Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Exploring the Efficacy of Hormone Replacement Therapy: A Network Meta-Analysis
*Corresponding author: Dr Peter Phiri, Director of Research & Innovation/ Visiting Fellow, Research & Innovation Department, Southern Health NHS Foundation Trust, Clinical Trials Facility, Tom Rudd Unit Moorgreen Hospital, Botley Road, West End, Southampton SO30 3JB, United Kingdom.
Received: March 17, 2024; Published: May 24, 2024
DOI: 10.34297/AJBSR.2024.22.002994
Abstract
Background: Hormone Replacement Therapy (HRT) is a treatment for menopausal conditions. Studies showing benefits of HRT in preventing chronic diseases lead to development of clinical guidelines by the American College of Physicians. This study aims to assess the effectiveness of HRT treatments across cardiometabolic measures including Triglycerides (TG), Follicle-Stimulating Hormone, LDL cholesterol, HDL cholesterol, and Estradiol in menopausal women. Our systematic review is aimed at reporting gaps in scientific knowledge.
Methods: A systematic methodology designed and published in PROSPERO (CRD42022346057) to report network meta-epidemiology analysis was utilised. We used databases by PubMed, Web of Science, ScienceDirect, EMBASE and MEDLINE for studies published between 30th of April 1980-2022. Effects of HRT treatments were explored using a Mixed Treatment Comparison (MTC) model. Fixed and random-effects models were used to address heterogeneity in published studies. Publication bias was assessed and corrected using funnel plots and Egger’s test.
Results: Of 45 eligible studies, our findings indicate a significant statistical heterogeneity between HRTs and reduction of TG, SFH, LDL-C alongside increase of HDL-C and Estradiol among menopausal women. The analysis suggests a lack of direct evidence to support their efficacy in reducing TG, SFH and LDL-C levels or to substantiate HRT’s effectiveness in increasing HDL-C and Estradiol. The results showed no significant publication bias in the meta-analysis of included studies.
Conclusion: Our findings indicate the use of HRT interventions among menopausal women may reduce TG, FSH and LDL-C levels and increase levels of HDL-C and estradiol via oral and oral+ transdermal administration. Our study reaffirms efficacy of HRT in supporting favourable lipid profile among menopausal women whilst highlighting the need for robust and inclusive epidemiology studies and clinical trials that are inclusive to all ethnicities to further develop clinical guidelines and policies.
Introduction
The use of Hormone Replacement Therapy (HRT) to ease the symptoms of menopause started in the 1960s with the Food and Drug Administration (FDA) approving oestrogen as a treatment for hot flushes experienced by menopausal women [1-4]. HRT remained controversial from social and medical perspectives with scholars arguing that menopause was being medicalised as supported by pharmaceutical advertising and in opposition to it being a natural part of the female lifecycle. The use of HRT was influenced by the 1960s feminist movement that changed women’s life expectancy and status with most European countries encouraging the concept of “feminine forever” based on the best-selling book with the same name by Wilson, et al., (1966) [1]. By the 1970s, oestrogen supplements were linked to increased risk of endometrial cancer as observed by Ziel, et al., and Finkle, et al., (1975) [1]. In the following years, Woodruff, et al., and colleagues (1994) showed reducing oestrogen doses and combining these with progesterone could minimise the risk of endometrial cancer [2,3]. The FDA approved HRT for treatment of hot flushes and prevention of osteoporosis in 1988. Several observational studies around the same time showed the benefits of HRT in preventing chronic diseases and the “feminine forever” concept switched to “healthy forever” with the development of the first HRT guidelines by the American College of Physicians (1992) [2-4].
Research on HRT started to gain momentum in the 1990s with the general increase in women’s health policies, in particular the signing of the Beijing declaration and the Platform for Action by the World Health Organisation’s (WHO) that included an agenda for women’s empowerment through health to support several critical areas of concern [5,6]. However, research into clinical management and treatments for menopause remains limited compared to other diseases such as diabetes. Characteristic symptoms of menopause include hot flushes, night sweats, sleep issues, vaginal atrophy, fatigue, and mood changes. Menopause has also been associated with chronic conditions such as osteoporosis and cardiovascular events with declining oestrogen levels [6]. The first Randomised Clinical Trial (RCT) explored cardiovascular diseases among 2736 post-menopausal women with confirmed coronary heart disease [7]. Following a 4-year follow up period, no differences were found between the treatment and placebo groups although the HRT group showed an increase in coronary heart disease or non-fatal myocardial infarction at 12 months which declined over the next 3 years [8]. The Women’s Health Initiative (WHI), which was a large, randomised study further assessed the effect of HRT on common causes of disability and death among post-menopausal women where 16,608 post-menopausal women were randomised to receive 0.625mg of Conjugated Equine Oestrogens (CEE) and 2.5mg of medroxyprogesterone acetate whilst 10,739 women without uteri received 0.625mg of conjugated equine oestrogen or placebo (Rossouw, et al., 2002) [8]. The initial results published in 2002 showed the group with an intact uterus had an increased incidence of breast cancer and coronary heart disease with a reduction in osteoporotic fractures and colorectal cancer [9]. This study was discontinued with the findings generating concerns among HRT users and lead to amendments in the clinical guidelines for prescribers [10]. The results from the oestrogen only arm based on women with a hysterectomy showed preliminary findings in 2004 which indicated a small increased risk of ischemic stroke without any further statistically significant cardiovascular benefits. Following these reports, the regulatory authorities in the United Kingdom (UK) issued safety restrictions on HRT with recommendations to doctors to prescribe lowest effective dose of HRT for the shortest time required to relieve menopausal symptoms and prevention of osteoporosis as a second line treatment [10,11]. The regulators also recommended that HRT should not be used in asymptomatic post-menopausal women [12]. A re-analysis of the WHI data using a meta-analysis showed that the use of HRT among women between 50-59 years or those with early onset of menopause was associated with benefit of reduction in coronary diseases and all-cause mortality. This was followed by findings from Schierbeck, et al., (2012), a study from Denmark that showed healthy post-menopausal women’s risk of heart disease was reduced after taking combined HRT for 10 years [13].
