which are critical processes for endometrial development and receptivity [14,15]. This pilot study aims to evaluate the efficacy of ABCD-Endosera, a next generation growth factor that concentrates preparation [16,17] in improving endometrial thickness and live birth rate among patients with thin endometrium.
Methods
Study Design and Participants
This was an IEC approved (Protocol No.: SGN/CHE/ETR/0119), self-controlled pilot study conducted between July 2019-Sep 2021. A total of 50 patients with a history of thin endometrium undergoing IVF (freeze all approach) were enrolled. After obtaining informed consent, infertile women of age less than 39 years who have EMT persistently less than 7mm on baseline endometrial evaluation before IVF despite standard HRT and normal endometrial cavity during hysteroscopic evaluation and women with a history of cycle cancellation during FET cycles due to persistent thin endometrium were included in the study.
a) Inclusion Criteria
1. Patients in previous frozen embryo transfer cycles had their uterine lining which was persistently thin (characterized by endometrial thickness<7mm), presented with inadequate response to treatments to increase blood flow to the endometrium include extended estrogen doses, low-dose aspirin, sildenafil, or Vitamin E and therapies like PRP and G-CSF.
2. Experienced one or more unsuccessful IVF cycles, despite having good quality embryos ready for transfer.
3. Women with normal transvaginal ultrasounds and no significant issues in their uterus or nearby areas were included.
4. Patients tested negative for genital tuberculosis using Acid-Fast Bacillus (AFB) culture.
b) Exclusion Criteria
Patients were excluded if they had a history of intrauterine adhesions, endometrial cavity infections, pelvic cancer, severe endometriosis, submucosal uterine myomas or endometrial polyps and adenomyosis. Endometrial thickness was measured with vaginal ultrasound at its thickest part in the longitudinal axis of the uterus and was performed by the same investigator using a computerized vaginal ultrasound. This investigator was blind to the conditions of patients. Thin endometrium was defined as the endometrium thickness <7mm on the day when progesterone was given in HRT cycles [18].
Preparation of Endometrium
All the patients underwent standard Hormone Replacement Therapy (HRT) protocols for endometrial preparation. The protocol involved the incremental use of oral estradiol valerate 2mg/day during days 1-7, 4mg/day during days 8-12, 6mg/day during days 13 to embryo transfer [38]. Transvaginal ultrasound was used to monitor endometrial thickness, and once it reached 7 mm, 10 mg of micronized progesterone acetate was started. When endometrial thickness failed to reach over 7mm, patients were consulted to make decision tocancel the cycle, proceed to a new FET cycle or undergo embryo transfer regardless of thin endometrium. The decision to receive ABCD-Endosera treatment or not was based on the patients' preferences.
Embryo Transfer and Outcome Measures
In this study Single Embryo Transfer (SET) was employed as the chosen method for transferring embryos in all patients. The transferred embryos were blastocysts that had undergone the process of freezing and thawing. These blastocysts were of high quality, specifically graded as 4 AA/AB based on the guidelines outlined in the Istanbul Consensus workshop [18]. This approach not only minimizes the risks associated with multiple pregnancies but also ensures the transfer of the most viable embryos. SET allows for a precise evaluation of the individual embryos' potential for successful implantation, thereby optimizing the chances of achieving favorable pregnancy outcomes. Post-embryo transplantation, the luteal phase was supported through a combination of daily intramuscular injection of 50mg progesterone and nightly administration of 200mg vaginal progesterone soft capsules. Serum Human Chorionic Gonadotropin (HCG) levels were measured 14 days after embryo transfer. Vaginal ultrasonography was conducted 35 days after transfer in cases of biochemical pregnancy, while the presence of an intrauterine fetal heartbeat was used to define a clinical pregnancy. The primary outcome and endpoint of the study focused on endometrial thickness. The secondary endpoints included the clinical pregnancy rate, defined as the presence of a gestational sac and fetal heartbeat on transvaginal ultrasound five weeks after embryo transfer. Additional secondary outcome measures encompassed the miscarriage rate and ongoing pregnancy rate.
