Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Exploring the Causality between Psoriasis and Chronic Kidney Disease: A Two-Sample Mendelian Randomization Study
*Corresponding author: Yingxin Xu, Peking University Shenzhen Hospital Clinical College, Anhui Medical University, Shenzhen 518036, China, The Fifth Clinical Medical College, Anhui Medical University, Hefei 230000, China and Peking University Shenzhen Hospital, Shenzhen 518036, China.
Received: April 30, 2024; Published: May 06, 2024
DOI: 10.34297/AJBSR.2024.22.002964
Abstract
Object: To study whether psoriasis has a causal effect on the risk of chronic kidney disease (CKD) using two-sample Mendelian randomization (MR) analysis.
Methods: We selected single nucleotide polymorphisms (SNPs) as genetic instrumental variables (IVs) from a genome-wide association study (GWAS) study of psoriasis. Summary data from the largest GWAS meta-analysis of kidney function was utilized as the outcome dataset. Inverse-variance weighted (IVW) was selected to severe as the primary approach of analysis.
Results: We did not find MR association between genetically determined psoriasis and CKD (OR: 0.996; 95% CI: 0.960-1.033; P=0.820), eGFR (OR: 1.000; 95% CI: 0.997-1.001; P=0.244), and BUN (OR: 0.998; 95% CI: 0.994-1.001; P=0.225).
Conclusion: Our MR results showed that no genetic evidence of a causal relationship between psoriasis and CKD risk.
Keywords: Psoriasis, Chronic kidney disease, Mendelian randomization, Linkage disequilibrium score, Causal association
Introduction
Chronic kidney disease (CKD) is distinguished by abnormal microstructures and functioning of the renal due to a variety of reasons for more than 3 months, including evidence as follows: Minimum of one kidney damage marker including albuminuria is found and (or) a decline in estimated glomerular filtration rate (eGFR) drops to less than 6 0mL/min·1.73m2 [1,2]. Renal function continues to decline as CKD progresses, and patients who have reached the end-stage renal disease (ESRD) often require replacement kidney treatment [3,4]. Studies show that the incidence of people with CKD is increasing at an alarming rate every year and has developed into an immense public health problem around the world [1,5]. It is estimated that 11-14% population throughout the world are suffering from CKD, representing approximately 700million patients [2,6,7]. Worryingly, the mortality rate is particularly high for people with CKD. It is estimated that CKD, which is a crucial cause of both chronic renal failure and uremia, contributes to the deaths of 1.2million people every year [8]. In addition, the number of deaths caused by CKD is significantly rising at a pace that is just next to HIV infection [1]. It is anticipated that CKD would move up to the fifth spot on the list of main causes of death around the world within the next 20 years [9]. Due to the fact that the pathophysiology of CKD is extremely convoluted and remains to be discovered, there is a severe lack of effective medical treatment for delaying the development of CKD and avoiding harmful effects [10]. Therefore, it is essential to do research on the causes of CKD for develop more effective strategies for avoiding the development of the condition.
Previous researches have revealed a number of traditional risk factors that related to CKD, including diabetes, hypertension, and glomerulonephritis [1]. Among those factors, the potential role of psoriasis related inflammation in the increased risk of CKD has aroused interest of researchers [11]. Psoriasis is a chronic skin condition related to the immune system that is defined by the formation of well-defined red areas on the skin covered with scales [12] and is often accompanied by comorbidities such as hypertension, diabetes mellitus [13]. Over 60 million people throughout the world suffer from psoriasis [14]. Numbers of researches indicate that psoriasis has an important part in the development and progression of other several conditions, such as cardiovascular disease, lung cancer, and inflammatory bowel disease [15-17], and has gradually become a research focus on the occurrence and development of CKD. Numerous researches have revealed that patients who suffer from psoriasis have a greater rate of CKD morbidity than those in general [18,19]. According to the research results of a meta-analysis included four retrospective cohort studies, psoriasis was found to be linked to a considerably elevated risk of CKD [20]. A similar recent meta-analysis reached the same conclusion [21]. Nonetheless, it is challenging to ascertain whether causal link exists on the basis of observational association alone. Furthermore, the reason of the elevated risk of CKD in those suffering from psoriasis is still unclear. Although psoriasis-related systemic inflammation is one of possible explanations, the common comorbidities in psoriasis patients and use of drugs used to treat psoriasis [22] might also contribute to the development of CKD. Because these potential biases were not fully adjusted in earlier researches, it is still not known whether or not psoriasis has a causal role in the onset of CKD.
According to mendel's second law in which genes are randomly dispersed during meiosis, similarly, genetic variations in a population are also distributed at random. Mendelian randomization (MR) analysis is an approach that takes single nucleotide polymorphisms (SNPs) as genetic instrumental variables (IVs) on the principle of this law, similar to randomized controlled trial (RCT) [23]. Because of this, the method plays a more substantial causal reasoning ability in estimating causality and is viewed as a credible strategy to disentangle the causative link of interested exposure on clinical outcomes [24,25]. In our research, we utilized data from earlier genome-wide association studies (GWASs) to carry out two-sample MR in order to examine the potential causality between psoriasis and CKD. In addition, we analyzed the cross-trait genetic correlation between psoriasis and CKD using linkage disequilibrium regression score (LDSC).
