Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Market Dynamics – Emergence of Mesenchymal Stem Cell-Conditioned Media as a Novel Treatment for Acne
*Corresponding author: Jonathan R T Lakey, Department of Surgery and Biomedical Engineering, University of California Irvine, California, USA.
Received: May 25, 2024; Published: June 04, 2024
DOI: 10.34297/AJBSR.2024.22.003005
Abstract
Acne vulgaris and its consequential scarring inflict significant psychological and emotional burdens on those affected, profoundly impacting their overall well-being. As a chronic inflammatory condition, acne ranks as the eighth most prevalent disease globally, affecting approximately 9.4% of the population. Its incidence is escalating, affecting both adolescents and adults alike. Notably, over 85% of adolescents encounter acne, often commencing during preadolescence and persisting into adulthood. The severity of acne directly correlates with the degree of impairment and influence on an individual’s life. Moreover, acne can lead to enduring physical and psychological scars.
Despite the availability of various treatment methods, there remains a persistent demand for safer and more efficacious therapies. This paper aims to delve into the market dynamics propelling innovation within the global acne treatment sector. Key factors to be explored include market size and growth, the escalating prevalence and associated costs, heightened demand for effective treatments, and the emergence of innovative therapies, such as those utilizing mesenchymal stem cell-conditioned media (MSC-CM). Addressing these market forces is Global Innovative Health Solutions, which introduces its groundbreaking therapy: SCM-Blend Acne Cream.
Keywords: Mesenchymal stem cell, MSC, Conditioned media, MSC-CM, Acne, Acne vulgaris, Acne cream, Treatment methods, Market dynamics
Introduction
Acne vulgaris (hereafter referred to as acne), a global issue impacting nearly everyone at some point in their lives, carries significant mental health consequences and financial burdens. Although not life-threatening, acne can profoundly impact a patient’s quality of life, self-esteem, and mental well-being [1-3]. Traditionally viewed as an adolescent condition, recent research and clinical practice over the past two decades have highlighted its prevalence among adults as well [4-9]. Moreover, acne has been linked to diminished health-related quality of life (HRQoL), with negative effects comparable to severe, life-threatening illnesses [4,10,11]. Facial acne, typically prominent on the cheeks, chin, and forehead, is characterized by erythema, post-inflammatory hyperpigmentation, and scarring [11]. Acne scarring and post-inflammatory hyperpigmentation can greatly affect a person's appearance and mental well-being, including damages to self-perception, social relationships, and overall quality of life, particularly among adolescents [3,10,12-16]. Studies by Lewis-Jones, et al. and Finlay, et al. showed that patients with chronic skin diseases like acne experience greater impairment in quality of life compared to those with other skin conditions [17,18]. The more severe the acne, the greater the impairment and impact [10,19-23]. For instance, the quality of life of Malaysian adolescents was impacted proportionally to the acne severity [22]. As per Mallon, et al., acne is not a trivial problem as acne patients in their study showed comparable levels of social, psychological and emotional issues to those suffered from chronic asthma, epilepsy, diabetes, and more [10]. Regional and cultural differences do not lessen the impact of acne on both quality of life and psychological functioning as demonstrated in Egyptian patients [21].
The development of acne arises from the blockage of skin pores due to a combination of bacteria, hair follicles, sebum (skin oil), dirt, and dead skin cells, leading to inflammation. Four primary pathogenic processes contribute to the formation of acne lesions: altered follicular keratinization resulting in comedones, increased and altered sebum production controlled by androgens, colonization of follicles by Propionibacterium acnes bacteria, and complex inflammatory mechanisms involving both innate and acquired immunity [24,25]. Acne, a chronic inflammatory disease of the pilosebaceous unit, manifests as non-inflammatory lesions (open and closed comedones) and inflammatory lesions (papules, pustules, nodules), often resulting in varying degrees of scarring [24,26-28]. Inflammatory acne occurs when bacteria colonize closed comedones, leading to sebum breakdown and an inflammatory response by neutrophils, macrophages and lymphocytes, resulting in papules (relatively deep) and pustules (more superficial), which are erythematous lesions. Deeper and more severe forms of inflammatory acne, such as nodules and cysts, can be painful and may lead to scarring.
Obstruction and inflammation of the pilosebaceous unit, comprising the hair follicle, hair shaft, and accompanying sebaceous gland, is central to the development of acne, which primarily affects the face and upper trunk [24,26,27,29-32]. Acne lesions range from comedones to papules, pustules, nodules, and cysts, with two subtypes identified in postadolescent acne: persistent and late-onset [33]. Capitanio, et al. showed that 85% of patients with post-adolescent acne had mostly comedonal acne [34]. Persistent acne, continuing from adolescence into adulthood, is predominant in adult female patients, whereas late-onset acne begins after the age of 25 years [30,33].