Intervention
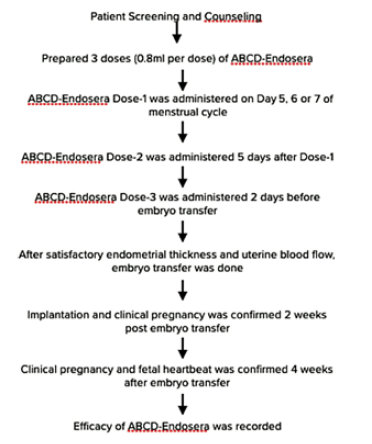
ABCD-Endosera was prepared from autologous blood, as previously reported. [16,17] After obtaining informed consent, ABCD-Endosera was prepared from concentrated platelets, prepared from fresh peripheral blood collected from the peripheral vein which was then subjected to proprietary centrifugation-based selective enrichment protocol. As presented in Figure 1, three doses of ABCD- Endosera (0.8 mL per dose) were prepared from 30mL of peripheral blood. For patients receiving ABCD-Endosera treatment, the infusion protocol involved administering the first dose of 0.8 ml of ABCD-Endosera intrauterine infusion between days 5-7 of the menstrual cycle. The second dose was administered five days after the first dose, and the third dose was given 48 hours before embryo transfer. The ABCD Endosera infusion into the uterine cavity was performed using a Tomcat catheter for each administration [16,17]. Endometrial and sub endometrial blood flow, crucial for successful implantation, was monitored using color Doppler in 2D mode on a transvaginal scan. Prior to ABCD- Endosera instillation, these patients had no visible endometrial/sub endometrial blood flow, whereas a noticeable improvement in blood flow was evident post-treatment as per the different zones of vascularity with reference to the Applebaum criteria. [19] Biochemical pregnancy was confirmed by positive serum beta-human chorionic gonadotropin (β-hCG) two weeks post-embryo transfer, and Transvaginal ultrasound (TVS) was performed after another two weeks to confirm clinical pregnancy.
Outcome Measures
The primary outcome of this study was to evaluate the effect of intrauterine infusions of ABCD-Endosera on EMT in patients presenting with refractory thin endometrium. The secondary outcomes encompassed assessing implantation rates, clinical pregnancy rates, live birth rates, and the reporting of adverse effects. (Tables 1,2)
Statistical Analysis
Data were collected using MS Excel and analyzed using SPSS statistical software (SPSS, Chicago, IL, USA) version 28.0. Categorical variables were expressed as numbers and percentages, while continuous variables were expressed as mean [standard deviation (SD)].
Results
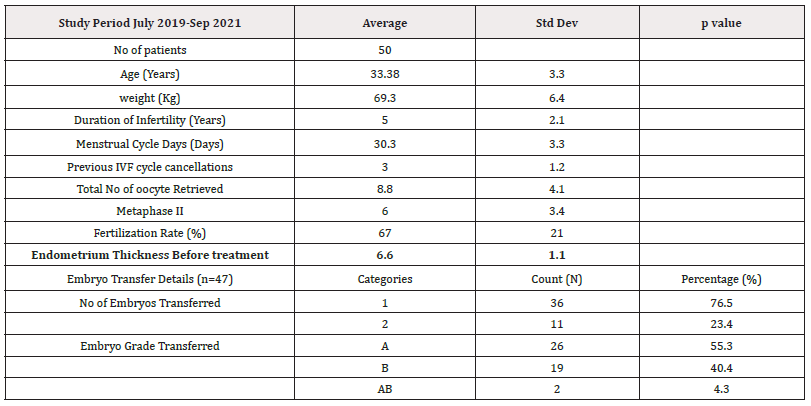
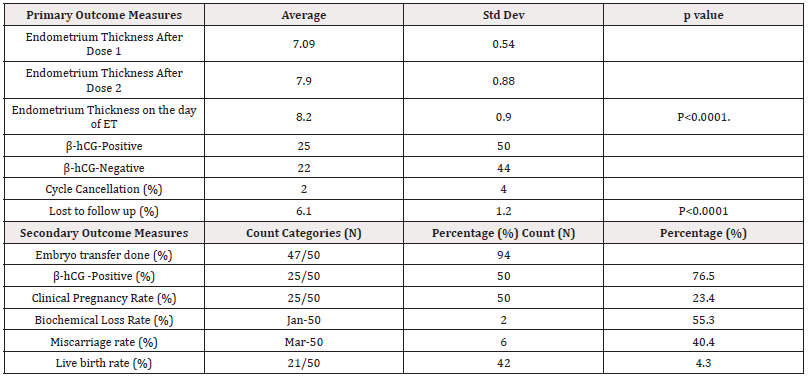
The study included 50 patients with a history of refractory thin endometrium (Table-1). The average age of the patients was 33.38 years (±3.3), and they had an average weight of 69.3 kg (±6.4). They had an average infertility duration of 5 years (±2.1), with a menstrual cycle duration averaging 30.3 days (±3.3). The patients had undergone an average of 3 intrauterine insemination (IUI) procedures (±1.2) and 3 previous IVF attempts (±1.2). The average number of oocytes retrieved was 8.8 (±4.1), and the number of Metaphase II was 6.0 (±3.4). The fertilization rate was 67% (±21%). The endometrial thickness before the treatment was 6.6 mm (±1.1). This value significantly increased to 7.09 mm (±0.54) after dose 1, 7.9 mm (±0.88) after dose 2, and 8.2 mm (±0.9) on the day of embryo transfer, showing a significant difference (p < 0.0001) with a mean increase of 1.6 mm. Secondary outcomes indicated that embryo transfer was carried out in 94% (47/50) of the patients, with 4% (2/50) experiencing cycle cancellation, and 2% (1/50) lost to follow-up. The clinical pregnancy rate stood at 50% (25/50), while 44% (22/50) did not result in pregnancy. One patient (2%, 1/50) had a biochemical pregnancy, and three patients (6%, 3/50) experienced a miscarriage. The live birth rate was 42% (21/50) (Table-2). The procedure was well tolerated without severe adverse events.