Materials and Methods
Study Design and Data Resources
Our two-sample MR study used genetic variation to investigate the possible causative relationship of psoriasis with eGFR (a major renal function trait) and blood urea nitrogen (BUN) (a secondary renal function trait). For SNPs to be usable as IVs in MR Analysis, 3 critical assumptions must be true [23]: The correlation assumption, the exclusion limitation assumption, and the independence assumption. Firstly, there should be a strong connection between the instrument and the exposure (correlation). Secondly, SNP does not directly affect outcome unless it is affected by exposure, i.e. pleiotropy should not be present (exclusion limitation). Thirdly, genetic variation does not correlate with potential confounding factors between exposure and outcome (independence). Reporting for this study in accordance with the most recent STROBE-MR regulations [26].
Summary statistics of the genome-wide association study (GWAS) for psoriasis was derived from the FINNGEN Consortium (Release 8, http://www.finngen.fi)), analyzing a total of 8, 383 clinically diagnosed psoriasis cases according to the ICD-10 and 347, 694 controls. Information on kidney function related SNPs was sourced from the CKDGen consortium, a network of researchers from all over the world working together to carry out the most extensive genome-wide association meta-analysis of CKD that has been done to date. Taking into consideration factors such as ethnicity, sample size, update frequency of the data and equencing depth, we chose to use the genome-wide genetic dataset on kidney function published by Wuttke, et al. in 2019 [27]. The meta-analysis of the GWAS of CKD that was included in this dataset aggregated data from a total of 22 cohorts and comprised 41395 cases of CKD as well as 439303 controls. This meta-analysis included only patients that satisfied the diagnostic criteria for moderate to advanced CKD, i.e., eGFR<60mL/min.1.73m2 in all cases. The summary statistics for both psoriasis and CKD datasets were collected from persons of European ancestry.
Selection of Genetic IVs
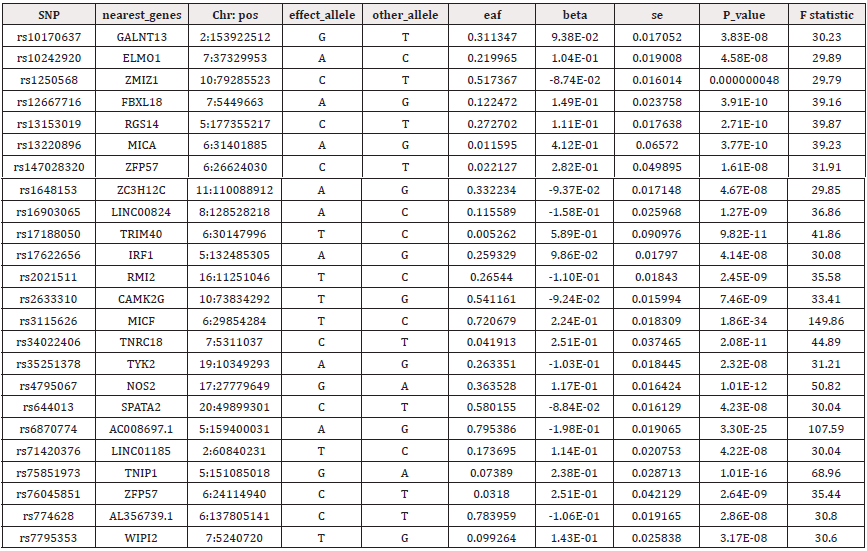
Firstly, to satisfy the correlation assumption, the genetic IVs assessing the causality between psoriasis and risk of CKD were derived from the FINNGEN consortium GWAS analysis, in which SNPs at the genome-wide significance level (p<5×10-8) suggested is associated with psoriasis. Secondly, by computing pair-wise linkage disequilibrium and weeding out SNPs with r2≥0.001 in the specific genomic region (kb=10,000). Thirdly, we omitted palindromic SNPs (SNPs in which the allele comprises of self-complementary base sequences) after harmonizing the SNPs-exposure and SNPs-outcome. Finally, in order to ensure the reliability of MR estimation, the MR pleiotropy residual sum and outlier (MR-PRESSO) method was applied to identify potential outliers and remove the outliers [28].
Weak tool bias was avoided through the application of the F-statistic for assessing the strength between genetic IVs and interested exposure [29]. The formula for calculating the F-statistic is as follows: F=R2(N-K-1)/[K(1-R2)]. Here, R2 indicates the level to which the genetic IVs explain SNPs in terms of exposure, N denotes sample size of GWAS associated with exposure, and K represents the number of used SNPs. When the F-statistic is greater than 10, we regularly regard the association between IVs and exposure to be robust and the findings of the MR study could be shielded from the effects of weak tool bias [30].
MR Analyses
During MR analysis, according to the existence of heterogeneity, different inverse-variance-weighted (IVW) (fixed-effects model or random-effects model IVW) was selected to severe as the primary approach of analysis [31]. In order to conduct a comprehensive and accurate investigation of causal relationship, MR-Egger [32], Weighted median [33], Weighted mode, and Simple mode [34] were severed as complementary methods of MR. Despite the fact that the MR-Egger technique has a limited statistical ability, it is still able to provide an estimate when taking into account multiple effects [32]. The Weighted median method can yield reliable conclusions if it is assumed that more than half of the IVs are valid in the MR analysis, even if there are invalid IVs [33].