Acne affects both men and women and is one of the most common externally visible skin diseases encountered by individuals aged 15 to 40 in the United States (U.S.) [4]. Despite being commonly associated with adolescence, many adults still grapple with acne beyond their teenage years, experiencing it differently based on various factors. Adult acne is defined by Preneau, et al. as onset ranging from 20-25 years [35]. The average age of adult onset was found to be 25±6 years, and one-third (33.7%) were diagnosed with acne as adults [11]. In adult women, acne pathogenesis is notably complex, with androgens playing a significant role [11,36-38]. This is evidenced by the response to hormonal treatments, notably used for hyperandrogenism disorders like polycystic ovary syndrome (PCOS), indicating the role of androgens in conditions such as acne, alongside the use of hormone-based therapies such as oral contraceptives and anti-androgen medications for managing symptoms in women with normal androgen levels [39]. PCOS is a common endocrine disorder that can cause hyperandrogenism, which is an excessive production of androgens (male hormones) in females [40]. One of the main physical manifestations of hyperandrogenism in PCOS is acne, but it’s important to note that not all individuals with PCOS will experience acne, as the symptoms can vary among individuals [40]. Additionally, the absence of acne in androgen-insensitive women and the association between rising dehydroepiandrosterone sulfate levels and acne onset in premenarchal girls and some PCOS patients further underscore the role of androgens [37,41-43].
Despite its prevalence and impact, adult acne lacks a clear age-based definition, resulting in inconsistent age demarcations in the literature [11,35]. Nonetheless, the global acne market persists in its pursuit of new treatments to enhance quality of life. This paper will explore the market dynamics driving innovation in the global acne market, such as market size and growth trends, escalating prevalence and costs, as well as the mounting demand for minimally invasive and efficacious treatments. Moreover, it will explore innovative therapies like mesenchymal stem cell-conditioned media (MSC-CM). Global Innovative Health Solutions (GIHS) emerges as a significant contributor to the expanding acne market, introducing its latest product, SCM-Blend Acne Cream. This Acne Cream is uniquely formulated with GIHS’s proprietary MSC-CM, known as the SCM Blend, presenting a promising new therapeutic approach.
Market Size and Growth Trends
The global acne treatment market is on the verge of significant growth between 2023 and 2032, primarily propelled by the high prevalence of acne-related skin conditions, rising awareness, and ongoing advancements in effective treatment options [44]. According to a market research study published by Custom Market Insights, this market is expected to achieve a Compound Annual Growth Rate (CAGR) of approximately 6.5% during 2023-2032, with a projected value of USD 18.77 Billion by 2032 [44]. Fortune Business Insights reports similar trends, forecasting a CAGR of 5.1% to 5.2% during the same period, with the market size reaching USD 17.48 billion by 2032 [45,46]. In addition, The Brainy Insights estimates a CAGR of 4.2%, projecting the market to reach USD 18.5 billion by 2032 [47]. Several factors contribute to the growth of the global acne treatment market, including the rise in the young population, increasing awareness, the development of effective treatments, and the escalating prevalence of skin problems worldwide. Despite common side effects, the demand for acne treatment remains high [44].
Acne, affecting a significant portion of the global population, has been categorized into moderate acne, mild acne, and moderate to severe acne, with the moderate acne segment holding the largest share of 43.72% in 2019 [44,48]. The 18 to 44 age group dominates the market, driven by hormonal changes and lifestyle factors [45,49]. Lifestyle changes, such as smoking, drinking, and excessive consumption of junk food, contribute to conditions like skin allergies and acne, accelerating the growth of the acne medication market [49].
Skin care clinics dominate the end-user segment, with a 38% market share in 2022, expected to grow rapidly due to increasing skin problems in urban areas [50]. Other end-users include hospitals and specialty centers. Retail pharmacies dominate distribution channels and are projected to grow significantly due to consumer preferences and rising urbanization and telemedicine [45,49].
Regionally, the Asia-Pacific market is anticipated to register the highest growth rate due to a high incidence of acne, a younger population, rising healthcare expenditure, increased government initiatives, and the presence of numerous pharmaceutical companies [48]. However, North America leads the global acne treatment market, with a 35.9% share in 2022 and 45% in 2023, followed by Europe, due to factors such as a growing population suffering from acne, beauty-related concerns, and investments in research and development [48-50]. In North America, the U.S. market size for acne treatment was USD 5.00 billion in 2022, projected to reach USD 7.27 billion by 2030, with a CAGR of 4.7% [51]. Additionally, Canada's acne treatment market is growing due to rising awareness, access to advanced treatments, and a culture that values healthcare and wellness [52].
In conclusion, the global acne treatment market is witnessing substantial growth, fueled by increasing awareness of skin health, new product developments, and lifestyle changes. With advancements in treatment options and rising demand, the market is expected to continue its upward trajectory. GIHS aims to establish itself as a dominant player in the growing global acne market with its newly developed SCM Blend-Acne Cream, aimed at alleviating the multiple burdens of this skin disease.
Escalating Prevalence and Costs
Acne is a widespread issue affecting individuals globally, encompassing both adolescents and adults. Acne is a common long-lasting skin condition characterized by inflammation, ranks as the eighth most prevalent disease worldwide, affecting 9.4% of the population [53]. It poses a significant concern, with nearly all adolescents aged between 15 and 17 experience some degree of acne [24,54]. Its prevalence is on the rise, particularly among adolescents, with studies indicating that 85% to 100% of adolescents experience acne, often beginning in preadolescence and continuing into adulthood [55-59].
