Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Evaluation of Skin Fibrotic Response to Injury: A Literature Review
*Corresponding author: Frederick H. Silver, Department of Pathology and Laboratory Medicine, Rutgers Robert Wood Johnson Medical School, New Jersey, USA.
Received: April 18, 2024; Published: April 23, 2024
DOI: 10.34297/AJBSR.2024.22.002938
Abstract
Fibrosis is a critical process in tissue repair and wound healing, marked by collagen deposition and extracellular matrix alterations. This review explores fibrosis in wound healing, differentiating collagen deposition in repair, regeneration, and tissue fibrosis. Collagen deposition in repair and regeneration aims to restore tissue integrity, while in cancer-associated fibrosis, interactions between cancer cells and stromal cells, especially cancer-associated fibroblasts (CAFs), drive collagen-rich extracellular matrix deposition. Distinguishing between wound healing-related and cancer-related fibrosis is crucial for targeted therapies. A variety of cells, including mesenchymal stem cells (MSCs), macrophages, and myofibroblasts, play pivotal roles in collagen deposition, tissue remodeling, and wound contraction. Their activities vary among wound types, influencing healing outcomes.
Mechanosignaling, mediated by integrins and focal adhesion complexes, regulates cellular behaviors in wound healing. Differences in fibrosis mechanotransduction pathways exist between cancerous and non-cancerous lesions, impacting tissue repair. Growth factors such as EGF and FGF-β, along with molecules such as beta-catenin and WNT signaling, have critical roles in regulating mechanotransduction in wound healing and cancer. The conversion of E-cadherin to N-cadherin in focal adhesions, collagen mutations affecting integrin binding, and experimental models contribute to understanding fibrosis.
Knowledge gaps persist, warranting further research to explore noninvasive tools such as vibrational optical coherence tomography (VOCT) for fibrosis assessment. Differentiating fibrosis in wound healing and cancer lesions is crucial for clinical management. Insights from this review can provide information for targeted therapies to modulate fibrotic responses, prevent excessive scarring, and optimize tissue regeneration.
Keywords: Wound healing, Skin fibrotic response, Fibrosis, Collagen, EGF, FGF, Beta-catenin, cadherins, WNT signaling
Introduction
Fibrosis, marked by excessive collagen deposition and extracellular matrix alterations, is a critical process in tissue repair and wound healing. It holds vital importance in both research and clinical practice, with fibrotic disorders contributing significantly to mortality rates in the United States. An estimate of 45% of all deaths are due to or are associated with fibrotic disorders [1]. This review aims to comprehensively explore fibrosis in wound healing, with a specific focus on differentiating collagen deposition in repair, regeneration, and tissue fibrosis.
In repair and regeneration, collagen deposition is a normal aspect of the healing process, striving to restore tissue integrity and function [2,3]. Repair entails granulation tissue formation with collagen deposition, angiogenesis, and inflammatory cell infiltration [4]. This tissue is replaced over time by connective tissue and epithelial cells during the remodeling phase in the form of scar tissue [5]. Tumor stroma resembles scar tissue in terms of the presence and active participation of CAFs, endothelial and immune cells, and extracellular matrix (ECM) [6,7].
Repair leads to tissue fibrosis, involving excessive and improper collagen deposition, culminating in dense, stiff fibrotic scars [6]. This occurs when the original tissue structure is significantly lost, often due to persistent inflammation or prolonged injury. An imbalance between collagen synthesis and degradation results in an accumulation of collagen-rich extracellular matrix components, leading to excessive cross-linking and functional impairment in scar tissue.
In the context of skin cancer, fibrosis is unique, primarily driven by interactions between cancer cells and stromal cells, particularly cancer-associated fibroblasts (CAFs) [6]. CAFs foster collagen-rich extracellular matrix deposition in the tumor microenvironment, promoting tumor growth and invasion [6]. Understanding these differences in collagen deposition is essential for targeted therapeutic development.
The significance of fibrosis in wound healing extends to clinical management, where it can impair tissue function and lead to debilitating scars, particularly in chronic wounds and fibrotic disorders [7]. Distinguishing between wound healing-related and cancer-related fibrosis is crucial, as they occur in distinct contexts with different implications [6,7].
Exploring the roles of various cells, including monocytes, in fibrosis is essential. Differentiating between benign and cancerous fibrosis provides valuable insights into their pathophysiological mechanisms and may guide condition-specific therapeutic strategies. Given the importance of fibrosis in wound healing and its clinical impact, this review aims to assess the existing literature, identify knowledge gaps, and provide insights for future research and clinical management.
Methods
A comprehensive literature review was conducted to gather relevant information on the fibrotic response in wound healing, specifically focusing on the skin. The review process involved an extensive search of the PubMed database, utilizing key search terms such as "skin fibrotic response," "skin fibrotic response healing," and "skin fibrotic response cancer." The objective was to identify studies that investigated fibrosis in wound healing and its association with skin-related conditions, including cancer. Inclusion criteria was set to include all study types published in the English language, providing a broad scope of research encompassing experimental studies, clinical trials, observational studies, and reviews. Papers not published in English or those not specifically evaluating fibrosis in the context of wound healing were excluded from the review. The retrieved articles were then assessed based on their titles and abstracts to determine their relevance to the research objective. The screened papers were then reviewed in full text, and those not found to meet inclusion and exclusion criteria were excluded.