To date, the optimum use of HRT and its benefits and risks remains a topic of ongoing discussion between users, clinicians, researchers and policy makers. Given the lack of consensus on the optimal use of HRT, there is a real need to assess the effectiveness of HRT interventions. Our study examines the effectiveness of HRT interventions as it relates to cardiometabolic profiles specifically, Triglycerides (TG), Follicle-Stimulating Hormone (FSH), LDL Cholesterol (LDL-C), HDL Cholesterol (HDL-C), and Estradiol.
Methods
A systematic methodology was developed and published as a protocol in PROSPERO (CRD42022346057) as part of a wider exploratory study on menopausal women (MARiE) project. The Network Meta-Analysis (NMA) was conducted to assess the effectiveness of HRT interventions on cardiometabolic profile, considering the variations in their implementation. A NMA approach was chosen over a pairwise meta-analysis due to its ability to simultaneously compare multiple interventions and their ranking based on relative effectiveness by incorporating direct and indirect evidence.
Aim
This study aimed to explore the effects of HRT on Triglycerides (TG), Follicle-Stimulating Hormone (FSH), LDL Cholesterol (LDL-C), HDL Cholesterol (HDL-C), and Estradiol.
Eligibility Criteria
All clinical trials and observational studies reporting efficacy or effectiveness of HRT in relation to cardiometabolic parameters in menopausal women, peer reviewed and published in English from the 30th of April 1980 until the 30th of April 2022 were included. Studies that did not report statistical measures were excluded from the meta-analysis to enable subsequent comparisons by Mixed Treatment Comparison (MTC) model.
Search Strategy and Data Extraction
We used multiple databases of PubMed, Web of Science, ScienceDirect, EMBASE and MEDLINE to gather the initial data with keywords of hormone replacement therapy, menopause, HRT and cardiometabolic disease in women. A study specific data extraction template was developed using Microsoft excel to extract the study ID, type of HRT, treatment arms, outcome measures, sample size, odds ratios, effect estimates, and standard errors associated with the effect estimates. The screening and data extraction was performed by 3 authors independently.
Statistical Analysis Plan
The NMA simultaneously estimated treatment effects for all interventions using a Mixed Treatment Comparison (MTC) model, which accounts for both direct and indirect evidence by incorporating common comparators. Direct comparison involved comparing two interventions within a study using directly collected data. In contrast, indirect comparison involved comparing two interventions within the same study where no direct comparison was available, but the comparison could be made by combining data from multiple studies. Heterogeneity refers to the variability or differences in treatment effects observed across different studies included in the analysis. It indicates that the effects of interventions may vary among studies, suggesting the presence of diverse factors or characteristics that influence treatment outcomes. We assessed heterogeneity using the I² statistic and the Q-test. The I² statistic quantifies the proportion of total variation across studies that can be attributed to heterogeneity, while the Q-test evaluates the statistical significance of this heterogeneity. A higher value of I² was used to demonstrate significant statistical heterogeneity among the studies [14,15,16]. Model selection was completed based on the heterogeneity observed. This model accounts for both within-study and between-study variability, if treatment effects may vary across the included studies. The fixed-effects model was used in the presence of weak statistical heterogeneity [14]. To determine the statistical significance of the treatment effects, we used mean difference. The analysis was performed using R, involving the estimation of treatment effects, model fitting, and result presentation. Publication bias was assessed using funnel plots and Egger’s test [16].
Results
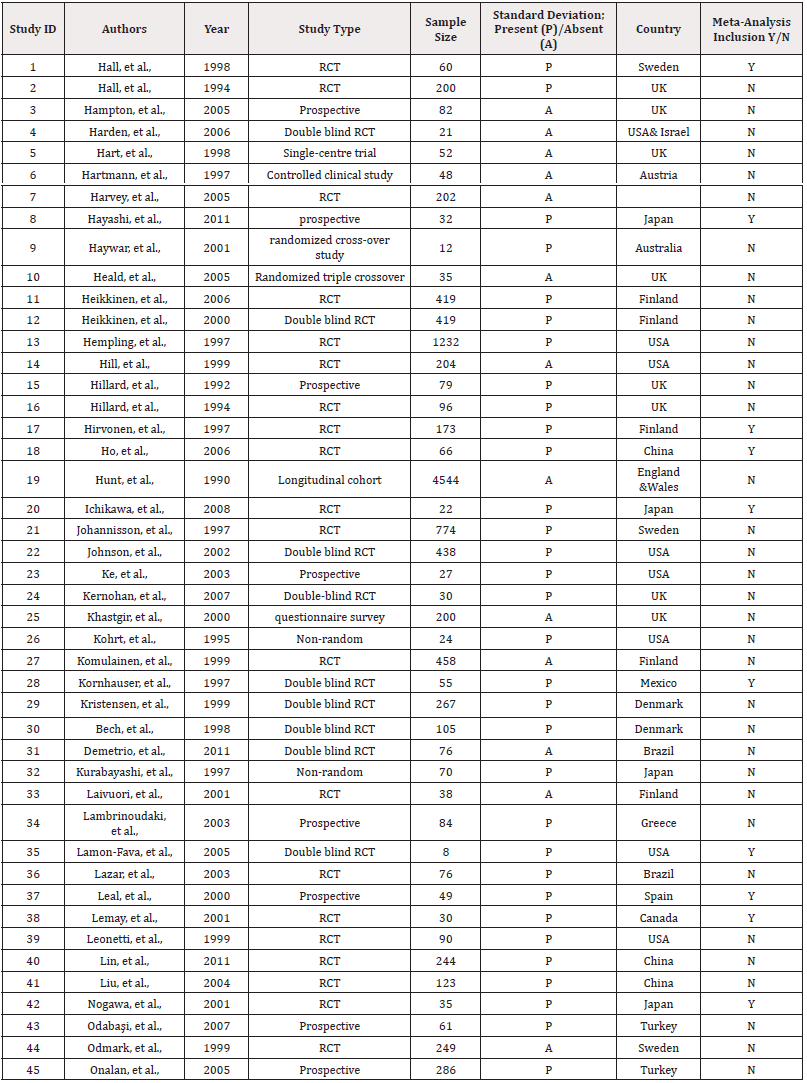
Of the 45 systematically included studies (Table 1), 10 were selected for a network meta-analysis. Studies were excluded from the meta-analysis due to the absence of quantifiable measures such as Standard Deviation (SD), lack of common denominators and insufficient common statistical details (Figure 1).