Acne ranks among the top 10 most prevalent conditions worldwide and consistently features as one of the top three most prevalent skin conditions in large studies conducted in the UK, France, and the U.S. [53,60-62]. In Western countries, it affects 79%-95% of adolescents, while in northeastern China, 51.3% of adolescents are affected [63,64]. The Global Burden of Disease (GBD) study reports that approximately 85% of young adults aged 12–25 years are affected by acne [65]. Other studies have corroborated these findings [66,67]. Research by Bhate, et al. found that about 85% of individuals aged 12 to 24 experience at least minor acne [57]. Acne can occur at any life stage, persisting into one's 30s and 40s [11,30,68].
While the causes of acne are multifactorial, including dietary, genetic, and environmental factors, the onset of puberty serves as a common trigger. Androgen production during puberty stimulates sebum production and keratinocyte hyperproliferation, partly contributing to the prevalence of acne in this population, regardless of socioeconomic status, nationality, or gender [69]. Other factors contributing to acne pathogenesis encompass genetics, as evidenced by twin studies, a family history of severe acne, and dietary factors such as glycemic index, including chocolate consumption, and dairy consumption [64,70-79]. Furthermore, environmental factors such as smoking, occlusive cosmetics, and occupational exposures contribute to acne pathogenesis [80-83].
In recent decades, the occurrence of acne in adults has been rising, particularly among women [7,68]. Despite traditionally being viewed as an adolescent condition, acne persists into adulthood. In women aged 20 to 29 years, the prevalence rate is 50.9%, compared to 26.3% in those aged 40 to 49 years. [54]. Furthermore, women are affected at higher rates than men in the same age groups, with 50.9% of women affected compared to 42.5% of men in the younger age group, and 26.3% of women affected compared to 12.0% of men in the older age group [54]. Moreover, several studies show that approximately 15% to 41% of women are affected, with environmental factors such as ultraviolet light, pollution, and lifestyle stressors contributing to its prevalence [7,11,30,68,84-88]. Women are disproportionately affected by acne compared to men, with approximately 12% to 22% of U.S. women experiencing adult acne, compared to 3% of men [7-9]. Collier, et al. also found that women were being more affected than men in all age groups 20 years and older [54]. Female patients account for a significant majority of dermatologist visits for acne, particularly among those older than 25 years [89,90]. Milder cases of acne may also be more prevalent in adult women [11,35].
The healthcare costs associated with acne are substantial, with an estimated financial burden of $3 billion per year in the United States [57]. In 2013 alone, the costs related to treatment and lost productivity among individuals seeking medical care for acne surpassed $1.2 billion, with nearly $400 million attributed to lost productivity [48,91]. The direct cost of acne treatment in the U.S. exceeds $1 billion annually, with over $100 million spent on over-the-counter medications [92]. In 2013, more than 5.1 million people sought medical treatment for acne, predominantly children and young adults [91]. Furthermore, acne can result in enduring physical and psychological scars. Despite its prevalence as the most common skin condition in the U.S., finding a minimally invasive, long-lasting solution for effective acne and scar treatment remains a formidable challenge. However, companies like GIHS are pioneering efforts to address this persistent issue. Moreover, the impact of acne transcends mere financial burdens, affecting over 50 million individuals annually in the U.S. alone [45,48,51,57,93]. This significant contribution to the burden of acne treatment underscores its paramount importance within the healthcare landscape, particularly in the American market.
Rising Demand for Better Treatments
Psychosocial and Emotional Distress Affecting Well-Being
The demand for an efficient and effective treatment solution for acne extends far beyond financial concerns, as it arises from the significant physical and psychosocial distress experienced by affected individuals. In a 2016 study conducted by Hazarika, et al., which surveyed 100 acne patients, physical symptoms such as itch, soreness, pain, and stinging were reported by 78% of the participants [94]. Additionally, Tasoula, et al. and Reich et al. also observed the presence of itch symptoms in their respective studies [94-96].
While acne is not life-threatening or physically debilitating, its impact on social and psychological functioning can be severe. A large Greek survey conducted among 1,531 adolescents, with a similar distribution of genders aged between 11 and 19, revealed a self-reported acne prevalence of 51.2%, affecting both genders equally [96]. Among them, 71.2% reported mild acne, while 28.8% reported moderate to severe acne. The survey highlighted that acne significantly affects the quality of life of young adolescents in Greece, with more severe acne associated with greater effects on self-esteem, body image, and relationships with others. The impact of acne on quality of life was significantly associated with its severity, as adolescents with moderate to severe acne experienced more profound psychosocial and emotional distress. Among those surveyed, 31.4% reported feelings of unworthiness and teasing, 21.3% made modifications to their dressing style, 19.2% experienced challenges in personal and social lives, 14% avoided swimming and other sports activities, and between 19.4% and 21.4% reported negative effects on personal activities, including hobbies and school work. Peer pressure was found to affect body image proportionally to acne severity, with feelings of embarrassment and decreased self-esteem observed in a high percentage of individuals with acne.