Sensitivity Analysis
In order to perform sensitivity analysis, this study included a number of different methods. (1) Heterogeneity between estimates of individual of IVs was evaluated by Cochrane’s Q-statistic. In the absence of heterogeneity, the fixed-effects model IVW was severed as the main approach of analysis; otherwise, the random-effects model [35]. (2) Horizontal pleiotropy of IVs was investigated by MR-Egger intercept approach [32]. (3) Asymmetry in the funnel plots was also used to investigate the possibility of horizontal pleiotropy. (4) The leave-one-out analysis was performed to investigate whether the findings might be attributed to the high influence SNP.
All MR studies were carried out using R software (version 4.3.0) and a series of R packages “Two-Sample-MR” (version 0.5.6) and “MR-PRESSO” (version 1.0).
Genetic Correlation Analysis
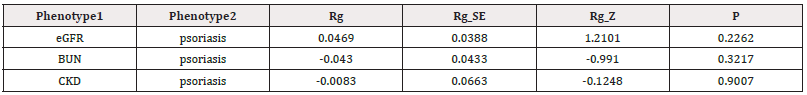
We utilized LDSC to calculate the co-heritability between psoriasis and CKD through investigating the genetic correlation (Rg) and the p-value [36].
Results
Genetic IVs Selected in MR
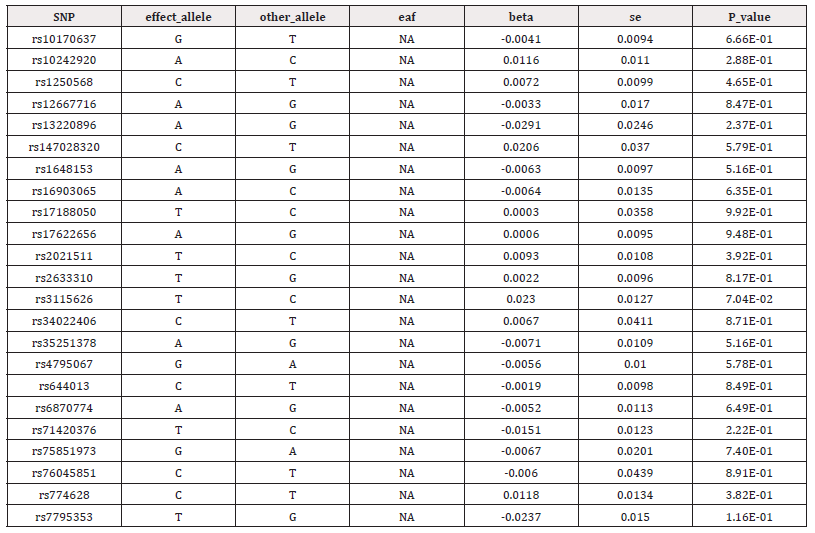
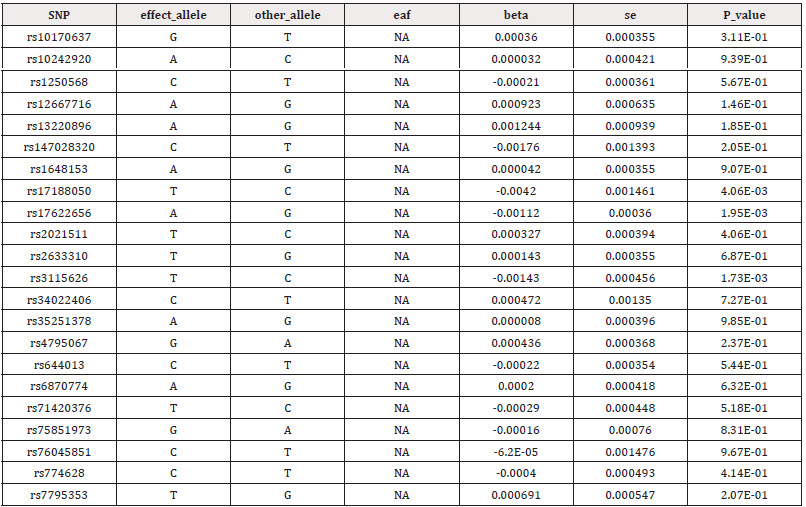
In this study, we obtained 24 SNPs associated with psoriasis after a generally accepted genome-wide threshold of significance (p<5×10-8, r2<0.001, kb = 10,000) (Supplementary Table S1). After excluding outliers identified by MR-PRESSO and removal of palindromic sequence SNPs by a harmonizing process, we selected the remaining SNPs as genetic IVs. Details of genetic IVs of CKD and its renal function features were shown in supplementary Table S2-4. The smallest F-statistics of SNPs was 30, larger than the empirical standard of 10, suggesting that MR estimates are not affected by weak tool bias (Supplementary Table S1).