Leal, et al., and colleagues (2000) reported the changes in estradiol and triglycerides separately for participants with and without hot flushes. To better identify these changes across all studies, we conducted a meta-analysis with studies that recorded participants with and without hot flushes, as part of a combined analysis. A similar approach was applied in the context of studies that recorded the presence or absence of hot flushes with Follicle-Stimulating Hormone (FSH) and estradiol. A similar methodology was used by Nogawa, et al., and colleagues (2001). Hall, et al., (1998), Ichikawa et al (2020) and Leal, et al., (2000) assessed the use of transdermal 17β-estradiol and oral administration of Medroxy Progesterone Acetate (MPA). Within the context of the meta-analysis, this was categorised as "oral +transdermal." "Serm" refers to the Selective Oestrogen Receptor Modulator (SERM) raloxifene (Table 1).
Role of HRT on Reducing Triglycerides (TG) levels
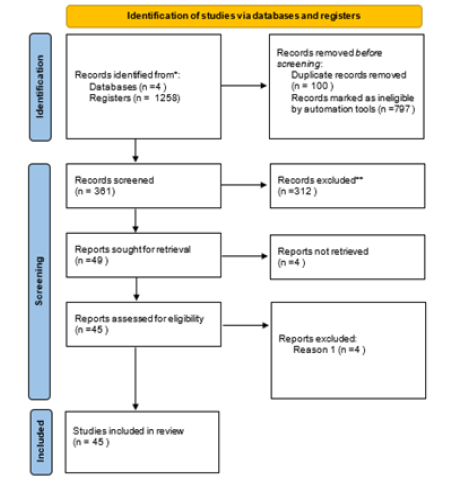
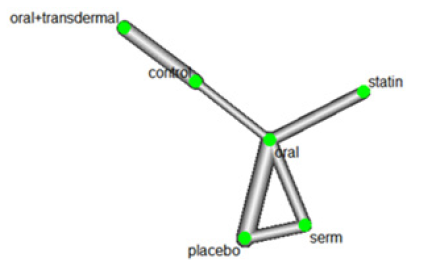
Efficacy of HRT was assessed based on reducing Triglycerides (TG) levels. Figure 1 shows the network of interventions, including oral, SERM, statin, oral plus transdermal control. Figure 1 illustrates the direct relationships among various interventions in different papers. Each node represents an intervention, and the thickness of the connecting lines indicates the number of studies. This graph provides a clear visualization of the direct relationships between interventions. The value of 77.1% of I^2 with a p-value of <0.0001 (Figure 2) indicates a significant statistical heterogeneity. Based on the identified statistical heterogeneity, we used a random-effects model to address heterogeneity and reduce bias. In network meta-analysis, heterogeneity refers to the variability in treatment effects across different studies. When there is substantial heterogeneity in the model, we assign lower weights to studies with greater heterogeneity in the random-effects model, effectively down-weighting their contribution to the overall effect estimate. This approach aims to minimize bias in estimating the overall treatment effect by accounting for the differences among studies and avoiding undue influence from any single study (Figure 2).
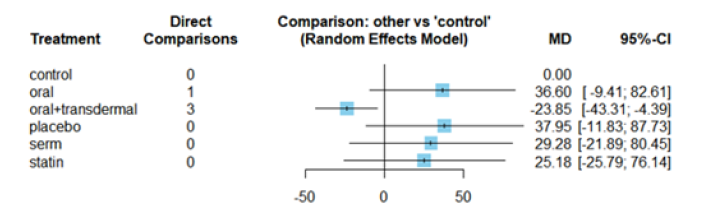
The MDs of all interventions compared directly and indirectly with reference to the control group consisted of participants who did not receive any medication during the experiment (Figure 3). Among the interventions directly compared to the control group, only the oral +transdermal intervention showed a statistically significant difference where the TG levels reduced with an effect size of -23.85. This is because the 95% CI of [-43.31, -4.39] does not include 0, indicating a significant decrease in TG levels with this intervention. In contrast, interventions such as placebo, SERM and statin lacked direct comparisons to the control group. This suggests a lack of direct evidence to support their efficacy in reducing TG levels among menopausal women (Figure 3).
The Egger's test p-value was 0.0850, indicating no significant publication bias in the meta-analysis (Figure 4).
Efficacy of HRT on Reducing Follicle-Stimulating Hormone (FSH)
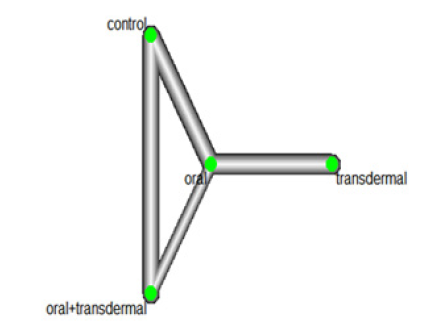
We assessed the efficacy of HRT in reducing FSH levels. Figure 4 illustrates the network of interventions, including oral, transdermal, oral +transdermal, and control. The control group consisted of participants who did not receive any medication during the experiment. A significant heterogeneity was observed with I^2 value of 52.9% and a p-value <0.0001, leading us to utilize the random-effects model (Figure 5).
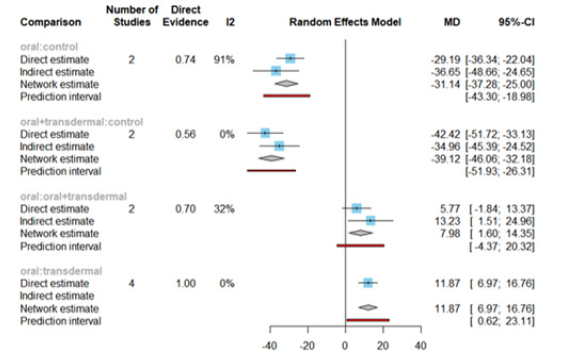
The Mean Differences (MDs) of all interventions compared directly and indirectly with the control group as the reference (Figure 6). Among the interventions directly compared to the control group, the oral intervention demonstrated a statistically significant difference in FSH levels, with an effect size of -31.14. Similarly, the oral +transdermal intervention also exhibited a significant difference in FSH levels, with an effect size of -39.12. These effect sizes were accompanied by 95% Confidence Intervals (CI) that did not include 0, indicating a significant decrease in FSH levels associated with these interventions (Figure 6).