Findings from the Greek study were consisitent with other research studies. As high as 75% of acne patients reported interpersonal problems with partner, close friends and relatives [94]. Additionally, a significant number of patients (68%) complained about the negative influence of acne on their social/leisure activities [94]. Social avoidance and withdrawal behaviour documented by Yolac, et al. and Fried, et al. can lead to the development of a permanent avoidant personality trait [97-99]. Avoidance of swimming, exercise and other sports due to acne was reported in several studies [19,100]. Between 14.4% and 25% of individuals reported difficulties in sports activities due to acne, while between 21.4% and 57% felt that acne affected their schoolwork and personal activities during holidays, including social interactions with the opposite sex [94,96,100,101]. Moreover, 69% reported avoidance behaviour, anger, and frustration affecting daily activities such as shopping, household chores, and gardening [94]. While major sleep disruption was not detected, Tasoula, et al. observed a significant percentage (16.6%) experienced it, higher than in the study by Walker et al. (6%) [19,96]. Lack of confidence, social dysfunction, and reduced employment opportunities were also documented [99,102,103]. Female adults with acne agreed that physical appeal is advantageous to getting hired and finding life partners [11,104,105]. Acne also affected adolescents' dressing styles and choices, with approximately one-fourth facing difficulty in dressing due to acne [106,107]. Social inhibition or phobia accompanying acne was evident, with nearly 20% of acne adolescents experiencing problems in relationship building due to their acne lesions [96,108]. Developmental issues of socialization, body image, and sexuality were documeted [94,102]. Adolescents often resort to coping mechanisms such as avoiding eye contact, growing hair long to cover the face, or using makeup to minimize the appearance of acne lesions due to fear of scrutiny by others [109-111].
Severe acne has been associated with increased anxiety, depression symptoms, and overall impact on patients' lives [112]. Studies have shown a heightened prevalence of mood disorders, psychiatric hospitalizations, school absenteeism, unemployment, and even suicidal ideation among individuals with severe acne [113,114]. Moreover, anxiety and depression were prevalent among acne patients, with up to 9% reporting suicidal ideation [115-118]. However, Magin, et al. found that feelings of unworthiness due to negative peer appraisal were more prominent than anxiety and depression, leading to impairment of self-esteem and self-image [96,99,119]. Furthermore, Singam, et al. demonstrated that patients with coexistent acne are more likely to experience primary hospital admissions for various mental health disorders, including depression, schizophrenia, and substance use disorders, compared to those without acne [59,113].
Though prevalent among acne adolescents, with almost half experiencing an impact on their self-esteem, embarrassment and self-esteem issues were felt by adults as well [10,94,112,120]. Studies have highlighted acne's particularly troublesome impact on patients between 20 and 40 years old, including distress related to their appearance compared to younger age (16-19 years old) groups [111,121-123]. Emotional effects and quality of life did not differ between males and females, with both genders equally affected by their skin problems [96,124-126]. However, studies from different regions showed varying impacts, possibly due to cultural differences [21,117,127]. Overall, acne significantly affects quality of life, leading to disturbances in body image, emotional stress, and social isolation [122,128-131]. Given the severe impacts of acne on individuals, there is an urgent need to develop more effective acne treatments.
Acne Scars Diminishing Well-Being
Acne scars, a persistent complication, represent a daunting consequence due to their potential to cause facial disfigurement and psychological distress, particularly when located on the face after lesions have healed [67,132,133]. This issue significantly impacts patients' quality of life [134-136]. Acne lesions typically manifest on the cheeks and forehead and can lead to permanent scaring, which tends to worsen with age due to natural lipoatrophy, further accentuating the scars [26,137]. Scarring often occurs early in acne and may affect up to 95% of patients, with its severity linked to both the severity of acne and delay before treatment [138]. Adults with acne demonstrate a higher prevalence of acne scars (59%) compared to the non-adults (32%) [139]. Despite varying degrees of scar formation, scars remain a significant concern for individuals due to their impact on appearance and psychological well-being [140].
Scar formation in acne can be categorized into two main stages: excessive tissue formation leading to keloid or hypertrophic scars, and tissue loss or damage resulting in atrophic scars [26,141]. Among these, facial atrophic scars are the most common [89,142-144]. These scars are crucial considerations in acne management and treatment due to their significant impact on physical and emotional well-being. Atrophic scars, which account for more than 80% of acne scars, are primarily of the ice pick (~60%), boxcar (~25%), or rolling (~15%) types [66,145-147]. Atrophic acne scars are a consequence of inflammation that damages deep dermal structures, resulting in irregular collagen synthesis and breakdown, causing skin indentation due to tissue contraction [142]. Acne scars are enduring outcomes of acne, with prevalence rates as high as acne itself, affecting 47% of acne sufferers [139]. Main risk factors include male gender, family history of acne, and acne severity [139]. Genetic factors, such as SELL and TGFB2, have been implicated in scarring, further highlighting a genetic influence [148]. Acne scars are prevalent across all levels of acne severity, with severe acne particularly prone to scar formation due to intense skin inflammation [139,149]. Geographical variations in acne scar prevalence have been noted, with Africa (31%) demonstrating lower rates compared to Asia (52%) and Europe (51%) [139,150]. Despite dark-skinned individuals' heightened susceptibility to keloid and hypertrophic scars, they are less prone to atrophic acne scars, possibly due to genetic factors [150-152]. In addition, traditional observations suggest a higher prevalence of acne scars in men (58%), potentially due to hormonal and glandular differences [89,153,154]. Early intervention and comprehensive acne management are crucial in mitigating the risk of scarring, considering the multifaceted nature of acne scars influenced by genetic predisposition, acne severity, and gender differences.