Causal Relationship between Psoriasis and CKD, BUN, and eGFR
Cochrane’s Q-statistic revealed no heterogeneity between psoriasis and CKD (IVW: QCKD=14.173, PCKD=0.895; MR-Egger: QCKD=14.028, PCKD=0.868), and BUN (IVW: QBUN=12.827, PBUN=0.938; MR-Egger: QBUN=12.176, PBUN=0.935), while heterogeneity was found between psoriasis and eGFR (IVW: QeGFR=37.767, PeGFR=0.014; MR-Egger: QeGFR=35.436, PeGFR=0.018) (Table 1).Therefore, the association between psoriasis and CKD, and BUN was analyzed by fixed-effects model IVW, and psoriasis and eGFR was analyzed by random-effects model IVW. We did not find MR association between genetically determined psoriasis with CKD (OR: 0.996; 95% CI: 0.960-1.033; P=0.820) and BUN (OR: 0.998; 95% CI: 0.994-1.001; P=0.225) based on the fixed-effects model IVW (Table 2). Similarly, no association was found between psoriasis and eGFR using random-effects model IVW (OR: 1.000; 95% CI: 0.997-1.001; P=0.244) (Table 2). The association was consistent in complementary methods of MR using MR-Egger, Weighted median, Weighted mode and Simple mode (Table 2). At the same time, the MR-Egger intercept approach manifested that the IVs of psoriasis in CKD and its renal function features did not exhibit horizontal pleiotropy (PCKD=0.707; PeGFR=0.265; PBUN=0.429) (Table 1). The funnel plots were symmetric indicating no horizontal pleiotropy (Figures 1-3). Leave-one-out analysis found that no high influence SNP had a significant impact on the results (Figures 1-3).

Table 1: Pleiotropy and heterogeneity test of the psoriasis genetic IVs from CKD GWAS.
Note*: Abbreviations: df, degree of freedom; MR, Mendelian randomization; Q, heterogeneity statistic Q; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; BUN, blood urea nitrogen.
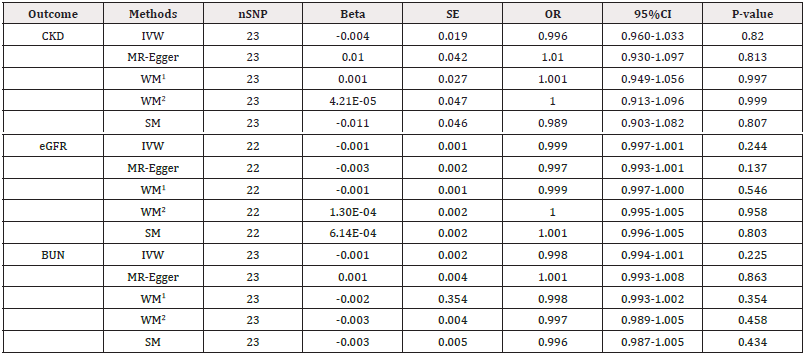
Table 2: Mendelian randomization estimates of psoriasis on the risk for CKD and its renal function trait.
Note*: Abbreviations: CI, confidence interval; SE, standard error; IVW, inverse variance-weighted; SNP, single-nucleotide polymorphism; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; BUN, blood urea nitrogen; OR, odds ratio; WM1, Weighted median; WM2, Weighted mode; SM, Simple mode.
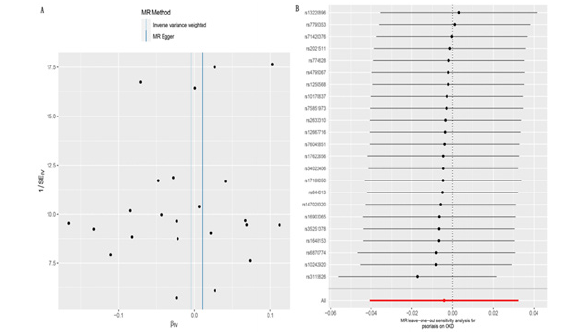
Figure 1: (A) The funnel plot of Mendelian randomization analysis for psoriasis on the chronic kidney disease. (B) Leave-one-out sensitivity analysis for psoriasis on the chronic kidney disease.
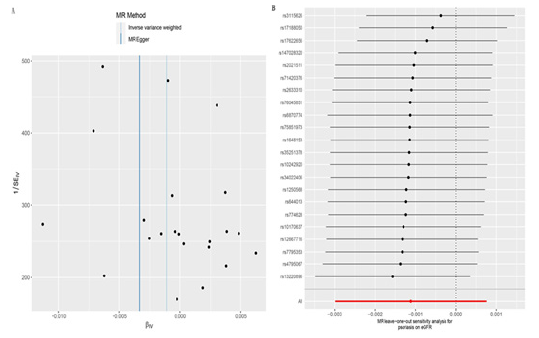
Figure 2: (A) The funnel plot of Mendelian randomization analysis for psoriasis on the estimated glomerular filtration rate. (B) Leave-one-out sensitivity analysis for psoriasis on the estimated glomerular filtration rate.