Discussion
Fibrosis in Wound Healing versus Cancer Lesion Fibrosis
Collagen deposition occurs during normal wound healing, foreign body responses to implants, cancer associated fibrosis, genetic connective tissue disorders, keloid formation, and burn wound contractures. While the structural role of collagen involves energy storage and transmission, providing mechanical support for tissues and preventing premature mechanical failure, it is also a natural substrate for cellular attachment during skin regeneration and repair. The processes of collagen deposition, collagen synthesis and secretion can go awry resulting in hypertrophic scarring, keloid formation, cancer associated fibrosis, tissue failure as a result of genetic connective skin disorders, and burn wound contractures [8,9]. The cellular and molecular mechanisms driving collagen deposition are intricate, involving the activation and differentiation of diverse cell types. Fibroblasts, myofibroblasts, and other stromal cells play pivotal roles in collagen synthesis and deposition [8]. Moreover, growth factors, cytokines, and signaling pathways, such as transforming growth factor-beta (TGF-β), have been implicated in regulating wound healing-related fibrosis [9]. A profound comprehension of these mechanisms is indispensable for the development of precisely targeted therapies to modulate fibrotic responses and facilitate proper wound healing.
Comparative analysis of fibrosis across various tissue types reveals notable divergences in fibrotic processes and outcomes. Skin fibrosis, for instance, may manifest distinct characteristics in contrast to fibrosis in other anatomical regions [10]. These disparities extend to the underlying mechanisms, cellular interactions, and molecular signaling pathways, emphasizing the need for tissue-specific strategies in comprehending and addressing fibrosis.
Distinguishing between fibrosis linked to epithelial-mesenchymal transition (EMT) in wound healing and fibrosis associated with cancer-associated fibroblasts (CAFs) and cellular mutations in skin cancer assumes paramount significance. EMT signifies a process wherein epithelial cells undergo a phenotypic shift towards a mesenchymal state. EMT has been associated with both physiologic regeneration and pathological fibrotic conditions, such as in the context of acne [11]. Conversely, fibrosis linked to CAFs and cellular mutations in skin cancer contributes to tumor progression and invasion, entailing intricate interactions among cancer cells, stromal elements, and the extracellular matrix [12].
A grasp of these distinctions offers valuable insights into the distinct cellular origins, molecular pathways, and therapeutic avenues for each condition. Keloids and hypertrophic scars stand apart from typical scars in terms of their pathogenesis and clinical presentation. Keloids are characterized by excessive collagen deposition extending beyond the wound boundaries, yielding raised, thickened, and often pruritic scars [10]. In contrast, hypertrophic scars remain confined within the original wound borders and tend to regress over time. The mechanisms underpinning keloid and hypertrophic scar formation encompass dysregulated collagen synthesis, altered cellular signaling, and irregular wound healing responses [10]. Substantial further inquiry is imperative to elucidate the specific factors contributing to keloid and hypertrophic scar development, as well as to identify efficacious treatment modalities.
Wound contracture affecting joints is seen during the healing of a burn that reduces the original wound size and appears to involve myofibroblasts. Myofibroblasts are important cells that contain a contractile element, alpha-smooth muscle actin (SMA), which contributes to the contracture of a scar [11]. During re-epithelization of a wound, myofibroblasts usually disappear ending further contracture. However, if this process continues through the re-epithelization of wound healing after a severe burn, a debilitating wound may result leading to extremely limited range of motion and poor cosmetic outcomes [12]. The balance between contraction of myofibroblasts and re-epithelialization determines the flexibility and quality of a healed wound and determines level of scar formation (Table 1, Figure 1).
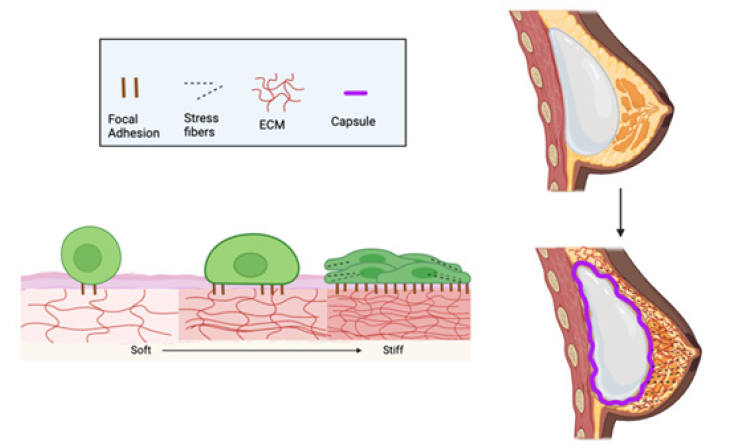
Figure 1: Diagram illustrating how substrate stiffness can affect the cellular response during healing resulting in increased focal adhesions and accumulation of stress fibers within cells. (Left) When fibroblasts and inflammatory cells accumulate in a wound they initially attach to the exposed tissue after necrotic tissue is removed. They then form focal adhesions through integrin mediated MAP kinase pathways leading to stress fiber formation and the production of stiff collagen matrices. (Right) An example of this occurs when an implant is placed in the breast. This leads to the formation of a collagenous capsule around the implant as a result of a foreign body response. The foreign body response leads to capsular contraction around the implant which can cause pain and implant movement. Figure 1 was created under a license from Bio render.
Granulation Tissue in Wound Healing
Granulation tissue deposition is a hallmark of the proliferative phase in wound healing, marked by the formation of new blood vessels and connective tissue components [13]. However, the presence and extent of granulation tissue are subject to variability, contingent upon factors like wound type and severity, underlying conditions, and individual healing responses. In minor injuries, such as those affecting the epidermis and superficial dermis, substantial granulation tissue formation may not be a requisite for healing. Such wounds may promptly progress to the remodeling phase, where collagen fibers reorganize and mature. This accelerated process, stemming from an efficient inflammatory response and rapid tissue repair, results in wound resolution without the deposition of prominent granulation tissue [13].