Current Treatment Methods with Side Effects: Non-Surgical vs. Surgical
Facial acne scars prompt a range of treatment methods, including both surgical and non-surgical approaches. Non-surgical options involving topical medications and non-invasive procedures show promise, while surgical options like punch excision and subcutaneous excision address deeper scars. Even mild acne demonstrates a significant prevalence of scars, emphasizing the importance of treating all forms of acne to prevent scarring [139]. Due to their significant impact and difficulty in treatment, acne scars are gaining more attention. Risk factors for scarring include severe acne, duration of acne, family history of atrophic scars, and lesion manipulation [155]. Although treatments like laser resurfacing, micro-needling, chemical peels, and fillers exist, completely eliminating acne scars remains challenging. Various post-acne scar treatments exist, each with differing efficacy, side effects, and downtime. While recent advancements offer promising options, it's essential to consider associated risks and potential adverse effects before treatment initiation. Current methods include traditional approaches like skin abrasion, chemical peeling, and micro-needle therapy, as well as newer options like mesenchymal stem cells (MSC) and laser therapy. Laser therapy, categorized as ablative and non-ablative fractionated lasers, offers effective treatment, although associated with potential short-term adverse reactions, including pain, erythema, and inflammation [156].
Different types of laser treatment are utilized for acne scar management. Ablative lasers, such as 10600 nm CO2 and 2940 nm pulsed Er: YAG lasers, vaporize the skin surface, promoting tissue removal and forming scabs post-treatment [140,157]. Several studies on ablative lasers have yielded mixed clinical efficacy results [158-161]. Despite being effective, CO2 lasers can lead to short-term adverse reactions like erythema and pigmentation [162-164]. Non-ablative lasers, including pulsed dye, 1320 nm Nd: YAG, 1450 nm diode, and 1550 nm erbium glass lasers, are effective in reducing boxcar-type atrophic acne scars [165]. For instance, 675 nm lasers stimulate collagen resynthesis, resulting in significant scar improvement without adverse reactions [165-167]. Studies also show the effectiveness of 1320 nm Nd: YAG lasers in treating atrophic acne scars, especially in younger patients [168-170]. Combining 1550 nm fractionated lasers with tretinoin has proven more effective than laser monotherapy for ice-pick-type scars, illustrating combination therapy often yields optimal results [170].
Other non-surgical treatments for acne scarring include chemical peels, fillers, micro-needling, and thread lifting. However, thread lifting has the least evidence regarding its clinical efficacy for acne scarring [171,172]. It involves inserting barbed threads under the skin to lift tissues and stimulate collagen synthesis [173]. Initially using non-absorbable threads, now semi-permanent absorbable threads are preferred due to lower infection risk, despite a shorter duration [171-173]. Minor complications may include mild erythema, small hemorrhage, small ecchymosis, transitory esthesia, and mild tumefaction [173]. Research using absorbable polydioxanone (PDO) threads showed improvement in boxcar scars without adverse events, but larger studies are needed to further assess effectiveness and safety [174].
Micro-needling therapy involves creating tiny holes in the skin to promote collagen production and skin regeneration, effectively improving rolling and boxcar scars with minimal side effects [175-177]. However, it is less effective for ice-pick scars and does not cause pigment deposition [178-182]. Combining micro-needling with other treatments like platelet-rich plasma (PRP) or chemical peels can enhance results, offering a versatile and safe approach to acne scar treatment [180,181,183-186]. Nevertheless, side effects such as erythema, edema and pain are commonly reported [187].
Skin fillers are a common method to address atrophic acne scars by injecting medical materials into depressed areas to level them with the surrounding skin. These fillers come in short-acting, semi-permanent, and permanent types, each with varying durations and biodegradability [188,189]. Short-term fillers like hyaluronic acid (HA) and exogenous collagen last 6 to 18 months, providing immediate volume increase and long-term collagen synthesis stimulation [190-193]. Semi-permanent fillers like poly-L-lactic acid (PLLA) and calcium hydroxylapatite (CaHA) stimulate collagen production but may cause adverse reactions [194-198]. Permanent fillers such as polymethylmethacrylate (PMMA) offer long-lasting results but require careful monitoring for complications [188,199].
Chemical peels utilize acids like alpha-hydroxy acids (AHAs) such as glycolic and lactic acid, beta-hydroxy acids (BHAs) like salicylic acid, and trichloroacetic acid (TCA) to promote skin exfoliation and collagen production [200,201]. Emerging agents like pyruvic acid and mandelic acid are also increasingly used for this purpose [171,200]. Different peeling strengths can address various skin issues, including acne scars, photoaging, dyschromia, and precancerous lesions [202]. Combining acids like salicylic and mandelic peels (SMPs) has proven effective for acne scar treatment [203-206]. Deeper scars, especially ice-pick scars, may require stronger agents like TCA (>50%) [165,204,205]. Chemical peels offer fast recovery, are cost-effective, and can be used alone or with other treatments to address various scar types [200,204,207-211].