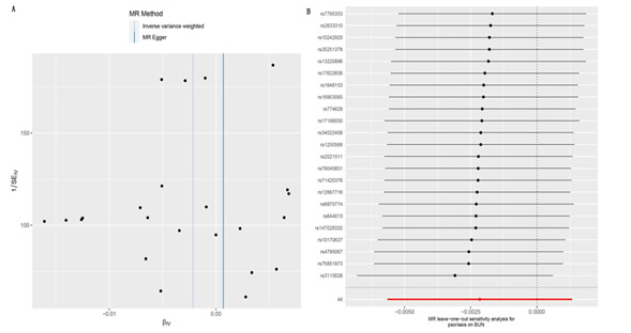
Figure 3: (A) The funnel plot of Mendelian randomization analysis for psoriasis on the blood urea nitrogen. (B) Leave-one-out sensitivity analysis for psoriasis on the blood urea nitrogen.
Genetic Correlation between Psoriasis and CKD
Since the epidemiological connection between psoriasis and CKD may stem from shared susceptibility genetic variations, we carried out LDSC analysis to determine whether there was genetic correlation between psoriasis and CKD. However, we did not observe proof of genetic correlation between psoriasis and CKD and its renal function features (Table 3). It has indicated that shared genetic factors are not to blame for the greater prevalence of CKD in psoriasis patients.
Discussion
This investigation into the causal association between psoriasis and CKD is the first to use MR analysis. MR analysis utilizing five different approaches (fixed-effects model IVW or random-effects model IVW, MR-Egger, Weighted median, and Simple mode) yielded consistent conclusions, suggesting no causality between psoriasis and CKD. The LDSC analysis demonstrated that psoriasis and CKD have no genetic correlation. This investigation elucidated the effect of psoriasis on the risk of CKD from a standpoint of genetic.
Psoriasis has been thought of a systemic disease, with effects well beyond the skin [37]. Recently, investigators have switched their attention to pathological albuminuria in psoriasis patients by measuring 24-hour albuminuria, 24-hour microalbuminuria, and albuminuria, but data are limited. Evidence from observational study has indicated an increased microalbuminuria, a marker of renal impairment, in patients with psoriasis [38]. Dervisoglu E, et al. reported no significant distinction in 24-hour proteinuria and albuminuria between patients with psoriasis and controls [39] whereas Ren F, et al. has observed increased 24-hour microalbuminuria and 24-hour proteinuria, which increased the risk of subclinical renal dysfunction [40]. The majority of investigations observed people with psoriasis had a significantly greater prevalence of pathological albuminuria in comparison to controls [39,40], whereas only a minority have found no difference [41]. However, all of the aforementioned studies were cross-sectional and had limited sample sizes. The outcome of these studies were albuminuria assay results of various types instead of the CKD diagnosis. To address these knowledge empty spaces, two large population-based cohort studies from United Kingdom and China have evaluated the relationship between psoriasis and CKD [19,42]. Both investigations have showed that patients with psoriasis had a higher risk of CKD compared to without psoriasis. Although both researches have made effort to adjust for potential confounds, selection bias and reverse causality are still problems inherent to observational study design. It is challenging to distinguish the association between two variables is mediated by confounders or there is a causal association.
Our MR study is the first to use the method that reduces inherent biases in observational studies for investigating the causality between psoriasis and CKD. Our results did not support the causal relationship from psoriasis to CKD. In addition, the shared genetic contributions cannot account for the association observed in the observational studies. However, there are series of potential causes for elevated risk of CKD in psoriasis patients.
First, the conventional CKD risk factors may confuse this non-causal relationship. Hypertension and diabetes are well-known conventional risk factors for CKD [1], and these can act as confounding factors. High prevalence of hypertension and diabetes are observed in patients suffered from psoriasis [13,43,44]. On account of most investigations did not adequately adjust the confounding factors, which can impact the observed association between psoriasis and CKD.
Second, systemic inflammation caused by psoriasis is a crucial factor in the genesis and progression of CKD [45]. On the one hand, atherosclerotic damage to the renal has been identified as one of the most common reasons of CKD [46], and numerous investigations have observed that psoriasis patients are more likely to have a significant atherosclerotic burden due to chronic damage to endothelial cells caused by chronic inflammation [47,48]. On the one hand, over-activation of adaptive immune components is thought to be essential to the pathogenesis of psoriasis, such as over-activation of Th1 and Th17 lymphocytes, which are important immune cells known to be involved in the pathogenesis of psoriasis [49]. Th17 and IL-17 can also directly produce renal inflammation through the activation of neutrophils or through their participation in macrophage-mediated tissue destruction [50]. Additionally, inflammatory factors such as TNF-α and IL-12, IL-23 can promote kidney damage during the inflammatory process of psoriasis [51,52], of which TNF-α has been identified as an independent predictor of progression of CKD [53]. Another piece of evidence comes from a RCT in which mean eGFR levels were improved persistently in advanced CKD patients treated with the targeted anti-inflammatory drug bardoxolone methyl [54].
Thirdly, drugs used in systemic treatment for psoriasis may cause kidney damage, especially methotrexate and cyclosporine. Renal injury caused by methotrexate is associated with the accumulation of the drug and its metabolites in the renal tubules, resulting in direct damage to the tubular cells [55,56]. Cyclosporine can mediate kidney blood flow hypoperfusion, leading to renal tubule cell apoptosis, renal tubule atrophy and interstitial fibrosis, which directly leads to kidney damage [57]. In addition, cyclosporine increases the risk of high blood pressure, a risk factor for CKD [58]. Therefore, long-term drugs therapy may cause the increased risk of CKD in people with psoriasis.