The Egger's test p-value was 0.2018, indicating no significant publication bias in the meta-analysis.
Efficacy of HRT on Reducing LDL cholesterol (LDL-C)
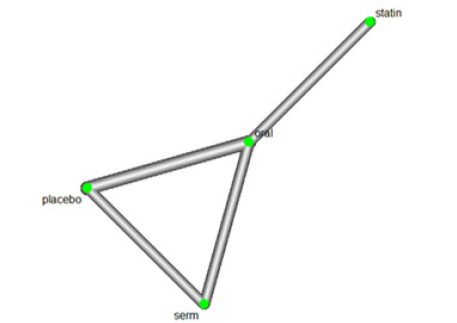
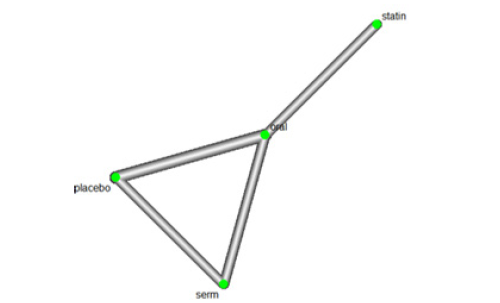
We investigated the efficacy of Hormone Replacement Therapy (HRT) in reducing LDL Cholesterol (LDL-C) levels. The network of interventions, encompassing oral, Selective Oestrogen Receptor Modulators (SERMs), statins, and placebo (Figure 7).
The observed I^2 value of 73.9% and a p-value less than 0.0001 indicated statistically significant heterogeneity, leading us to employ the random-effects model (Figure 8).
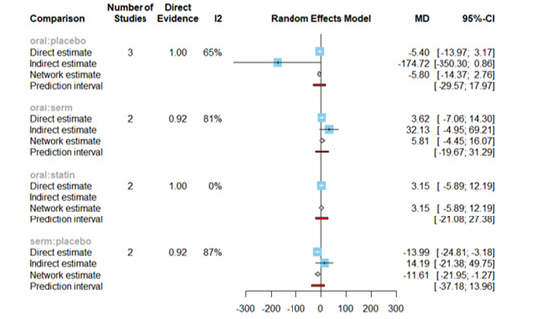
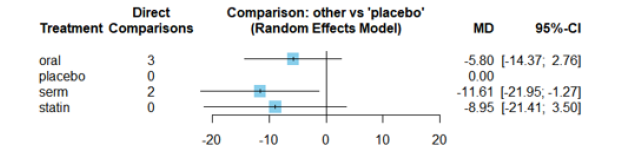
The Mean Differences (MDs) of all interventions compared directly and indirectly with the placebo group serving as the reference (Figure 9). Among the interventions directly compared to the placebo group, the SERM intervention exhibited a statistically significant difference in LDL-C levels, with an effect size of -11.61. This significant effect is attributed to the fact that the 95% Confidence Interval (CI) does not include 0, indicating a notable decrease in LDL-C levels associated with this intervention. Conversely, interventions such as oral and statins lacked direct comparisons to the placebo group. This suggests a scarcity of direct evidence to substantiate their effectiveness in reducing LDL-C levels (Figure 9).
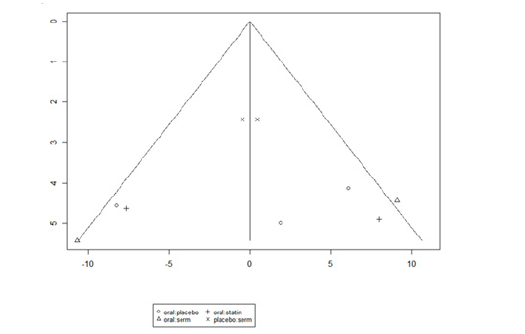
The funnel graph in figure 10 indicates no significant publication bias in the meta-analysis (Figure 10).
Efficacy of HRT on Increasing HDL Cholesterol (HDL-C)
We investigated the efficacy of Hormone Replacement Therapy (HRT) in increasing HDL Cholesterol (HDL-C) levels. Figure 11 presents the network of interventions, encompassing oral, Selective Oestrogen Receptor Modulators (SERMs), statins, and placebo (Figure 11).
The results of the network meta-analysis for the change in HDL-C level are demonstrated in Figure 12. The observed I^2 value of 56.8% and a p-value less than 0.0001 indicated significant statistical heterogeneity, leading us to employ the random-effects model (Figure 12).
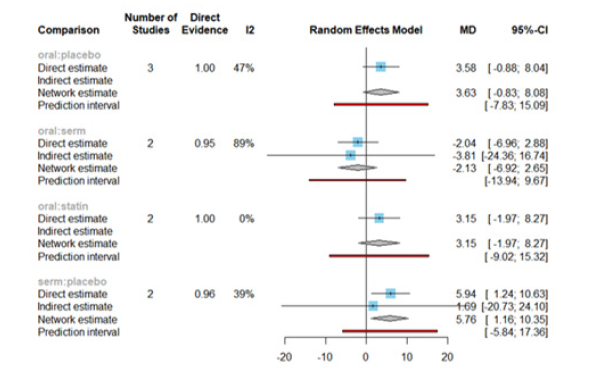
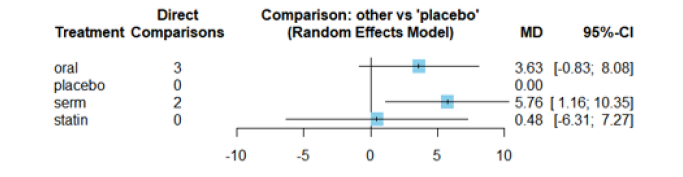
Figure 13 displays the Mean Differences (MDs) of all interventions compared directly and indirectly with the placebo group serving as the reference. Among the interventions directly compared to the placebo group, the SERM intervention exhibited a statistically significant difference in HDL-C levels, with an effect size of 5.76. This significant effect is attributed to the fact that the 95% Confidence Interval (CI) does not include 0, indicating a notable increasing in HDL-C levels associated with this intervention. Conversely, interventions such as oral and statins lacked direct comparisons to the placebo group. This suggests a scarcity of direct evidence to substantiate their effectiveness in reducing HDL-C levels (Figure 13).