Dermabrasion, a first major advanced treatment for scarring, involves controlled abrasion of superficial skin layers to stimulate re-epithelialization and collagen production [138,171,212,213]. While effective for shallow rolling and boxcar scars, it is less so for ice pick scars and may cause complications like pain and dyschromia [26,66,143,206,214-216]. Despite its historical effectiveness, dermabrasion is gradually being replaced by alternatives like chemical peels and lasers, which offer comparable or superior results with shorter recovery times [206].
Punch excision treatments, including punch elevation, excision, and grafting, involve using a circular blade to reach subcutaneous tissue [66]. These approaches effectively target deep ice pick and boxcar scars resistant to other treatments [182]. While punch elevation lifts scars without damage, excision may lead to visible ring scars and uneven texture if not done optimally [182,205,217-220]. Laser treatment can improve resulting uneven texture and linear scars [171,180,182,219,220]. Punch grafting offers a last resort for deep scars by inserting graft tissue into the punch site [171,221]. Despite being effective for specific scar types, punch techniques haven't seen recent advancements apart from adjunct laser therapies [171].
Subcutaneous excision, a surgical technique devoid of incisions, effectively targets atrophic acne scars and wrinkles, especially rolling scars, boasting improvement rates of up to 100% in standardized grading systems [222]. However, its efficacy is limited for boxcar scars, and complications such as nerve and blood vessel damage can lead to side effects like hematoma formation and excessive pigmentation [223-225]. Despite advancements in surgical instruments, complications remain a concern. Combining subcutaneous excision with treatments like micro-needling therapy, laser therapy, or hyaluronic acid injections enhances efficacy [222-224]. Studies highlight improved outcomes when combining subcutaneous excision with procedures like CO2 fractional laser or PRP [161,224,227,228]. Nonetheless, achieving optimal results necessitates careful surgical technique and a comprehensive understanding of facial anatomy.
Combined therapy for acne scars, utilizing multiple treatment methods simultaneously, has garnered attention for its ability to comprehensively address scars and enhance treatment efficacy. Common combinations, such as laser therapy with PRP injections, have demonstrated superior outcomes compared to laser therapy alone [229,230]. Studies have also highlighted the efficacy of PRP in combination with techniques like subscision and micro-needling therapy [186,224,231]. Combination therapy, integrating both surgical and non-surgical methods, is often utilized for optimal scar improvement. Further research is necessary to identify the most effective combinations among the wide array of available treatment options.
Each treatment modality presents unique benefits and risks, necessitating personalized approaches considering patient-specific factors like genetics, age, and skin type [171,232]. While experimental treatments hold potential, careful planning and consideration of efficacy, safety, patient preferences, and cost are crucial for optimal outcomes in treating acne scars.
Topical vs. Oral: Retinoids and Antibiotics
As antibiotic resistance continues to rise, acne treatment approaches have shifted away from widespread antibiotic use toward more limited usage, favoring isotretinoin for moderate to severe cases. However, due to the ongoing need for innovative therapies to address acne's widespread occurrence, effective patient counseling and regular evaluations are paramount [32]. Additionally, there is a growing demand for effective treatments to manage this globally prevalent condition.
Topical retinoids like adapalene, tretinoin, and tazarotene effectively treat acne by preventing comedone formation and reducing inflammation [233,234]. They are preferred for all types of acne, minimizing scarring and discoloration [235,236]. Patients usually start with low concentrations due to potential side effects like dryness and irritation, gradually increasing as tolerated. Oral isotretinoin, a systemic retinoid, is safe and effective for severe acne, also recommended for moderate cases resistant to treatment [25]. It reduces sebum production, acne lesions, and scarring, without increasing depression risk, though rare mood exacerbation cases have been reported [237-252]. Isotretinoin doesn’t associate with inflammatory bowel disease, but its safety and efficacy remain uncertain [25,67,253]. Treatment must consider reproductive plans, monitored through the iPLEDGE program, established by the FDA in 2006, mandating contraception use for fertile women, to prevent pregnancies while on isotretinoin therapy due to its severe teratogenic effects, including craniofacial, cardiac and thymic malformations [254-258]. Despite the strict monitoring, a noncompliance rate of 29% was reported [259]. Nonetheless, isotretinoin remains a cornerstone treatment.