Although the results of our MR study do not support the causal relationship between psoriasis genetic susceptibility and lifetime risk of CKD, the elevated risk of CKD in patients with psoriasis is a reality problem that cannot be ignored. It is possible that other factors are to blame, such as traditional CKD risk factors that psoriasis patients are born with, inflammatory factors, and medications. Our LDSC analysis results also confirm this point from the side. A 2015 Swedish registry study involving 193,829 people investigated the association between mild and severe psoriasis and 12 specific causes of death, kidney disease was identified as the second most associated disease with death, after liver disease [59].
Our MR analysis had several strengths; (1) The results we observed in the MR-Egger intercept method and the MR-PRESSO method are not exactly the same. The MR-Egger intercept method did not find the existence of pleiotropy, but abnormal SNPs were found in MR-PRESSO. (2) Using MR can reduce bias caused by reverse causality and unknown confounding factors, and applies to causal reasoning, which can be impossible to achieve in traditional observational studies. (3) The GWAS datasets for both psoriasis and CKD were collected from people of European descent, which may lessen the impact of demographic stratification on potential associations. (4) Data from large-scale GWAS datasets for psoriasis (n=356,077) and CKD (n=480,698) made it possible to boost ability of statistics to detect minor effects in complicated phenotypes.
Our MR study does contain a few restrictions that cannot be disregarded; (1) There may be racial differences in the genetic link between psoriasis and CKD, whereas our GWAS datasets were obtained from European population and may not be effectively extended to other populations. It is possible for hidden population structure to interfere with the connection between genetic IV and outcome. (2) The existence of potentially confounding factors is not something that can be fully eliminated by us, the biggest problem during MR studies is pleiotropy, horizontal pleiotropy emerges when genetic IV influences results through multiple pathways. Although we applied different MR methods as sensitivity analyses and obtained consistent results, it has been nearly impossible to entirely exclude the presence of pleiotropy in MR studies thus far. (3) Because the psoriasis and CKD datasets were derived from summary statistics, the relationship between different severity or types of psoriasis and different CKD stages could not be explored. (4) This study did not involve environmental factors and simply offered genetic evidence of non-causal association between psoriasis and CKD.
Conclusions
Our MR study does not support the causal relationship between psoriasis and CKD. Further investigation is still required to determine the mechanisms underlying the observational association between them. However, we cannot disregard psoriasis patients are at elevated risk of CKD because of traditional CKD risk factors that psoriasis patients are born with, inflammation burden, and drugs. For patients with psoriasis, early management of CKD is necessary.
Supplementary Materials
Supplementary Tables 1-4.
Author Contributions
Conceptualization, Hao Zhou; Data curation, Yajie Qi; Formal analysis, Yajie Qi; Investigation, Hao Zhou; Methodology, Hao Zhou and Yajie Qi; Software, Hao Zhou and Yajie Qi; Validation, Hao Zhou and Hui Qi; Visualization, Yajie Qi; Writing - original draft, Hao Zhou and Yajie Qi; Writing - review & editing, Yingxin Xu.
Fundings
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to re-analysis of previously collected and published data.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets for this study can be found in the FinnGen database (http://www.finngen.fi) and CKDGen consortium (ckdgen.imbi.uni-freiburg.de). All data were accessed on April 15, 2023.
Acknowledgments
We want to acknowledge the participants and investigators of the FinnGen study. We also thank the CKDGen consortium for providing the data set.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Webster AC, Evi V Nagler, Rachael L Morton, Philip Masson (2017) Chronic Kidney Disease. Lancet 389(10075): 1238-1252.
- Kalantar Zadeh K, Tazeen H Jafar, Dorothea Nitsch, Brendon L Neuen, Vlado Perkovic, et al. (2021) Chronic kidney disease. Lancet 398(10302): 786-802.
- Orlandi PF, Dawei Xie, Wei Yang, Jordana B Cohen, Rajat Deo, et al. (2020) Slope of Kidney Function and Its Association with Longitudinal Mortality and Cardiovascular Disease among Individuals with CKD. J Am Soc Nephrol 31(12): 2912-2923.
- Swartling O, Helena Rydell, Maria Stendahl, Mårten Segelmark, et al. (2021) CKD Progression and Mortality Among Men and Women: A Nationwide Study in Sweden. Am J Kidney Dis 78(2): 190-199.e1.
- (2020) Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 395(10225): 709-733.
- Hill NR, Samuel T Fatoba, Jason L Oke, Jennifer A Hirst, Christopher AO Callaghan, et al. (2016) Global Prevalence of Chronic Kidney Disease - A Systematic Review and Meta-Analysis. PLoS One 11(7): e0158765.
- Lv JC, Zhang LX (2019) Prevalence and Disease Burden of Chronic Kidney Disease. Adv Exp Med Biol 1165: 3-15.
- Xie Y, Benjamin Bowe, Ali H Mokdad, Hong Xian, Yan Yan, et al. (2018) Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int 94(3): 567-581.