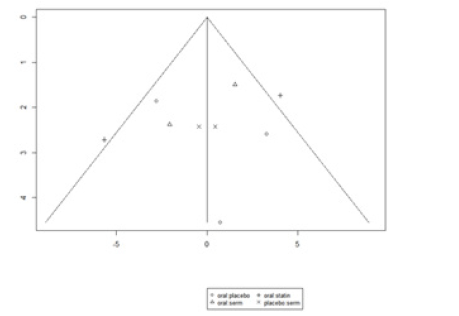
The funnel graph in figure 14 indicates no significant publication bias in the meta-analysis (Figure 14).
Efficacy of HRT on Increasing Estradiol
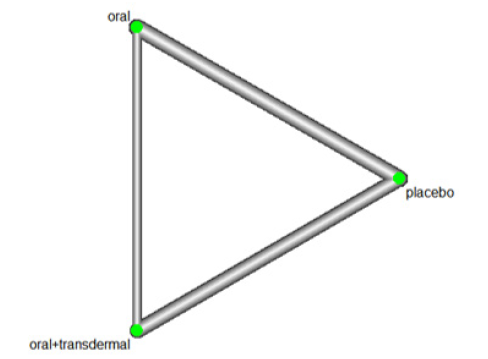
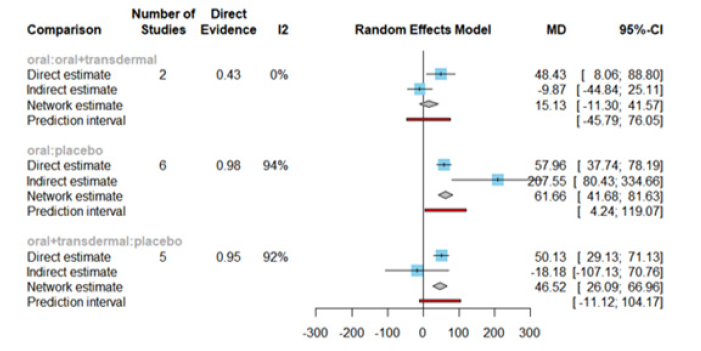
We investigated the effectiveness of Hormone Replacement Therapy (HRT) in increasing Estradiol levels. Figure 15 illustrates the network of interventions, including oral, oral+transdermal, and placebo (Figure 15).
Figure 16 presents the results of the network meta-analysis for changes in Estradiol levels. The statistical analysis revealed a significant heterogeneity, with an I² value of 93.3% and a p-value less than 0.0001, leading us to employ the random-effects model (Figure 16).
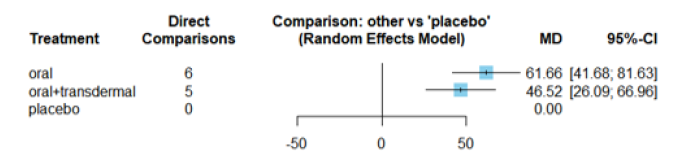
Figure 17 displays the Mean Differences (MDs) of all interventions compared directly and indirectly with the placebo group as the reference. Among the interventions directly compared to the placebo group, both the oral and oral +transdermal interventions demonstrated a statistically significant difference in Estradiol levels, with effect sizes of 61.66 and 46.52, respectively. These findings are supported by the fact that the 95% Confidence Interval (CI) does not include 0, indicating a significant increase in Estradiol levels with these interventions (Figure 17).
The Egger's test p-value was 0.6901, indicating no significant publication bias in the meta-analysis.
Summary
Table 2 showed the comparisons with statistically significant effect of HRT (Table 2).
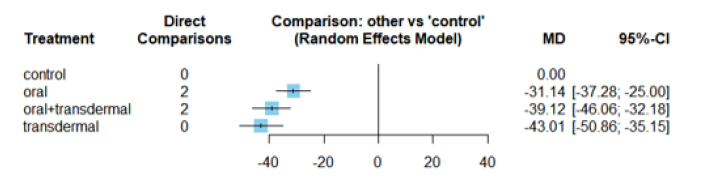
Compared with control group, oral +transdermal treatment provided a statistically significant decrease in TG. These significant comparisons showed that Oral +transdermal treatment had better effect in reduction of TG. Compared with control group, oral, Oral +transdermal and transdermal treatment showed a statistically significant reduction in FSH. These significant comparisons showed that transdermal treatment had better effect in reduction of FSH. In the evaluation of HRT effect on TG, oral +transdermal intervention demonstrated a significant decrease in the effect size indicating its efficacy in lowering TG levels. When exploring the impact of HRT on FSH, the transdermal intervention exhibited the most favourable effect followed by oral +transdermal and oral interventions, respectively. All three treatment regimens demonstrated a significant decrease in the effect size of FSH indicating a reduction in FSH levels. With regards to the influence of HRT on LDL-C and SERM exhibited a noteworthy decrease in the effect size of LDL-C. In contrast, regarding the impact of HRT on HDL-C, SERM showed an increase in the effect size of HDL-C suggesting an affect to increase HDL-C levels. Examining the influence of HRT on Estradiol levels as an oral regime showed the most beneficial effects followed by oral + transdermal combination.
Discussion
Main findings
Our study demonstrates effectiveness of HRT interventions across cardiometabolic measures including TG, FSH, LDL-C, HDL-C and estradiol in menopausal women. Specifically, various forms of HRT or SERM were found to be efficacious in reducing TG, FSH and LDL-C levels whilst increasing levels of HDL-C and estradiol. A previous trial conducted in Korea has shown similar results with levels of TG being lower in postmenopausal women undergoing HRT compared to controls thereby, potentially reducing risk of dyslipidaemia and consequent CVD [17]. As observed in the Framingham study, the risk of cardiovascular disease in women increases with age indicating a potential role of menopause [18]. This hypothesis was reaffirmed by findings showing a doubled risk of postmenopausal cardiovascular disease incidence compared to premenopausal groups within the Framingham female cohorts [18]. In alignment with Nie, et al., (2022), our findings corroborate benefits of menopausal hormone therapy in significantly reducing LDL-C levels and enhancing overall lipid profile in menopausal women [19]. We also found specifically that SERM impacted both, LDL-C and HDL-C levels significantly. Yang, et al., and colleagues (2021) found positive effects of Raloxifene use in women resulting in increased HDL-C and significantly decreased LDL-C [20]. Other SERMs such as Tamoxifen have also shown impact on lipid profiles with modest decreases in LDL-C and HDL-C levels whereas, Bazedoxifene with conjugated estrogen showed decreased LDL-C and increased HDL-C levels [21].