Topical antibiotics are effective against acne but face challenges due to emerging resistance, especially with erythromycin and clindamycin. [25,236,260-264]. Combining them with benzoyl peroxide is recommended to combat resistance [25,236,260,264-267]. Oral antibiotics like tetracyclines, preferred for moderate to severe cases, should be used cautiously to prevent resistance [25,236,264]. Antibiotic resistance affects over 2.8 million people in the US, with common side effects like nausea, vomiting, and diarrhea reported in around 7% of tetracycline users and 4% of macrolide users [25,45,262]. Minocycline and doxycycline are contraindicated during pregnancy due to the risk of tooth and bone discoloration, while their use during breastfeeding is generally considered safe [25,257,268-272]. Minocycline may cause fewer gastrointestinal side effects but can lead to tinnitus, dizziness, and skin pigmentation [25, 272]. Doxycycline is associated with sun sensitivity and stomach upset, primarily metabolized by the liver and safe for patients with kidney problems [25]. Limiting oral antibiotics may reduce risks like inflammatory bowel disease and infections [273-275]. Certain antibiotics like penicillin, erythromycin, and cephalosporin are considered safer during pregnancy, though caution is advised due to potential fetal cardiac malformations and hepatotoxicity [268,276-279]. Penicillin and cephalosporin are safe for use during breastfeeding but rarely used for acne due to uncertain effectiveness and potential side effects such as allergic reactions, nausea and diarrhea [25,268]. The effects of these antibiotics on oral contraceptive pills are uncertain, with limited evidence suggesting a potential risk of contraceptive failure [280-285]. However, other antibiotic classes are discouraged unless tetracyclines or macrolides (e.g., erythromycin) are contraindicated, and switching antibiotic classes should be approached cautiously to avoid promoting bacterial resistance [25,264,286].
Women over 25 years old often experience treatment failure, with a significant portion failing multiple courses of antibiotics or even isotretinoin [32,241,242,287,288]. Treating acne in adult women requires individualized approaches, considering patient preferences and reproductive factors. Screening for depression before and after isotretinoin initiation is recommended [289]. Despite advancements, the lack of a standardized acne grading system complicates treatment efficacy assessments, highlighting the need for further research in this area [25,290].
The prescription medicines segment, including antibiotics, retinoids, and other products commanded a market share of approximately 57% in 2022 [47]. Retinoids led among therapeutic classes, capturing about 29% of the market share in the same year, owing to their efficacy against moderate to severe acne by unclogging pores [47]. Equally dominant in 2022 was the antibiotics segment, driven by their approval for acne treatment and effectiveness in managing exudates [45,46]. In 2023, the topical segment emerged as the dominant force and is expected to maintain its lead due to increased product availability and patient preference for easy application [45]. Conversely, the oral segment is projected to grow due to rising acne prevalence and new product launches. Patients prioritize effective, comfortable, and easy-to-use acne treatments, regardless of whether antibiotics or retinoids dominate the market. However, this dominance comes with product limitations and suboptimal efficacy, necessitating a personalized medicine approach. In response to this demand, GIHS is launching its SCM-Blend Acne Cream, offering an enhanced acne treatment option that can be used either alone or in combination therapy.
Opportunities for Novel Therapies
Mesenchymal Stem Cells-Derived Conditioned Media (MSC-CM) as an Emerging Therapeutic
Managing post-acne scars poses significant challenges. Novel therapies utilizing mesenchymal stem/stromal cell conditioned media are being explored for acne scars, wound healing, and other skin conditions such as scalp psoriasis [160,291-301]. Recent research emphasizes that the therapeutic effects of mesenchymal stem cells (MSCs) stem from their secretion of paracrine factors, which trigger antiapoptotic events and facilitate tissue repair by maintaining an increased paracrine factor environment, thus making conditioned media derived from MSC cultures (MSC-CM), also known as the secretome of MSCs, a promising avenue for various regenerative therapies [302]. The stromal/stem cell secretome, comprising proteins, microRNA, growth factors, antioxidants, proteasomes, and exosomes, plays a pivotal role in therapeutic efficacy [303]. Conditioned media (CM) containing the secretome, serve as a rich source of paracrine factors, including vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), insulin-like growth factor-1 (IGF-1), IGF-2, and stromal cell-derived factor 1 (SDF-1), which promote tissue repair and regeneration [304-307].
Tissue regeneration and improvement in skin appearance quality may be facilitated by the presence of growth factors, chemokines, and cytokines in MSC-CM. Previous studies indicate that paracrine factors from MSCs in the CM play a pivotal role in wound healing [308,309]. Adipose-derived stem cell (ADSC)-CM has been shown to stimulate collagen synthesis, dermal fibroblast migration, and wound healing in animal models [310]. MSC-CM has also demonstrated efficacy in promoting skin burn wound recovery in rats, with factors like basic fibroblast growth factor (bFGF) being implicated in tissue regeneration [311,312]. Comparative studies suggest that wound healing with bone marrow-derived mesenchymal stem cell (BMMSC)-CM surpasses that with fibroblast-CM [308]. Stem cell-derived CM presents a viable alternative for cell-based therapies, given its effectiveness in healing various wounds [306,308,309,313]. MSC-CM's properties vary depending on factors such as cell source, culture conditions (hypoxic vs. normoxic), and timing of collection [314-318]. For example, hypoxic treatment, commonly used to enhance CM, has been shown to reduce oxygen levels and improve cellular functions [319]. CM obtained from MSCs cultured under hypoxic conditions has been shown to enhance cell proliferation and self-renewal capacities [319-322]. Allogeneic MSC-CM has demonstrated superior healing rates compared to xenogeneic MSC-CM, particularly under hypoxic conditions [319-321,323,324].