- Foreman KJ, Neal Marquez, Andrew Dolgert, Kai Fukutaki, Nancy Fullman, et al. (2018) Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 392(10159): 2052-2090.
- Wang YN, Shi Xing Ma, Yuan Yuan Chen, Lin Chen, Bao Li Liu, et al. (2019) Chronic kidney disease: Biomarker diagnosis to therapeutic targets. Clin Chim Acta 499: 54-63.
- Coimbra S, H Oliveira, P Rocha Pereira, A Figueiredo, A Santos Silva (2017) The crosstalk between renal function, inflammation and psoriasis vulgaris. Br J Dermatol 176(3): 829-831.
- Griffiths C, April W Armstrong, Johann E Gudjonsson, Jonathan NWN Barker (2021) Psoriasis. Lancet 397(10281): 1301-1315.
- Guenther L, Gulliver W (2009) Psoriasis comorbidities. J Cutan Med Surg 13 Suppl 2: S77-87.
- Parisi R, Ireny YK Iskandar, Evangelos Kontopantelis , Matthias Augustin , Christopher EM Griffiths, et al. (2020) National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ 369: m1590.
- Xiao Y, Danrong Jing, Zhenwei Tang, Cong Peng, Mingzhu Yin, et al. (2022) Serum Lipids and Risk of Incident Psoriasis: A Prospective Cohort Study from the UK Biobank Study and Mendelian Randomization Analysis. J Invest Dermatol 142(12): 3192-3199.e12.
- Freuer D, Jakob Linseisen, Christa Meisinger (2022) Association Between Inflammatory Bowel Disease and Both Psoriasis and Psoriatic Arthritis: A Bidirectional 2-Sample Mendelian Randomization Study. JAMA Dermatol 158(11): 1262-1268.
- Wang X, Xiulan Wang, Hongkang Wang, Mingxing Yang, Wen Dong, et al. (2023) Association between psoriasis and lung cancer: two-sample Mendelian randomization analyses. BMC Pulm Med 23(1): 4.
- Chiu HY, H L Huang, CH Li, YJ Yin, HA Chen, et al. (2015) Increased risk of glomerulonephritis and chronic kidney disease in relation to the severity of psoriasis, concomitant medication, and comorbidity: a nationwide population-based cohort study. Br J Dermatol 173(1): 146-154.
- Wan J, Shuwei Wang, Kevin Haynes, Michelle R Denburg, Daniel B Shin, et al. (2013) Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ 347: f5961.
- Ungprasert P, Raksasuk S (2018) Psoriasis and risk of incident chronic kidney disease and end-stage renal disease: a systematic review and meta-analysis. Int Urol Nephrol 50(7): 1277-1283.
- Jing X, Wen Zhuyuan, Chen Aijun, Xiong Jianxia, Huang Kun, et al. (2023) Association of psoriasis with chronic kidney disease and end-stage renal disease: a systematic review and meta-analysis. Front Med (Lausanne) 10: 1175477.
- Visconti L, Giuseppe Leonardi, Michele Buemi, Domenico Santoro, Valeria Cernaro, et al. (2016) Kidney disease and psoriasis: novel evidences beyond old concepts. Clin Rheumatol 35(2): 297-302.
- Emdin CA (2017) Mendelian Randomization. JAMA 318(19): 1925-1926.
- Smith GD, Ebrahim S (2003) 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 32(1): 1-22.
- Zheng J, Denis Baird, Maria Carolina Borges, Jack Bowden, Gibran Hemani, et al. (2017) Recent Developments in Mendelian Randomization Studies. Curr Epidemiol Rep 4(4): 330-345.
- Skrivankova VW, Rebecca C Richmond, Benjamin AR Woolf, James Yarmolinsky, Neil M Davies, et al. (2021) Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA 326(16): 1614-1621.
- Wuttke M, Yong Li, Man Li, Karsten B Sieber, Mary F Feitosa, et al. (2019) A catalog of genetic loci associated with kidney function from analyses of a million individuals. Nat Genet 51(6): 957-972.
- Verbanck M, Chia Yen Chen, Benjamin Neale, Ron Do (2018) Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet 50(5): 693-698.
- Burgess S, Thompson SG (2011) Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol 40(3): 755-64.
- Pierce BL, Habibul Ahsan, Tyler J Vanderweele (2011) Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol 40(3): 740-752.
- Lawlor DA, Debbie A Lawlor, Roger M Harbord, Jonathan AC Sterne, Nic Timpson, George Davey Smith, et al. (2008) Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med 27(8): 1133-1163.
- Bowden J, George Davey Smith, Stephen Burgess (2015) Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 44(2): 512-525.
- Bowden J, George Davey Smith, Philip C Haycock, Stephen Burgess (2016) Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol 40(4): 304-314.
- Hartwig FP, George Davey Smith, Jack Bowden (2017) Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol 46(6): 1985-1998.
- Bowden J, Fabiola Del Greco M, Cosetta Minelli, George Davey Smith, Nuala Sheehan, et al. (2017) A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med 36(11): 1783-1802.
- Bulik Sullivan B, Hilary K Finucane, Verneri Anttila, Alexander Gusev, Felix R Day, et al. (2015) An atlas of genetic correlations across human diseases and traits. Nat Genet 47(11): 1236-1241.