Discussion and Conclusion
In this self-controlled retrospective study, ABCD-Endosera derived from platelets was found to significantly improve endometrial thickness and live birth rate in patients with thin endometrium. These findings support previous research indicating the therapeutic potential of platelet-derived growth factors in the management of thin endometrium [6,7,9,10,16,17]. This approach directly harnesses the growth factors that are responsible for the efficacy of PRP, such as VEGF, PDGF, and TGF-β, which play critical roles in endometrial regeneration and tissue repair. The use of growth factors in other specialties, such as orthopedics and wound healing, has also demonstrated the potential of growth factors to promote tissue regeneration and repair [16-22]. Cryopreservation of platelet concentrates has been shown to retain the biological activity of growth factors, such as platelet-derived growth factor (PDGF) and transforming growth factor-beta (TGF-β), enabling their use in various clinical applications [23-28]. Several studies have demonstrated that the extraction and storage of growth factors from platelets are feasible and effective [29-37]. With concentrated growth factors preparation, the targeted growth factors can be more effectively delivered to the endometrial tissue, ensuring that the necessary factors are present in optimal levels to promote tissue regeneration and repair. In comparison to the results observed in conventional PRP based treatment, our study has shown better improvements in endometrial thickness and live birth rate among patients with thin endometrium [38].
Strengths
ABCD-Endosera is a more targeted and efficient alternative to PRP for treating thin endometrium. Unlike PRP, ABCD-Endosera does not contain pro-inflammatory sources, which may lead to more consistent results across patients. This can result in a more standardized, cost-effective and time-efficient treatment option for patients, without compromising efficacy. This approach appears to offer several advantages over PRP, including the direct delivery of essential growth factors, reduced variability between patients, and potential improvements in clinical practice efficiency by preparing multiple doses from a single blood aspiration. This feature reduces the number of patient visits to the hospital, thereby decreasing the overall burden on both the patient and healthcare system. Furthermore, by cryo-storing ABCD-Endosera for extended periods, the waiting time for patients can be significantly reduced, as doses can be prepared in advance and administered when needed.
Limitations
Despite promising results, our pilot study was limited by its small size and retrospective design. Future large-scale randomized controlled trials comparing ABCD-Endosera to other treatments are needed to optimize its protocols and investigate its synergistic effects with other therapies. These trials should also evaluate its long-term safety and efficacy, as well as the mechanisms by which growth factors promote endometrial regeneration and the optimal concentration for each growth factor.
Summary
In summary, this self-controlled retrospective study has shown promising results in the use of growth factor concentrate derived from platelets to improve endometrial thickness and live birth rate in patients with thin endometrium. The advantages of ABCD-Endosera over PRP, such as the direct delivery of essential growth factors, reduced variability between patients, and potential improvements in clinical practice efficiency, warrant further investigation in larger, well-designed trials.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
There was no support from any funding agency. This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Author contribution statement
Study design and concept: Mala Raj, Vasanthi Palanivel Manuscript writing, modification and editing: Vasanthi Palanivel, Mala Raj, Shrinivas Chari Review the manuscript and approve the release: Mala Raj, Shrinivas Chari, Vasanthi Palanivel.
Acknowledgements
We would like to thank all the staff from Firm Hospitals and Seragen Biotherapeutics Pvt Ltd, who helped the authors in conducting the study.
References
- Gargett CE (2007) Uterine stem cells: what is the evidence? Human Reprod Update 13(1): 87-101.
- Tetsuo Maruyama, Hirotaka Masuda, Masanori Ono, Takashi Kajitani, Yasunori Yoshimura (2010) Human uterine stem/progenitor cells: their possible role in uterine physiology and pathology. Reproduction 140(1): 11-22.