Conversely, deep chronic or non-healing wounds often exhibit a protracted inflammatory phase and impaired granulation tissue development. Factors like persistent infection, inadequate blood supply, or systemic illnesses can disrupt the natural course of wound healing, impeding granulation tissue formation [10]. Consequently, these wounds may exhibit delayed or incomplete healing, potentially leading to chronicity or excessive fibrosis [11]. In the case of wound healing after breast implantation, fibrous capsules can continue to grow around the implants and undergo capsular contraction for years, leading to pain and cosmetic changes requiring implant removal [13,14]. Despite the softness of breast implants, scar tissues continue to growth due to the hyperactivity of contractile myofibroblasts that secrete collagen and other ECM proteins [15,16]. Thus, the cycle of wound healing may repeat indefinitely, resulting in continued growth of scar tissue [15].
The stages of wound healing can diverge based on lesion nature and location. Variables such as wound depth, the presence of foreign bodies or necrotic tissue, and specific tissue involvement can dictate the processes and timeline of wound repair [15]. For example, wounds affecting deeper tissues like muscle or tendons may necessitate more intricate and extended healing processes in comparison to superficial wounds.
Furthermore, distinct wound types, encompassing acute traumatic wounds, surgical incisions, and chronic ulcers, may display variations in the inflammatory response, angiogenesis, and cellular events during healing [16]. The underlying pathophysiology and lesion-specific microenvironment significantly impact the phases and ultimate outcomes of wound healing.
The Role of Mesenchymal Stem Cells, Macrophages, and Myofibroblasts
The roles of mesenchymal stem cells (MSCs), M1 and M2 macrophages, and myofibroblasts in tissue deposition, collagen remodeling, and wound contraction are pivotal in the wound healing process. MSCs possess the potential to differentiate into various cell types, including fibroblasts, which are central to collagen synthesis and deposition [17]. Furthermore, MSCs exert immunomodulatory effects, foster tissue regeneration through paracrine actions, secrete growth factors, and interact with other cell types involved in wound healing.
Macrophages, fundamental to the inflammatory phase of wound healing, exhibit distinct M1 and M2 phenotypes. M1 macrophages contribute to the early inflammatory response and facilitate bacterial clearance, while M2 macrophages participate in tissue repair and extracellular matrix remodeling [18]. M2 macrophages release anti-inflammatory cytokines and growth factors, thereby promoting collagen synthesis and matrix remodeling [19]. Their presence is crucial for resolving inflammation and transitioning from the inflammatory to the proliferative phase of wound healing.
Myofibroblasts, contractile cells, significantly contribute to wound contraction, tensile strength, and tissue remodeling. Their differentiation from fibroblasts is triggered by various signals, including mechanical tension and growth factors like transforming growth factor-beta (TGF-β) [20]. Myofibroblasts express alpha-smooth muscle actin (α-SMA) and are key drivers of wound contraction through their contractile properties. However, when unregulated, these contractile cells are the cause of debilitating wound contracture in burn patients and capsular contraction of breast implants [21].
The presence of these cells and activities can influence collagen deposition and wound contraction. MSCs, via differentiation into fibroblasts, impact the quantity and quality of extracellular matrix collagen. Moreover, MSCs modulate the behavior of other cells, including macrophages and myofibroblasts, through paracrine signaling, further affecting collagen deposition and tissue remodeling [17]. Macrophages, particularly M2 macrophages, are actively involved in collagen synthesis and remodeling, with their secreted factors, such as TGF-β and other growth factors, promoting collagen fiber production and organization [18]. An imbalance between M1 and M2 macrophages can lead to compromised collagen deposition and hindered wound healing. Myofibroblasts, due to their contractile properties, are essential for wound closure and wound size reduction. Disruptions in myofibroblast recruitment or function can result in delayed wound contraction and impaired healing.
The roles and behaviors of MSCs, M1 and M2 macrophages, and myofibroblasts in tissue deposition, collagen remodeling, and wound contraction can vary among different wound types. Factors like the underlying pathophysiology, wound location, and comorbidities can influence these cells' functions [21]. In acute traumatic wounds or surgical incisions, MSCs, M2 macrophages, and myofibroblasts generally contribute to typical tissue repair, collagen synthesis, and wound contraction. However, in chronic wounds such as diabetic ulcers or venous ulcers, altered cell behavior may occur due to persistent inflammation and an impaired healing environment, resulting in impaired collagen deposition, delayed healing, and compromised wound contraction [22]. Specific wound types like burns or pressure ulcers present unique challenges and may exhibit variations in the inflammatory response and interactions with MSCs, macrophages, and myofibroblasts. Understanding these differences is paramount for developing tailored therapies to optimize healing outcomes in various wound contexts. Biofilm formation on wounds poses a significant obstacle to healing by shielding bacteria and perpetuating inflammation, disrupting typical healing processes. In acute wounds, MSCs, M2 macrophages, and myofibroblasts facilitate tissue repair, but chronic wounds such as diabetic or venous ulcers suffer from altered cell behavior due to prolonged inflammation, leading to impaired collagen deposition and delayed healing [19]. Different wounds, such as burns or pressure ulcers, present unique challenges with varied inflammatory responses and interactions with healing cells. Tailoring therapies to address biofilm-related impediments and leveraging the potential of cells involved in healing can optimize outcomes in diverse wound contexts.