As menopausal changes are characterised by reduced estrogen concentrations, the subsequent altered lipid profiles with elevated total cholesterol render a higher cardiovascular risk. Further research on use of lipid profile altering pharmacological interventions including HRT are key to improving our understanding of polypharmacy and its implications for menopause related comorbidity such as osteoporosis, CVD and climacteric symptoms [21]. Our analyses also found that HRT interventions affect FSH negatively and estradiol positively. The protective effects of estrogen have been recorded historically in terms of changes in serum lipids, nongenomic vasodilation and longer-term effects on vasculature. Santen, et al., (2010) reviewed HRT studies and found that estradiol intervention can prevent accelerated bone loss and delay atherosclerotic CVD events [22]. Our findings provide reaffirmation of changes in enhanced estradiol and reduced FSH post-HRT.
A key clinical consideration in HRT administration is regarding the optimal route of administration for menopausal women. Our study found oral +transdermal and oral methods to be the most effective routes of administration in modulating levels of TG, FSH and estradiol. Studies indicate an increased risk of thromboembolism and stroke from oral HRT administration [23-25]. However, this risk seems to be insignificant in women within the initial 10 years of menopausal change. Women in higher risk groups may opt for safer alternatives identified such as use of transdermal estrogen in combination with micronised progesterone as they have a smaller impact on biological coagulation and inflammation [26-29]. Within our findings, we identified a research gap in use of orals and statins to modulate LDL-C and HDL-C levels in menopausal women that would be beneficial to explore with direct comparators [30,31]. Similarly, studies investigating placebo, SERM and statins lacked direct comparisons to controls groups and there remains an opportunity to study evidence of their use in lowering of triglyceride levels as implicated in menopausal women [32]. Given the lack of consensus on HRT’s efficacy and clinical use for long-term health protection in menopausal women, our study reaffirms its efficacy in supporting a favourable lipid profile via improvements in biomarkers such as TG, FSH, LDL-C, HDL-C and estradiol within menopausal women. Our analyses suggest a positive impact of HRT use via oral and oral +transdermal methods on cardiometabolic factors within this patient population.
Strengths and Limitations
To our knowledge, this is the first meta-analysis conducted to identify and report HRT outcomes using existing peer review studies. The searches were inclusive of multiple databases including PubMed, Web of Science, ScienceDirect, EMBASE and MEDLINE and not limited by geography, therefore, improving our chances of a comprehensive literature review. However, there were attrition of sources that did not report statistical measures or were published after April 2022. Furthermore, 37 of the 47 studies reviewed could not be incorporated due to absence of quantifiable measures such as Standard Deviation (SD), lack of common denominators and insufficient common statistical details, constraining the generalisability of our findings. Upon further analyses of the excluded studies, the majority of trials focused on postmenopausal groups with limited evidence for perimenopausal and menopausal women. This is indicative of the need to study the impact of HRT across menopausal transitions and model the same.
Similarly, most studies were conducted in high-income countries whereas limited studies of this pool were in low to middle income countries. Within examined trials, race demographic breakdowns and differences were scarce with only a few studies noting a varied impact of the intervention between racial groups. This presents a limitation as it does not account for racial or cultural differences in terms of patient reporting and experience. For instance, Lin, et al., and colleagues (2011) highlighted the cultural contrast between Chinese and Caucasian patient reporting styles and how these differences impacted results seen in psychological and physiological outcomes [33]. As the majority of trials were based on biological markers, only a handful incorporated quality of life measures based on reports by menopausal women, which offer key data for tailored clinical management across populations. Finally, numerous studies commonly excluded patients with histories of non-communicable diseases such as diabetes, hypertension, thyroidism and depression amongst others which may represent common multimorbidity found in menopausal women. With multimorbidity impacting over 60% of aging women [34], HRT trials may be rendered less representative of the target population.
Implications and Recommendations
The use of HRT to manage difficult menopause symptoms has the potential not only to improve women’s quality of life, but to benefit the community economically. Previous findings show the economic burden in the US associated with menopause and postmenopausal management to be a direct cost of $248 per patient annually [35]. Though comparable to anxiety, hypertension and asthma, the cost to manage such symptoms was significantly lower than other chronic diseases, making it a worthwhile investment. Accounting for cost-benefit evaluation and the sizeable patient population, it is imperative that treatments such as HRT be available globally in an appropriate, tailored manner [36,37]. To ensure effective uptake and adherence to interventions such as HRT, they must be efficacious, safe, and acceptable by the patient populations being treated. Target populations may include women from different geographies and cultures, women in varying stages of menopause, women with comorbid disorders, women undergoing surgical menopause and transgender women.
The manifestation of menopausal symptoms differs by geography and clinical population, as women in LMICs have been shown to undergo menopause earlier than their counterparts in HICs. A study conducted in a city in Pakistan demonstrates how cultural outlook may mould patient communication and its lack of regarding symptomatology [38]. For instance, 60% of women perceived menopause to be a natural phenomenon providing the opportunity of rest and recuperation as opposed to seeking treatment. Plagued by financial challenges, risk of cancer and bleeding, women in Philippines also seek less medical help compared to their white counterparts with drug compliance and HRT awareness being severely low [38]. These cultural differences are only furthered when considering that type of patient reporting differs by geographies with Chinese women reporting more psychological outcomes compared to physiological measures than Caucasian counterparts [33]. In addition to communication, outlook and attitude, awareness regarding HRT is also a crucial factor to its acceptance and uptake as an intervention [39-42]. A Korean study suggested that less than half of women knew about preventive benefits of HRT in osteoporosis with less than a quarter knowing about CVD-related benefits [42].