- Tarek El Toukhy, Arri Coomarasamy, Mohammed Khairy, Kamal Sunkara, Paul Seed, et al., (2008) The relationship between endometrial thickness and outcome of medicated frozen embryo replacement cycles. Fertility and Sterility 89(4): 832-839.
- Richter KS, Bugge KR, Bromer JG, Levy MJ (2007) Relationship between endometrial thickness and embryo implantation, based on 1294 cycles of in vitro fertilization with transfer of two blastocyst-stage embryos. Fertility and Sterility 87(1): 53-59.
- Mouhayar Y, Franasiak JM, Sharara FI (2019) Obstetrical complications of thin endometrium in assisted reproductive technologies: a systematic review. Journal of Assisted Reproduction and Genetics 36(4): 607-611.
- Sunita R Tandulwadkar, Manasi V Naralkar, Akash D Surana, M Selvakarthick, Avinash H Khara (2017) Autologous in- trauterine platelet-rich plasma instillation for suboptimal endometrium in frozen embryo transfer cycles: a pilot study. Journal of Human Reproductive Sciences 10(3): 208-212.
- Yajie Chang, Jingjie Li, Yuqing Chen, Lina Wei, Xing Yang, et al., (2015) Autologous platelet-rich plasma promotes endometrial growth and improves pregnancy outcome during in vitro fertilization. International Journal of Clinical and Experimental Medicine 8(1): 1286-1290.
- Malanga GA, Goldin M (2014) PRP: review of the current evidence for muscu-loskeletal conditions. Current Physical Medicine and Rehabilitation Reports 2: 1-15.
- Zadehmodarres S, Salehpour S, Saharkhiz N, Nazari L (2017) Treatment of thin endometrium with autologous platelet-rich plasma: a pilot study. JBRA Assisted Reproduction 21(1): 54-56.
- Agarwal M, Mettler L, Jain S, Sandhya Meshram, Veronika Günther, et al., (2020) Management of a thin endometrium by hysteroscopic installation of platelet-rich plasma into the endomyometrial junc- tion: a pilot study. Journal of Clinical Medicine 9(9): 2795.
- Anitua E, Muruzabal A, Orive G (2014) Preservation of Biological Activity of Plasma and Platelet-Derived Eye Drops After Their Cryopreservation. Cornea 33(4): 340-348.
- Anitua E, Prado R, Orive (2012) Platelet-rich plasma: Preparation and formulation. Operative Techniques in Orthopedics 22: 25-32.
- Olivier Bausset, Laurent Giraudo, Julie Veran, Jeremy Magalon, Jean Marie Coudreuse, et al., (2012) Formulation and storage of platelet-rich plasma homemade product. BioResearch Open Access 1(3): 115-123.
- Cavalcante MB, Costa FDS, Araújo ACD, Barros CO, & Lôbo MDF (2020) Efficacy of autologous platelet-rich plasma in the treatment of thin en- dometrium. JBRA Assisted Reproduction 24(4): 422-427.
- Everts PA, Knape JT, Weibrich G, Schönberger JP, Hoffmann J, et al., (2006) Platelet-rich plasma and platelet gel: a review. Journal of Extra-corporeal Technology 38(2): 174-187.
- Pratap Kumar, Anjali Mundkur, D Sai Bhavna, Vasanthi Palanivel, Prashanth Adiga, et al. (2023) Intrauterine administration of autologous platelet-derived growth factor concentrate (aka autologous blood cell derivative) im- proves the endometrial thickness in 'thin' endometrium in the frozen embryo transfer cycle. J Obstet Gynecol India 73(Suppl 1): 108-114.
- Murdia K, Chandra V, Bhoi N, Gupta S, Gupta N, et al. (2023) Treatment of refractory thin endometrium with autologous blood cell derivative (ABCD-Endosera-Endosera): Advancing toward a next-generation of platelet-de- rived growth factors in frozen embryo transfer cycles: A pilot study. Fertil Sci Res 10(3): 151-157.
- Kasius A, Smit JG, Torrance HL, Eijkemans MJ, Mol BW, et al. (2014) Endometrial thickness and pregnancy rates after IVF: a systematic review and meta-analysis. Hum Reprod Update 20(4): 530-541.
- Khan MS, Shaikh A, Ratnani R (2016) Ultrasonography and Doppler Study to Predict Uter-ine Receptivity in Infertile Patients Undergoing Embryo Transfer. J Obstet Gynaecol India 66(Suppl 1): 377-382.
- Burnouf T, Goubran HA, Chen TM, Ou KL, El Ekiaby, et al. (2013) Blood-derived biomaterials and platelet growth factors in regenerative medicine. Blood Reviews 27(2): 77-89.