EMT Transition and Dysregulation of Mechanosignaling
The Epithelial-Mesenchymal Transition (EMT) is a fundamental process that plays a critical role in tissue development, wound healing, and is also implicated in pathological conditions such as cancer and scarring. EMT involves the transformation of epithelial cells into mesenchymal-like cells with enhanced migratory and invasive properties [6]. In wound healing, EMT contributes to tissue regeneration and repair by allowing epithelial cells to adopt a migratory phenotype, facilitating the formation of granulation tissue [22]. Epidermal keratinocytes become motile and possess mesenchymal traits during EMT. During wound repair, re-epithelialization may be thought of as a partial and reversible EMT process [23]. However, in the context of cancer and scarring, EMT can become dysregulated, thereby contributing to disease progression.
One intriguing facet related to EMT in cancer and scarring is its potential impact on mechanosignaling pathways. Mechanosignaling refers to the transmission of mechanical forces and cues that regulate cellular behaviors and tissue remodeling [24]. EMT has been shown to modulate mechanosignaling pathways, including the activation of focal adhesion kinase (FAK), Rho GTPases, and cytoskeletal remodeling [25]. Integrin-mediated mechanotransduction initiates a cellular response through the sensing of these mechanical signals by integrins, which transmits forces to FAKs, collagen, and stress fibers of intracellular proteins such as F-actin, talin, and vinculin. Furthermore, the binding of collagen and integrin produces stronger, load-bearing structures for efficient cell-matrix adhesion [26]. These mechanosignaling pathways with integrin-mediated mechanotransduction are pivotal in cell migration, invasion, and tissue contractility [27].
In cancer, dysregulated EMT can induce changes in mechanosignaling pathways that promote tumor cell invasion and metastasis. The acquisition of a mesenchymal phenotype enhances tumor cell contractility, enabling them to penetrate the extracellular matrix and invade neighboring tissues [28]. Furthermore, changes in mechanosignaling can impact the tumor microenvironment, influencing stromal cell behavior and altering the biomechanical properties of the tumor.
Similarly, in scarring and fibrosis, dysregulated EMT can contribute to excessive collagen deposition and tissue stiffening [27]. Changes in mechanosignaling pathways may also trigger the activation of myofibroblasts, contractile cells involved in tissue remodeling [21]. Dysregulated mechanosignaling can lead to increased myofibroblast contractility, excessive collagen production, and altered tissue architecture, ultimately resulting in the formation of fibrotic scars.
Understanding the interplay between EMT and mechanosignaling in cancer and scarring is of paramount importance. The specific molecular mechanisms and signaling pathways involved in these processes offer valuable insights into the development of targeted therapies aimed at modulating EMT and mechanosignaling to prevent or treat cancer metastasis and fibrotic disorders. Additional research is needed to fully elucidate the intricate connections between EMT, mechanosignaling, and disease progression, providing promising avenues for improving clinical interventions and patient outcomes.
Regarding the change in cadherins during fibrosis associated with cancer, it's important to note that EMT often involves alterations in cadherin expression [29]. Epithelial cells typically express E-cadherin, which plays a role in cell-cell adhesion and maintaining epithelial integrity. During EMT, there is a downregulation of E-cadherin and an upregulation of N-cadherin, a mesenchymal cadherin [29]. These changes in cadherin expression contribute to the loss of epithelial characteristics and the acquisition of mesenchymal properties by cells undergoing EMT. In the context of fibrosis associated with cancer, EMT can lead to similar changes in cadherin expression, which may promote cancer cell invasion and metastasis [29]. Understanding these cadherin changes is vital for targeting EMT-related processes in cancer treatment and metastasis prevention [30]. CAFs mediate increased ECM stiffness through inducing rigidly oriented collagen fibers that promotes EMT of cancer cells. The mechanical forces exerted by the stiffened environment induces TWIST1, an EMT- promoting transcription factor [30].
Mechanosignaling in Wound Healing & Differences in Fibrotic Mechanotransduction Pathways in Cancerous and Non-Cancerous Lesions
Mechanosignaling is a pivotal factor in wound healing, translating mechanical cues from the extracellular matrix (ECM) into biochemical signals that regulate cellular behavior and tissue remodeling. In non-cancerous skin lesions, such as acute wounds or surgical incisions, mechanosignaling pathways play a crucial role in coordinating cellular activities essential for effective wound healing [22]. These pathways encompass mechanosensors like integrins and focal adhesion complexes, which sense and respond to mechanical forces acting on the cells. Substrate stiffness is one of the factors that can impact cellular response, including growth, differentiation, and morphology. Yeung, et al. (2005) suggest that cell-cell interactions can override the influence of substrate on the cell. Moreover, in the case of capsular contraction of breast implants, the immune response to a foreign body mediates cellular response, forming the capsule [16,31,32]. Thus, substrate stiffness is not the only key influencer in cellular response.
In the context of wound healing, mechanotransduction pathways in non-cancerous skin lesions predominantly direct cell migration, proliferation, and tissue contraction. Mechanical forces stemming from ECM stiffness and tension trigger integrin clustering, subsequently activating downstream signaling molecules including Focal Adhesion Kinase (FAK), Rho GTPases, and Yes-associated protein (YAP)/transcriptional co-activator with PDZ-binding motif (TAZ) [12]. The activation of these pathways orchestrates cytoskeletal remodeling, cell polarization, and the secretion of growth factors and cytokines, facilitating wound closure and tissue regeneration [12,26].