- Duarte GV, Maria de Fátima SP Oliveira, Ivonise Follador, Thadeu Santo Silva, Edgar Marcelino de Carvalho Filho, et al. (2016) Diagnosis and underdiagnosis of comorbidities in psoriasis patients - need for a multidisciplinary approach. An Bras Dermatol 91(6): 743-747.
- Szepietowski JC, E Bielicka, F Wasik, W Kopec, T Szepietowski, et al. (2000) Microalbuminuria as a subclinical marker of renal impairment in subjects with psoriasis vulgaris. J Eur Acad Dermatol Venereol 14(6): 513-514.
- Dervisoglu E, Aysun Sikar Akturk, Kursat Yildiz, Rebiay Kiran, Ahmet Yilmaz, et al. (2012) The spectrum of renal abnormalities in patients with psoriasis. Int Urol Nephrol 44(2): 509-514.
- Ren F, Min Zhang, Liying Hao, Hong Sang (2017) Kidney involvement in psoriasis: a case-control study from China. Int Urol Nephrol 49(11): 1999-2003.
- Tehranchinia Z, Esmat Ghanei, Nahid Mohammadi, Masoud Partovi Kia, Hoda Rahimi, et al. (2018) No Relation between Psoriasis and Renal Abnormalities: A Case-Control Study. ScientificWorldJournal 2018: 5301631.
- Chi CC, Jui Wang, Yu Fen Chen, Shu Hui Wang, Fu Li Chen, et al. (2015) Risk of incident chronic kidney disease and end-stage renal disease in patients with psoriasis: A nationwide population-based cohort study. J Dermatol Sci 78(3): 232-238.
- Armstrong AW, Caitlin T Harskamp, Ehrin J Armstrong (2013) Psoriasis and the risk of diabetes mellitus: a systematic review and meta-analysis. JAMA Dermatol 149(1): 84-91.
- Armstrong AW, Caitlin T Harskamp, Ehrin J Armstrong (2013) The association between psoriasis and hypertension: a systematic review and meta-analysis of observational studies. J Hypertens 31(3): 433-442; discussion 442-443.
- Qian Q (2017) Inflammation: A Key Contributor to the Genesis and Progression of Chronic Kidney Disease. Contrib Nephrol 191: s72-83.
- Ozyilmaz A, Paul E de Jong, Ronald T Gansevoort (2012) Screening for chronic kidney disease can be of help to prevent atherosclerotic end-organ damage. Nephrol Dial Transplant 27(11): 4046-4052.
- Libby P, Paul M Ridker, Attilio Maseri (2002) Inflammation in atherosclerosis. Nature 420(6917): 868-874.
- Shahwan KT, Kimball AB (2015) Psoriasis and Cardiovascular Disease. Med Clin North Am 99(6): 1227-1242.
- Armstrong AW, Read C (2020) Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 323(19): 1945-1960.
- Dolff S, Oliver Witzke, Benjamin Wilde (2019) Th17 cells in renal inflammation and autoimmunity. Autoimmun Rev 18(2): 129-136.
- Mehaffey E, Majid D (2017) Tumor necrosis factor-alpha, kidney function, and hypertension. Am J Physiol Renal Physiol 313(4): F1005-F1008.
- Li H, Maria G Tsokos, Rhea Bhargava, Iannis E Adamopoulos, Hanni Menn Josephy, et al. (2021) IL-23 reshapes kidney resident cell metabolism and promotes local kidney inflammation. J Clin Invest 131(12): e142428.
- Amdur RL, Harold I Feldman, Jayanta Gupta, Wei Yang, Peter Kanetsky, et al. (2016) Inflammation and Progression of CKD: The CRIC Study. Clin J Am Soc Nephrol 11(9): 1546-1556.
- Pergola PE, Philip Raskin, Robert D Toto, Colin J Meyer, J Warren Huff, et al. (2011) Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med 365(4): 327-336.
- Wilsdon TD, Samuel L Whittle, Tilenka Rj Thynne, Arduino A Mangoni (2019) Methotrexate for psoriatic arthritis. Cochrane Database Syst Rev s1(1): CD012722.
- Yan K, Yuanjing Zhang 2, Ling Han 1, Qiong Huang 1, Zhenghua Zhang, et al. (2019) Safety and Efficacy of Methotrexate for Chinese Adults With Psoriasis With and Without Psoriatic Arthritis. JAMA Dermatol 155(3): 327-334.
- Elmets CA, Craig L Leonardi, Dawn M R Davis, Joel M Gelfand, Jason Lichten, et al. (2019) Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol 80(4): 1073-1113.
- Lowe NJ, J M Wieder, A Rosenbach, K Johnson, R Kunkel, et al. (1996) Long-term low-dose cyclosporine therapy for severe psoriasis: effects on renal function and structure. J Am Acad Dermatol 35(5 Pt 1): 710-719.
- Svedbom A, Johan Dalén, Carla Mamolo, Joseph C Cappelleri, Lotus Mallbris, et al. (2015) Increased cause-specific mortality in patients with mild and severe psoriasis: a population-based Swedish register study. Acta Derm Venereol 95(7): 809-815.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.