- Marx RE (2001) Platelet-rich plasma (PRP): what is PRP and what is not PRP. Implant Dentistry 10(4): 225-228.
- Gomes F, Cronemberger Andrade A, Wolf M, Hochmann S, Krisch L, et al. (2022) Synergy of Human Platelet-Derived Extracellular Vesicles with Secretome Proteins Promotes Regenerative Functions. Biomedicines 10(2): 238.
- Verma R, Kumar S, Garg P (2023) Platelet-rich plasma: a comparative and economical therapy for wound healing and tissue regeneration. Cell Tissue Bank 24: 285-306.
- Herrera Vizcaíno C, Dohle E, Al Maawi S, Booms P, Sader R, et al. (2019) Platelet-rich fibrin secretome induces three dimensional angiogenic activation in vitro. European Cells and Materials 37: 250-264.
- Scopelliti F, Cattani C, Dimartino V, Mirisola C, Cavani A, et al. (2022) Platelet De- rivatives and the Immunomodulation of Wound Healing. International Journal of Molecular Sciences 23(15): 8370.
- Singh N, Mohanty S, Seth T, Shankar M (2014) Autologous intrauterine platelet-rich plasma instillation for suboptimal endometrium in frozen embryo trans- fer cycles: A pilot study. Journal of Human Reproductive Sciences 10(3): 208-213.
- Brokhman I, Galea AM (2023) A novel method for the preparation and frozen storage of growth factors and cytokines obtained from platelet-rich plasma. Journal of Cartilage Joint Preservation® 3(2023): 100089.
- Hu S, Jin Z, Tang Q (2023) Effects of Intrauterine Infusion of Autologous Platelet-Rich Plasma in Women Undergoing Treatment with Assisted Reproductive Technology: a Meta-Analysis of Randomized Controlled Trials. Geburtshilfe Frauenheilkd 83(4): 453-462.
- Lin PY, Lee CI, Chen YC, Cheng EH, Huang CC, et al. (2023) Factors Affecting the Potential Efficacy of Intrauterine Platelet-Rich Plas- ma Infusion on Thin Endometrium in Women with Recurrent Implantation Failure. Journal of Personalized Medicine 13(9): 1419.
- Klatte Schulz F, Schmidt T, Uckert M, Scheffler S, Kalus U, et al. (2018) Comparative Analysis of Different Platelet Lysates and Platelet Rich Preparations to Stimulate Tendon Cell Biology: An In Vitro Study. Int J Mol Sci 19(1): 212.
- Iudicone P, Fioravanti D, Bonanno G, Miceli M, Lavorino C, et al. (2014) Pathogen-free, plasma-poor platelet lysate and expansion of human mesenchymal stem cells. J Transl Med 12:28.
- El Backly R, Ulivi V, Tonachini L, Cancedda R, Descalzi F, et al. (2011) Platelet lysate induces in vitro wound healing of human keratinocytes associated with a strong proinflammatory response. Tissue Eng Part A 17(13-14): 1787-1800.
- Meftahpour V, Malekghasemi S, Baghbanzadeh A, Aghebati Maleki A, Pourakbari R, et al. (2021) Platelet lysate: A promising candidate in regenera- tive medicine. Regen Med 16(1): 71-85.
- Ban Y, Yang X, Xing Y, Que W, Yu Z, et al. (2023) Intrauterine Infusion of Leukocyte-Poor Platelet-Rich Plasma Is an Effective Therapeutic Protocol for Pa-tients with Recurrent Implantation Failure: A Retrospective Cohort Study. J Clin Med 12(8): 2823.
- Burnouf T, Strunk D, Koh MB, Schallmoser K (2016) Human platelet lysate: Replacing fe- tal bovine serum as a gold standard for human cell propagation? Biomaterials 76:371-387.
- Schallmoser K, Henschler R, Gabriel C, Koh MBC, Burnouf T (2020) Production and Qual-ity Requirements of Human Platelet Lysate: A Position Statement from the Working Party on Cellular Therapies of the International Society of Blood Transfusion. Trends Biotechnol 38(1): 13-23.
- Altaie A, Owston H, Jones E (2016) Use of platelet lysate for bone regeneration-Are we ready for clinical translation? World J Stem Cells 8(2): 47-55.
- Madero S, Rodriguez A, Vassena R, Vernaeve V (2016) Endometrial Preparation: EffectofEstrogen Dose and Administration Route on Reproductive Outcomes in Ooc yte Donation Cycles with Fresh Embryo Hum Reprod 31(8): 1755-1764.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.