Conversely, mechanotransduction pathways in cancerous skin lesions exhibit distinct characteristics due to the dysregulated cellular behaviors linked with cancer progression. Cancer cells often display altered mechanical properties, mostly increased stiffness, and demonstrate aberrant mechanosignaling responses [31]. Dysregulation in mechanotransduction pathways can enhance tumor cell survival, invasion, and metastasis. In cancerous skin lesions, mechanosignaling pathways play a role in promoting cancer cell migration, invasion, and the remodeling of the tumor microenvironment [32]. Altered mechanosignaling can lead to heightened activation of focal adhesion complexes, increased contractility, and cytoskeletal reorganization, thereby facilitating tumor cell invasion through the ECM [33].
Increased collagen stiffness has been reported to increase FA complexes which provides sufficient traction for cell spreading, proliferation, and migration, ultimately leading to enhanced mechanotransduction and dramatic effects on cellular response [33]. Moreover, dysregulated mechanosignaling in cancer cells can influence intercellular communication, angiogenesis, and immune responses within the tumor microenvironment [33].
Furthermore, distinctions in fibrosis mechanotransduction pathways between cancerous and non-cancerous skin lesions are evident. In non-cancerous skin lesions, fibrosis is a tightly regulated process that aids in tissue repair. Mechanosignaling pathways involved in fibrosis, such as TGF-β/Smad and YAP/TAZ, are critical for activating and differentiating fibroblasts into myofibroblasts, leading to collagen production, tissue remodeling, and associations with cancerous lesions [12].
In cancerous skin lesions, fibrosis is often linked to tumor progression and unfavorable clinical outcomes. Fibrosis mechanotransduction pathways in cancer may encompass interactions among Cancer-Associated Fibroblasts (CAFs), tumor cells, and the ECM. CAFs can exhibit enhanced contractility and altered mechanosignaling responses, resulting in excessive collagen deposition and tissue stiffening, thereby contributing to tumor progression and treatment resistance.
Understanding the disparities in mechanotransduction pathways between cancerous and non-cancerous skin lesions is of paramount importance in developing targeted therapeutic approaches. Targeting specific mechanosignaling molecules or pathways involved in cancer-associated fibrosis offers novel prospects for intervention to curb tumor growth, invasion, and metastasis. Further investigations are warranted to elucidate the precise mechanisms and identify potential therapeutic targets within the fibrosis mechanotransduction pathways in cancerous skin lesions, ultimately enhancing patient outcomes and treatment strategies.
Mechanotransduction in Fibrosis and Wound Healing: Epidermal Growth Factor (EGF) and Fibroblast Growth Factor Beta (FGF-β)
Mechanotransduction, the process by which mechanical forces are converted into biochemical signals, plays a pivotal role in wound healing and fibrosis. Among the many molecular players, epidermal growth factor (EGF) and fibroblast growth factor beta (FGF-β) stand out as key regulators [5]. EGF released by platelets is involved in cell proliferation, migration, and tissue regeneration, making it vital during the initial phases of wound healing [5]. FGF-β, on the other hand, contributes to angiogenesis, stimulating the formation of new blood vessels crucial for delivering oxygen and nutrients to the healing tissue.
However, beyond growth factors like EGF and FGF-β, ion channels and receptors for growth factors and hormones also contribute significantly to this process, acting as sensors for mechanical cues, initiating signaling cascades that impact cell behavior [5]. Moreover, receptors for growth factors and hormones, including those found on MSCs, play essential roles in transducing mechanical stimuli into biochemical signals, influencing cellular activities crucial for healing and tissue regeneration [32]. MSCs themselves have been shown to release FGF-β during the healing process, contributing to the intricate network of signaling molecules that regulate tissue repair and regeneration [33]. Understanding these multifaceted interactions among ion channels, receptors, growth factors, and the role of MSCs in releasing FGF-β enhances our comprehension of the complex mechanisms underpinning effective wound healing and tissue regeneration.
Mechanical forces can induce the release of growth factors from inflammatory cells; however, prolonged forces can lead to chronic inflammatory responses, resulting in fibrosis and excessive scar formation [34].
In the context of skin cancer, dysregulation of EGF and FGF-β signaling pathways can occur. Tumor cells often overexpress growth factor receptors, that can be stimulated by mechanical forces and other cell surface recptors leading to uncontrolled cell division and migration [35]. This can disrupt the orderly process of wound healing and promote tumor invasion. Understanding the interplay between these growth factors and mechanotransduction pathways can facilitate understanding of how cancer lesions seize these mechanisms for their benefit.
The Role of Beta-Catenin and WNT Signaling in Wound Healing and Fibrosis
To advance our understanding of mechanotransduction in the context of wound healing and fibrosis, a meticulous examination of the roles played by beta-catenin and the WNT signaling pathway is imperative. These molecular components are pivotal contributors to the intricate network of mechanotransduction processes and warrant substantial consideration within our comprehensive review.
Beta-catenin, a multifunctional protein deeply involved in cell adhesion and transcriptional regulation, assumes a multifaceted role in mechanotransduction [36]. In the context of wound healing, beta-catenin operates as a molecular switch, facilitating the transition from an epithelial to a mesenchymal state, a transition commonly denoted as EMT [36]. EMT holds a fundamental role in the migration and proliferation of cells during tissue repair. Moreover, the influence of beta-catenin on cell-cell adhesion and signaling substantially impacts tissue regeneration by meticulously regulating cellular responses to mechanical cues.
Similarly, the WNT signaling pathway, with its intricate network of ligands and receptors, is intricately intertwined with mechanotransduction.