The differences in awareness persist globally with Belgian women who are non-HRT users reporting that over half would not opt for HRT due to fears of breast cancer risk, CVD risk and weight fluctuation [43]. It’s crucial to note that most clinical studies are conducted in HICs with dosages and routines tested within these populations [44]. This reflects the dire need for parity and representation in clinical trials as clinical management and lifestyle considerations may fundamentally differ geographically. For instance, estradiol in 2mg and 4mg is the preferred administration in Europe as opposed to conjugated estrogens with alcohol consumption being a key lifestyle consideration, which may not be applicable globally [45,46,36]. To account for these differences, future research study designs could accommodate for detailed information on race, ethnicities, comorbidity status, medical histories, and the stages of menopause, perimenopause, menopause, and post-menopause. Moreover, follow-up data could be gathered to monitor longitudinal change in menopausal women thereby, contributing to our understanding of HRT efficacy and effectiveness. Considering HRT efficacy and safety is strengthened, policies that prompt improved affordability and accessibility such as UK’s HRT Prescription Prepayment Certificate (PPC) and Australia’s Pharmaceutical Benefits Scheme remain imperative to intervention uptake, awareness and adherence [47,48].
Conclusion
We conclude that use of HRT intervention in menopausal women enhance overall lipid profile by reducing TG, FSH and LDL-C levels and increasing levels of HDL-C and estradiol via oral and oral +transdermal routes of administration. The requirement for robust, scientifically viable and clinically relevant clinical epidemiology studies and clinical trials is evident. These would need to use more inclusive approaches that demonstrate the efficacy, effectiveness, tolerability, and acceptance of the use of HRT across all ethnicities and races.
Declarations
None
Funding
Not applicable
Conflicts of interest
PP has received research grant from Novo Nordisk, and other, educational from Queen Mary University of London, other from John Wiley & Sons, other from Otsuka, outside the submitted work. SR reports other from Janssen, Lundbeck and Otsuka outside the submitted work. All other authors report no conflict of interest. The views expressed are those of the authors and not necessarily those of the NHS, the National Institute for Health Research, the Department of Health and Social Care or the Academic institutions.
Availability of Data and Material
All data used within this study has been publicly available. The authors will consider sharing the dataset gathered upon request.
Code Availability
Not applicable.
Author Contributions
GD developed the MARiE project as part of the ELEMI program. The first draft was written by GD and PP. The study design and statistical analysis plan was developed by GD and JQS. The analysis was performed by GD, RZ, XY, PJ and JQS. Editing and formatting was performed by PJ. All authors critically appraised and commented on all versions of the manuscript. All authors read and approved the final manuscript.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All authors consented to publish this manuscript.
Acknowledgements
The authors acknowledge support from Southern Health NHS Foundation Trust, Southern University of Science and Technology and University of Southampton. We would like to thank Dr Helen Kemp, who is our patient-public liaison and advocate for Menopause for the wider project that also contributed her views to this manuscript.
Research in Context
Evidence before This Study
Women’s health research overall is limited despite a growing need, in particular in relation to menopause and related treatments. The dearth of evidence linked to hormonal replacement treatments (HRT) and health outcomes is a concern for future proofing better precision treatments and optimal healthcare services.
Added Value of This Study
To our knowledge this is the first meta-analysis conducted to identify and report HRT outcomes in relation to its impact on lipid profile using existing peer review studies. This study also provides an evidence based meta-epidemiology outcomes that are applicable to the real-world. This study demonstrates the current knowledge and gaps in practice, allowing priorities to be considered by all stakeholders.
Implications of the Available Evidence
The findings of this study provide information to develop evidence-based policies and better processes related to polypharmacy and the use of cultural adaptions to optimise therapeutic benefit. The knowledge gaps indicate that current policies and guidelines in use are based on insufficient scientific base.
References
- Wilson RA (1996) In: In Feminine Forever. Evans M., editor. Lippincott & Co.
- Ziel HK, Finkle WD (1975) Increased risk on endometrial carcinoma among users of conjugated estrogens. N Engl J Med 293(23): 1167-1170.
- Woodruff JD, Pickar JH (1994) Incidence of endometrial hyperplasia in postmenopausal women taking conjugated estrogens (Premarin) with medroxyprogesterone acetate or conjugated estrogens alone. The Menopause Study Group. Am J Obstet Gynecol 170(5pt 1): 1213-1223.
- Lobo RA (2017) Hormone-replacement therapy: Current thinking. Nat Rev Endocrinol 13(4): 220-231.
- Lobo RA, Pickar JH, Stevenson JC, Mack WJ, Hodis HN (2016) Back to the future: Hormone replacement therapy as part of a prevention strategy for women at the onset of menopause. Atherosclerosis 254: 296-304
- Improving women’s health and Gender Justice since the 1995 Beijing Platform for Action [Internet]. World Health Organization.
- Hulley S, Grady D, Bush T, Furberg C, Herrington D, et al. (1998) Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA 280(7): 605-613.
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, et al. (2002) Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. JAMA 288(3): 321-333
- Hormone-replacement therapy: Updated advice [Internet].
- Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, et al. (2013) Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA 310(13): 1353-1368
- Salpeter SR, Walsh JM, Greyber E, Salpeter EE (2006) Brief report: Coronary heart disease events associated with hormone therapy in younger and older women. A meta-analysis. J Gen Intern Med 21: 363-366.
- Salpeter SR, Walsh JM, Greyber E, Ormiston TM, Salpeter EE (2004) Mortality associated with hormone replacement therapy in younger and older women: A meta-analysis. J Gen Intern Med19(7): 791-804.
- Schierbeck LL, Rejnmark L, Tofteng CL, Stilgren L, Eiken P, et al. (2012) Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: Randomized trial. BMJ 345: e6409
- Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11): 1539-1558.
- Schwarzer G, Carpenter JR, Rücker Gerta (2015) Meta-Analysis with R. Springer International Publishing.
- Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test BMJ 315(7109): 629-634.
- Ki EY, Hur SY, Park JS, Do Han K, Park YG (2015) Differences in the lipid profile and hormone replacement therapy use in Korean postmenopausal women: the Korea National Health and Nutrition Examination Survey (KNHANES) 2010–2012. Archives of Gynecol Obstet 294(1): 165-173.
- Kannel WB, Hjortland MC, McNamara PM, Gordon T (1976) Menopause and Risk of Cardiovascular Disease. Annals of Internal Medicine 85(4): 447-452.
- Nie G, Yang X, Wang Y, Liang W, Li X, et al. (2022) The effects of menopause hormone therapy on lipid profile in Postmenopausal women: A systematic review and meta-analysis. Front Pharmacology 13: 850815.
