Case Report 
 Creative Commons, CC-BY
Creative Commons, CC-BY
False Positive Troponin Due to Animal Induced Heterophile Antibody Case Report
*Corresponding author: Luke Valencia, DCLS Resident, MLS (ASCP)cm, University of Kansas Medical Center, Kansas City, USA.
Received: June 10, 2024; Published: June 13, 2024
DOI: 10.34297/AJBSR.2024.22.003022
Abstract
The report will investigate the intricacies surrounding a false positive troponin and explore the patient’s history, presenting symptoms, laboratory investigation, and confirmatory testing. The case underscores the importance of a comprehensive review and interdisciplinary team approach to even seemingly routine cardiac cases.
Introduction
Troponin assays are essential in an NSTEMI (Non-ST-segment Elevation Myocardial Infarction) work up; however, major limitations may restrict clinical utility in rare situations. The clinical laboratory will usually account for erroneous results arising from limitations like fibrin interference, hemolysis, and issues associated with preanalytical sample collection [1]. Unknown interfering substances pose a major limitation as they are rarely encountered. This case will delve into the work up of an unknown interference, an animal induced heterophile, discovered during a routine work up for chest pain.
Case
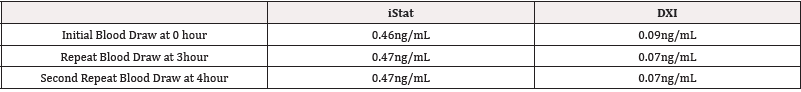
An 80-year-old male with history of ESRD (End Stage Renal Disease), atrial fibrillation, and asthma presented to the emergency room after a syncope event with shortness of breath and chest pain. A point of care device, iStat, troponin-I was ordered. The initial result showed a significant elevation in troponin whole blood concentration, 0.46ng/mL (normal <0.04ng/mL). The same specimen was sent to the laboratory for confirmation, where a discordant value of 0.09ng/mL was released. A significant elevation of 0.46ng/mL would indicate possible cardiac damage, while a value of 0.09 ng/ mL would be consistent with the patient’s ESRD. Two subsequent iStat troponins, performed at the three-hour and four-hour mark, again showed a significantly elevated troponin concentration of 0.47 and 0.47ng/mL with the confirmatory rerun on the laboratory analyzer (Beckman Coulter DXI TnI assay) being 0.07 ng/mL and 0.07 ng/mL (Table 1). The EKG did not support a STEMI diagnosis; however, an NSTEMI was unable to be ruled out with the conflicting troponin values. The Doctor of Clinical Laboratory Science (DCLS) resident was consulted for the discrepancies (Table 1).
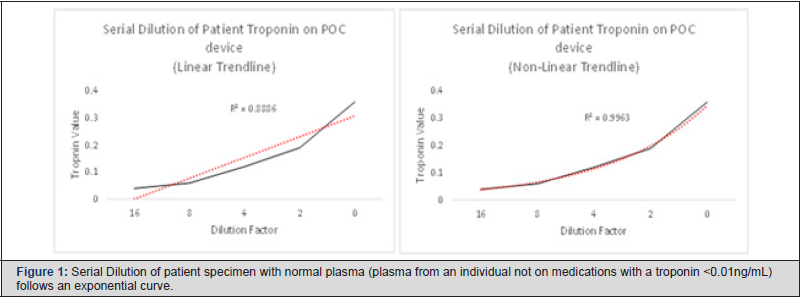
Review of reagent expiration with prior-run Quality Control (QC) and post-run QC within acceptable ranges ruled out instrument malfunction or reagent issues. Repeat runs by different users, on the same the specimens, and on different iStat devices further ruled out user error and ruled out instrument error. Preanalytical concerns were negated by consistent troponin results from different sample collections by different personnel and performed on different point of care devices. A CK-MB was performed and correlated with laboratory confirmed concentrations. Investigation led to the discovery of a possible heterophile antibody present in the patient’s blood. Discussion with the patient discovered he resided on a cow farm in his younger years. The iStat troponin Enzyme- Linked Immunosorbent Assay (ELISA) method utilizes bovine derived antibodies, while the confirmatory Beckman Coulter DXI troponin assay implements an anti-human cardiolipin mouse antibody [2]. Limitations of the iStat troponin assay note exposure to animals or recipients of immunoglobulins may lead to erroneous results [3]. Heterophile antibodies may be investigated through serial dilution of the suspected sample. The interfering anti-body should demonstrate a nonlinear trend line [4]. Serial dilution of the patient sample favored a non-linear result (Non-linear: R2=0.9963 vs. Linear:R3=0.8886) further supporting the picture of an interfering heterophile anti-body (Figure 1). Testing on plasma was used to maintain the integrity of the sample while the investigation was being conducted. Using plasma, rather than whole blood, on the point of care instrument resulted in a small decrease from initial whole blood troponin values; however, values correlated to initial results with consistent values on reruns. The investigation concluded that the patient was not having an NSTEMI. No medical intervention was needed. Resolution of symptoms occurred while the patient was under observation when the clinical investigation was being conducted. Patient was discharged and no adverse outcome occurred (Figure 1).
Discussion
Heterophilic antibodies have a prevalence of up to 3.1% in the general population [5]. In 2022, a systemic review concluded that human anti-mouse antibodies were the second most common cause of falsely reported elevated troponins [6]. Similarly, this case highlights a major limitation associated with the use of animal derived antibodies due to heterophilic antibodies. Animal derived antibodies are frequently used for troponin assays, as well as most chemistry assays in the clinical laboratory. Test values that do not correlate with the clinical presentation must be scrutinized, with limitations and interferes being reviewed prior to medical intervention. Troponin tests utilizing animal derived antibodies should be evaluated for heterophile interfere when conflicting results occur. Repeat of specimen testing by alternative methods should be considered when all other sources of error have been investigated. Laboratory specialists, such as the DCLS, may be utilized for investigation of discrepant laboratory results and challenging cases.
Conclusion
Heterophilic antibodies pose a risk to patient care. Clinicians should be aware of the limitations associated with assays utilizing animal derived antibodies. When discrepant results arise, animal exposure should be investigated when other sources of error have been eliminated.
Limitations
It should be noted that the presence of a heterophilic antibody, although strongly suspected from repeat testing, alternative testing, and dilutional study, was not conclusively confirmed. Further investigation using a heterophilic antibody blocking agent or elution should be considered when available [5].
Future Directions
Discrepant values that do not support clinical presentation should be investigated. If a heterophilic antibody is suspected, proper documentation on patient records is needed to prevent future misinterpretation of laboratory values. Assays with similar animal derived antibodies should be substituted with alternative methods.
Disclaimer
Written consent was obtained from the individual(s) AND/OR minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Acknowledgements
None.
Conflict of Interest
None.
References
- Chaulin AM (2022) False-Positive Causes in Serum Cardiac Troponin Levels. J Clin Med Res 14(2): 80-87.
- Beckman Coulter (2024) DXI TnI package insert. High-volume Immunoassay Analyzer UniCel DxI 800 Access Immunoassay System Beckman Coulter.
- Abbot Point of Care. iStat package insert. i-STAT cTnI Test Cartridge | Abbott Point of Care.
- Dasgupta A, Wahed A (2014) Chapter 2 - Immunoassay Platform and Designs. In: Clinical Chemistry, Immunology and Laboratory Quality Control: A Comprehensive Review for Board Preparation, Certification and Clinical Practice. Elsevier.
- Mair J Hammarsten O (2023) Potential analytical interferences in cardiac troponin immunoassays. Lab Precis Med 8: 12.
- Nevraumont A, Deltombe M, Favresse J, Louise Guillaume, Virginie Chapelle, et al. (2022) Interferences with cardiac biomarker assays: understanding the clinical impact. Eur Heart J 43(24): 2286-2288.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.