Throughout the wound healing process, WNT signaling effectively orchestrates various cellular behaviors, including cell migration, proliferation, and differentiation [37]. Furthermore, this pathway interfaces with beta-catenin, operating as a potent regulator of EMT and fibrosis. While these mechanotransduction actors are fundamental in normal wound healing, their dysregulation can yield profound disparities in fibrosis associated with skin cancers [38]. Within the cancer context, mutations in beta-catenin or aberrant activation of the WNT signaling pathway can precipitate uncontrolled cell proliferation, invasion, and metastasis. These alterations disrupt the finely tuned mechanisms of mechanotransduction that govern tissue homeostasis and repair, ultimately contributing to the pathogenesis of cancer [38].
As we delve deeper into the ramifications of beta-catenin and WNT signaling on mechanotransduction in wound healing and fibrosis, it becomes increasingly apparent that these molecular entities play a central role in orchestrating cellular responses to mechanical stimuli. Understanding the intricate ways in which they influence these processes gives invaluable insights into both normal and pathological tissue repair. Furthermore, it unveils novel avenues for targeted interventions. By crafting therapeutic strategies designed to modulate beta-catenin and WNT signaling, we have the potential to enhance wound healing and mitigate fibrosis associated with skin cancers. This knowledge holds a prominent position in translational research, harboring the potential to bridge the gap between fundamental mechanotransduction science and enhanced clinical outcomes for patients with a diverse array of skin conditions.
Additionally, the application of Hippo pathway inhibitors to treat BCCs that do not heal after excision serves as a promising example of how comprehending mechanotransduction can lead to innovative clinical interventions, particularly through the inhibition of WNT signaling [39].
Conversion of E-Cadherin to N-Cadherin in Focal Adhesions
Another critical aspect of mechanotransduction in wound healing and its relation to skin cancer involves the conversion of E-cadherin to N-cadherin within focal adhesions [40]. E-cadherin is typically responsible for maintaining cell-cell adhesion in epithelial tissues. During wound healing, E-cadherin can be downregulated, leading to the loss of epithelial characteristics and enabling cells to adopt a more migratory, mesenchymal phenotype - a hallmark of EMT [40]. N-cadherin, associated with a more motile phenotype, can then take its place, facilitating cell movement during tissue repair [40].
In skin cancer, this process can become dysregulated, contributing to increased tumor cell mobility and invasion. The switch from E-cadherin to N-cadherin in focal adhesions allows cancer cells to detach from the primary tumor site and infiltrate neighboring tissues [41]. Investigating the precise mechanisms behind this cadherin switch within focal adhesions is crucial to understanding how cancer lesions manipulate mechanotransduction pathways to drive their behavior.
Collagen Mutations and Integrin Binding
Collagen mutations also warrant attention when considering their potential impact on mechanotransduction pathways in wound healing and skin cancer. Collagen is a major component of the ECM and serves as a substrate for integrin binding - a key event in mechanotransduction [42]. Integrins are cell surface receptors that interact with the ECM, transmitting mechanical signals into intracellular responses [42].
In cases where collagen mutations affect the binding affinity of integrins, this can disrupt the formation of focal adhesions and downstream mechanotransduction processes [43]. Such disruptions may downregulate cell-mediated mechanotransduction pathways, impairing the ability of cells to sense and respond to mechanical cues in the microenvironment [43]. This can have implications for both normal wound healing, where proper ECM remodeling is crucial, and skin cancer, where changes in the tumor microenvironment can influence tumor progression and metastasis (Table 2).
Experimental Models for Fibrosis
Experimental models play a vital role in studying fibrosis in wound healing, allowing researchers to investigate cellular and molecular processes, test potential therapeutic interventions, and explore the efficacy of targeted approaches. Various animal models, such as mice, rats, and pigs, have been utilized to simulate wound healing and fibrotic responses [44]. In-vitro models using cell cultures and tissue engineering techniques also provide valuable insights into specific cellular interactions and signaling pathways involved in fibrosis [45].
Knowledge Gaps and Further Investigations
Despite significant advancements in understanding fibrosis in wound healing, several knowledge gaps and areas for future research remain. Further investigations are needed to unravel the precise mechanisms underlying EMT in regeneration, repair, and pathological tissue fibrosis. Additionally, elucidating the genetic and molecular alterations associated with fibrosis in wound healing, particularly in skin cancer, can aid in the development of targeted therapies and personalized treatment approaches. Moreover, there is a need to explore novel experimental models and techniques that more accurately recapitulate the complex microenvironment of fibrosis in wound healing.
In addition to the findings and implications discussed, it is important to consider the role of noninvasive tools in the study and clinical assessment of fibrosis in wound healing and cancer. One such tool is vibrational optical coherence tomography (VOCT). VOCT holds promise for noninvasive assessment and monitoring of fibrosis in wound healing and cancer [46]. It can provide real-time, three-dimensional imaging of tissue structures, allowing researchers and clinicians to visualize and analyze the collagen deposition, organization, and remodeling processes associated with fibrosis [46].
Additionally, VOCT's molecular sensitivity enables the identification and characterization of specific biomarkers and molecular alterations related to fibrosis, aiding in early detection and personalized treatment approaches [47]. The integration of VOCT into clinical practice can have significant implications, potentially offering an alternative to invasive procedures, while reducing patient discomfort and the risk of complications. VOCT's ability to provide quantitative and objective measurements can enhance diagnostic accuracy and facilitate treatment decision-making. Moreover, the longitudinal monitoring capabilities of VOCT can enable the assessment of treatment response and disease progression over time, guiding therapeutic interventions and optimizing patient outcomes.