- Yang F, Li N, Gaman MA, Wang N (2021) Raloxifene has favorable effects on the lipid profile in women explaining its beneficial effect on cardiovascular risk: A meta-analysis of randomized controlled trials. Pharmacol Res 166: 105512.
- Alomar SA, Găman MA, Prabahar K, Arafah OA, Almarshood F, et al. (2022) The effect of tamoxifen on the lipid profile in women: A systematic review and meta-analysis of randomized controlled trials. Exp Gerontol 159: 111680.
- Santen RJ, Allred DC, Ardoin SP, Archer DF, Boyd N, et al. (2010) Postmenopausal Hormone Therapy: An Endocrine Society Scientific Statement. J Clin Endocrinol Metab 95(7_supplement_1): s1-s66.
- Leal M, Díaz J, Serrano E, Abellán J, Carbonell LF (2000) Hormone replacement therapy for oxidative stress in postmenopausal women with hot flushes. Obstetrics and Gynecol 95(6 Pt 1): 804–809.
- Nogawa N, Sumino H, Ichikawa S, Kumakura H, Takayama Y, et al. (2001) Effect of long-term hormone replacement therapy on angiotensin-converting enzyme activity and bradykinin in postmenopausal women with essential hypertension and normotensive postmenopausal women. Menopause 8(3): 210-215.
- Hall G, Ulla Pripp, Schenck Gustafsson K, Landgren BM (1998) Longterm effects of hormone replacement therapy on symptoms of angina pectoris, quality of life and compliance in women with coronary artery disease. Maturitas 28(3): 235-242.
- Irahara M, Kuwahara A, Iwasa T, Ishikawa T, Ishihara O, et al. (2017) Assisted reproductive technology in Japan: a summary report of 1992-2014 by the Ethics Committee, Japan Society of Obstetrics and Gynecology. Reproductive Medicine and Biology 16(2): 126-132.
- Carlsten H A randomised controlled trial evaluating the effects of hormone replacement therapy (HRT) on bone mineral density (BMD) and disease course in postmenopausal women with rheumatoid arthritis (RA).
- Hampton NRE, Rees MCP, Lowe DG, Rauramo I, Barlow D, et al. (2005) Levonorgestrel intrauterine system (LNG-ius) with conjugated oral equine estrogen: A successful regimen for HRT in perimenopausal women. Human Reproduction 20(9): 2653-2660.
- Harden CL, Herzog AG, Nikolov BG, Koppel BS, Christos PJ, et al. (2006) Hormone replacement therapy in women with epilepsy: A randomized, double-blind, placebo-controlled study. Epilepsia 47(9): 1447-1451.
- Hartmann BW, Huber JC, Kirchengast S, Söregi G, Albrecht AE (1997) Effect of hormone replacement therapy on growth hormone stimulation in women with premature ovarian failure. Fertility and Sterility 68(1): 103-107.
- Harvey J, Scheurer C, Kawakami F, Quebe Fehling E, de Palacios PI, et al. (2005) Hormone replacement therapy and breast density changes. Climacteric 8(2): 185-192.
- Hart DM, Farish E, Fletcher CD, Barnes JF, Hart H, et al. (1998) Long-term effects of continuous combined HRT on bone turnover and lipid metabolism in postmenopausal women. Osteoporosis International 8(4): 326-332.
- Lin S, Sun L, Lin J, Yang XQ, Zhang L, et al. (2011) Estradiol 1 mg and drospirenone 2 mg as hormone replacement therapy in postmenopausal Chinese women Climacteric 14(4): 472-481.
- Xu X, Jones M, Mishra GD (2020) Age at natural menopause and development of chronic conditions and multimorbidity: results from an Australian prospective cohort. Hum Reprod 35(1): 203-211.
- Assaf A, Bushmakin A, Joyce N, Louie M, Flores M, et al. (2017) The Relative Burden of Menopausal and Postmenopausal Symptoms versus Other Major Conditions: A Retrospective Analysis of the Medical Expenditure Panel Survey Data. Am Health Drug Benefits 10(6): 311-321.
- Unger CA (2016) Hormone therapy for transgender patients. Transl Androl Urol 5(6): 877-884.
- Jaff N (2023) Does one size fit all? the usefulness of menopause education across low and middle-income countries. Maturitas 173: 90.
- Jalbuena JR N (1996) A32 menopausal medicine under difficult conditions: The Philippine experience. Maturitas 27:9-10.
- Naftolin F, Tan O (2011) Faculty opinions recommendation of Postmenopausal hormone therapy: An endocrine society scientific statement. Faculty Opinions – Post-Publication Peer Review of the Biomedical Literature.
- Oliver Williams C, Glisic M, Shahzad S, Brown E, Pellegrino Baena C, et al. (2018) The route of administration, timing, duration and dose of postmenopausal hormone therapy and cardiovascular outcomes IN WOMEN: A systematic review. Hum Reprod Update 25(2): 257-271.
- Shufelt CL, Manson JE (2021) Menopausal Hormone Therapy and Cardiovascular Disease: The Role of Formulation, Dose, and Route of Delivery. J Clinical Endocrinol Metab 106(5): 1245-1254.
- Krishna S (2002) Attitudes towards menopause and hormone replacement therapy in different cultures. I Congress Series 1229: 207-214.
- Depypere H, Pintiaux A, Desreux J, Hendrickx M, Neven P, et al. (2016) Coping with menopausal symptoms: An internet survey of Belgian postmenopausal women. Maturitas 90: 24-30.
- Stevenson JC, Arkadi Chines, Pan K, Ryan KA, Mirkin S (2015) A Pooled Analysis of the Effects of Conjugated Estrogens/Bazedoxifene on Lipid Parameters in Postmenopausal Women From the Selective Estrogens, Menopause, and Response to Therapy (SMART) Trials. J Clin Endocrinol Metab 100(6): 2329-2338.
- Kilim SR, Srinivasa Rao Chandala (2013) A Comparative Study of Lipid Profile and Oestradiol in Pre- and Post-Menopausal Women. J Clin Diagn Res 7(8): 1596-1568.
- Mendelsohn ME (2002) Protective effects of estrogen on the cardiovascular system. The American Journal of Cardiology 89(12A): 12E-17E.
- (2023) New Scheme for Cheaper Hormone Replacement Therapy Launches.
- Pharmaceutical Benefits Scheme - Services Australia.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.