Conclusion
Understanding the distinction between fibrosis in wound healing and cancer lesion fibrosis has significant implications for clinical management. It has the potential to aid in accurate diagnosis, appropriate treatment selection, and improved patient outcomes. This knowledge can inform the development of targeted therapies to modulate fibrotic responses in wound healing, prevent excessive scarring, and promote optimal tissue regeneration. Additionally, understanding the mechanisms underlying fibrosis in cancer lesions can guide the creation of therapeutic strategies to target the tumor microenvironment and mitigate the pro-fibrotic effects associated with cancer-associated fibroblasts. Furthermore, mechanotransduction plays a pivotal role in both wound repair and cancer-related fibrosis, as the process encompasses physical forces acting on cell surface mechanosensors that link mechanical impact to cytoskeleton structures. Mechanotransduction may activate intracellular signaling cascades and yield pleiotropic mediators or engage developmental pathways with complex genetic programs and cellular outcomes.
Further research is needed to address the identified knowledge gaps and expand the understanding of fibrosis in wound healing and cancer. Future studies may focus on identifying the intricate cellular and molecular mechanisms underlying epithelial-mesenchymal transition in regeneration, repair, and pathological tissue fibrosis. Additionally, investigations into the genetic and molecular alterations associated with fibrosis in wound healing and skin cancer can provide insights into personalized treatment approaches and targeted interventions. The clinical relevance of this research lies in its potential to improve the management of fibrotic conditions associated with wound healing and cancer. By elucidating the underlying mechanisms and identifying therapeutic targets, this knowledge can contribute to the development of innovative treatments and interventions aimed at reducing scar formation, promoting tissue regeneration, and improving the prognosis of patients with fibrotic disorders. By advancing the current understanding of fibrosis in wound healing and its association with cancer, we can pave the way for more effective clinical management and improved patient outcomes.
Declarations
All authors have made substantial contributions to the literature review. Writing: I.J.T. and A.L.; Literature search, I.J.T. and A.L.; Review and Editing, I.J.T. and A.L.; Supervision, F.H.S. All authors have read and agreed to the published version of the manuscript.
Availability of Data and Materials
Not applicable.
Financial Support and Sponsorship
This research received no external funding.
Conflicts of Interest
All authors declared that there are no conflicts of interest.
Ethical Approval and Consent to Participate
Not applicable.
Copyright
©The Author(s) 2021.
References
- Wynn TA (2004) Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol 4(8): 583-594.
- Piersma B, Hayward MK, Weaver VM (2020) Fibrosis and cancer: A strained relationship. Biochim Biophys Acta Rev Cancer 1873(2): 188356.
- Rybinski B, Franco Barraza J, Cukierman E (2014) The wound healing, chronic fibrosis, and cancer progression triad. Physiol Genomics 46(7): 223-244.
- Gurtner GC, Werner S, Barrandon Y, Longaker MT (2008) Wound repair and regeneration. Nature 453(7193): 314-321.
- Rodrigues M, Kosaric N, Bonham CA, Gurtner GC (2019) Wound Healing: A Cellular Perspective. Physiol Rev 99(1): 665-706.
- Haensel D, Dai X (2018) Epithelial-to-mesenchymal transition in cutaneous wound healing: Where we are and where we are heading. Dev Dyn 247(3): 473-480.
- Gál P, Brábek J, Holub M. et al. (2022) Autoimmunity, cancer and COVID-19 abnormally activate wound healing pathways: critical role of inflammation. Histochem Cell Biol 158(5): 415-434.
- Xu S, Xu H, Wang W, et al. (2019) The role of collagen in cancer: from bench to bedside. J Transl Med 17(1): 309.
- Mathew Steiner SS, Roy S, Sen CK (2021) Collagen in Wound Healing. Bioengineering (Basel) 8(5): 63.
- Kwan PO, Tredget EE (2017) Biological Principles of Scar and Contracture. Hand Clin 33(2): 277-292.
- Junker JP, Kratz C, Tollbäck A, Kratz G (2008) Mechanical tension stimulates the transdifferentiation of fibroblasts into myofibroblasts in human burn scars. Burns 34(7): 942-946.
- Van De Water L, Varney S, Tomasek JJ (2013) Mechanoregulation of the Myofibroblast in Wound Contraction, Scarring, and Fibrosis: Opportunities for New Therapeutic Intervention. Adv Wound Care (New Rochelle) 2(4): 122-141.
- Guimier E, Carson L, David B, Lambert JM, et al. (2022) Pharmacological Approaches for the Prevention of Breast Implant Capsular Contracture. J Surg Res 280: 129-150.
- Foroushani FT, Dzobo K, Khumalo NP, Mora VZ, de Mezerville R, et al. (2022) Advances in surface modifications of the silicone breast implant and impact on its biocompatibility and biointegration. Biomater Res 26(1): 80.
- Noskovicova N, Hinz B, Pakshir P (2021) Implant Fibrosis and the Underappreciated Role of Myofibroblasts in the Foreign Body Reaction. Cells 10(7): 1794.
- Headon H, Kasem A, Mokbel K (2015) Capsular Contracture after Breast Augmentation: An Update for Clinical Practice. Arch Plast Surg 42(5): 532-543.
- Bagalad BS, Mohan Kumar KP, Puneeth HK (2017) Myofibroblasts: Master of disguise. J Oral Maxillofac Pathol 21(3): 462-463.
- Goel A, Shrivastava P (2010) Post-burn scars and scar contractures. Indian J Plast Surg 43(Suppl): S63-S71.
- Bochaton Piallat ML, Gabbiani G, Hinz B (2016) The myofibroblast in wound healing and fibrosis: answered and unanswered questions. F1000Res 5: F1000.
- Noskovicova N, Hinz B, Pakshir P (2021) Implant Fibrosis and the Underappreciated Role of Myofibroblasts in the Foreign Body Reaction. Cells 10(7): 1794.
- Hwang K, Sim HB, Huan F, et al. (2010) Myofibroblasts and Capsular Tissue Tension in Breast Capsular Contracture. Aesth Plast Surg 34(6): 716-721.
- Yan C, Grimm WA, Garner WL, et al. (2010) Epithelial to mesenchymal transition in human skin wound healing is induced by tumor necrosis factor-alpha through bone morphogenic protein-2. Am J Pathol176(5): 2247-2258.
- Stone RC, Pastar I, Ojeh N, et al. (2016) Epithelial-mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res 365(3): 495-506.
- Tang VW (2020) Collagen, stiffness, and adhesion: the evolutionary basis of vertebrate mechanobiology. Mol Biol Cell 31(17): 1823-1834.
- Knapik DM, Perera P, Nam J, et al. (2014) Mechanosignaling in bone health, trauma and inflammation. Antioxid Redox Signal 20(6): 970-985.
- Hoffman BD, Grashoff C, Schwartz MA (2011) Dynamic molecular processes mediate cellular mechanotransduction. Nature 475(7356): 316-323.
- Schwartz MA (2010) Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb Perspect Biol 2(12): a005066.
- Sun Z, Guo SS, Fässler R (2016) Integrin-mediated mechanotransduction. J Cell Biol 215(4): 445-456.
- Piersma B, Hayward MK, Weaver VM (2020) Fibrosis and cancer: A strained relationship. Biochim Biophys Acta Rev Cancer 1873(2): 188356.
- Wei SC, Fattet L, Tsai JH, et al. (2015) Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat Cell Biol 17(5): 678-688.
- Yeung T, Georges PC, Flanagan LA, et al. (2005) Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton 60(1): 24-34.
- Yi B, Xu Q, Liu W (2021) An overview of substrate stiffness guided cellular response and its applications in tissue regeneration. Bioact Mater 15: 82-102.
- Handorf AM, Zhou Y, Halanski MA, Li WJ. (2015) Tissue stiffness dictates development, homeostasis, and disease progression. Organogenesis 11(1): 1-15.
- Wong VW, Paterno J, Sorkin M, et al. (2011) Mechanical force prolongs acute inflammation via T-cell-dependent pathways during scar formation. FASEB J 25(12): 4498-4510.
- Corchado Cobos R, García-Sancha N, González-Sarmiento R, Pérez-Losada J, Cañueto J (2020) Cutaneous Squamous Cell Carcinoma: From Biology to Therapy. Int J Mol Sci 21(8): 2956.
- Huang P, Yan R, Zhang X, Wang L, Ke X, et al. (2019) Activating Wnt/β-catenin signaling pathway for disease therapy: Challenges and opportunities. Pharmacol Ther 196: 79-90.
- Mascharak S, Talbott HE, Januszyk M, et al. (2022) multi-omic analysis reveals divergent molecular events in scarring and regenerative wound healing. Cell Stem Cell 29(2): 315-327.
- Sun Q, Rabbani P, Takeo M, et al. (2018) Dissecting Wnt Signaling for Melanocyte Regulation during Wound Healing. J Invest Dermatol 138(7): 1591-1600.
- Bednarski IA, Ciążyńska M, Wódz K, et al. (2021) Hippo Signaling Pathway as a New Potential Target in Non-Melanoma Skin Cancers: A Narrative Review. Life (Basel) 11(7): 680.
- Kaszak I, Witkowska Piłaszewicz O, Niewiadomska Z, Dworecka-Kaszak B, Ngosa Toka F, et al. (2020) Role of Cadherins in Cancer-A Review. Int J Mol Sci 21(20): 7624.
- Jakob T, Brown MJ, Udey MC (1999) Characterization of E-cadherin-containing junctions involving skin-derived dendritic cells. J Invest Dermatol 112(1): 102-108.
- Qiu Y, Mekkat A, Yu H, et al. (2018) Collagen Gly missense mutations: Effect of residue identity on collagen structure and integrin binding. J Struct Biol 203(3): 255-262.
- Ward DF Jr, Williams WA, Schapiro NE, et al. (2007) Focal adhesion kinase signaling controls cyclic tensile strain enhanced collagen I-induced osteogenic differentiation of human mesenchymal stem cells. Mol Cell Biomech 4(4): 177-188.
- Takeo M, Lee W, Ito M (2015) Wound healing and skin regeneration. Cold Spring Harb Perspect Med 5(1): a023267.
- Low JS, Mak KK, Zhang S, et al. (2021) In vitro methods used for discovering plant derived products as wound healing agents - An update on the cell types and rationale. Fitoterapia 154: 105026.
- Silver FH, Shah RG, Richard M, Benedetto D (2019) Comparative "virtual biopsies" of normal skin and skin lesions using vibrational optical coherence tomography. Skin Res Technol 25(5): 743-749.
- Silver FH, Deshmukh T, Kelkar N, Ritter K, Ryan N, et al. (2021) The "Virtual Biopsy" of Cancerous Lesions in 3D: Non-Invasive Differentiation between Melanoma and Other Lesions Using Vibrational Optical Coherence Tomography. Dermatopathology (Basel) 8(4): 539-551.





 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.