Differentiating MSC-CM from culture media alone is vital, as the former demonstrates superior efficacy in wound healing and scar reduction [321,325,326]. MSC-CM has shown greater hypertrophic scar reduction compared to culture media alone, with topical application post-laser treatment resulting in less erythema and pigmentation, suggesting that the observed effects of MSC-CM in these conditions are likely attributable to its secretome rather than the culture media alone [160,327-330]. Despite its potential, CM necessitates more frequent administration compared to MSC due to shorter half-lives of cytokines and growth factors [306].
Combining MSC-CM with conventional therapy may yield better outcomes, with various routes of administration showing promise, particularly topical and subcutaneous methods, which are the least invasive [299,300,331-333]. MSCs primarily isolated from adipose tissue, bone marrow, and umbilical cord blood have been utilized, with MSC-CM also showing potential benefits in atrophic scars and hypertrophic scars, particularly when combined with botulinum toxin type A (botox) [160,291-293,322,327-329,334,335]. Micro-needling combined with topical stem cell products, like amniotic fluid-derived mesenchymal stem cell-conditioned media (AF-MSC-CM), offers a novel approach for scar treatment. In a study comparing efficacy, significant improvement was noted with AF-MSC-CM compared to microneedling alone [292]. Similarly, the application of human stem cell-conditioned media following fractional CO2 laser procedure demonstrated atrophic scar improvement [293]. However, mixed results were observed with topical stem cell-conditioned medium after fractional carbon dioxide laser (FCL) compared to combined FCL and PRP or FCL alone for treating atrophic acne scars [291]. While SC-CM may enhance FCL efficacy, PRP seems to be the preferable option [291]. While adverse events are more commonly associated with laser therapy than MSC-CM treatment, further clinical studies are needed to fully assess the effects of MSC-CM on scar improvement. Nonetheless, combined therapy with laser therapy has shown promise in reducing acne scars [160,291-293].
Innovative Treatment Approach: GIHS’s SCM-Blend Acne Cream
Emerging therapies for acne scars, such as stem cells and cytokines, including MSCs, growth factors, and conditioned media, show promise in improving outcomes and reducing scars. Despite this progress, treating acne and its resultant scars remains challenging due to their prevalence and the absence of a universal solution.
According to a patient study published in the Journal of International Medical Research in November 2022, over 45% of patients discontinued therapy prematurely due to unresponsiveness and side effects associated with acne treatment products [51]. Addtionally, Fortune Business Insights reported that approximately 37% of patients discontinued using acne treatment products due to side effects [51].
In response to these challenges, GIHS offers a unique and effective solution: SCM-Blend Acne Cream. GIHS, a technology company with a vision of building a ‘Foundation for the Future,’ focuses on innovation in regenerative medicine, particularly stem cell therapeutics, to address human diseases. Led by Dr. Jonathan Lakey, a renowned researcher in stem cells and islet transplant for diabetics, GIHS aims to bring health and happiness to people through scientific innovation. Dr. Lakey's recent publication on Neurocream, with MSC-CM as the therapeutic agent, showed a 50% reduction in foot pain, tingling, and swelling in diabetic patients using the Neurocream, highlighting the potential benefits of stem cell therapeutics beyond diabetes [336]. As the Chief Scientific Officer and Co-Founder of GIHS, Dr. Lakey's expertise in stem cells is integral to the development and commercialization of stem cell therapeutics, including the SCM-Blend Acne Cream for acne patients.
Currently, GIHS is conducting a pilot study on the SCM-Blend Acne Cream (Table 1), with preliminary results showing visible improvement in acne for treated patients (Figures 1,2). No adverse effects have been reported so far, indicating the cream's safety and efficacy. Patient adherence to topical agents like the SCM-Blend Acne Cream is crucial for treatment success. Studies have shown that over 75% of patients adhere to topical agents, surpassing the average adherence rates of 50% to 60% [337-339]. Given the preference for non-invasive topical treatments in the acne treatment market, GIHS is well-positioned to drive market growth with its SCM-Blend Acne Cream. By offering a comfortable and effective solution, GIHS aims to make impactful changes and improve patients' quality of life with its new creation, the SCM-Blend Acne Cream.
Conclusion
Early intervention is crucial to prevent worsening acne and scar formation, emphasizing the importance of prompt and effective acne treatment. Facial acne not only affects physical appearance but also disrupts emotional and social functioning. Although adult acne tends to be mild or moderate, its predominantly inflammatory nature can make it resistant to conventional treatments like antibiotics and isotretinoin, leading to increased scarring [241,242]. Overall, acne is a multifaceted condition that imposes significant burdens on both individuals and society. Its far-reaching effects underscore the importance of developing effective treatment strategies and providing support for those affected by this common dermatological condition [59,113]. New therapies for treating acne are constantly being researched and developed due to various market dynamics, including market size and growth trends, increasing prevalence and awareness, and the demand for better treatments. Many of these treatments are still undergoing testing, but early studies indicate promising results, such as GIHS’s SCM-Blend Acne Cream. To offer an effective solution to the acne problem, GIHS proposes a novel treatment with its SCM-Blend Acne Cream. GIHS offers a new treatment option to patients by providing effective and customizable modalities tailored to their individual needs. This facilitates advancements in personalized medicine within both clinical and cosmetic dermatology.
References
- Aktan S, Özmen E and Sanli B (2000) Anxiety, depression, and nature of acne vulgaris in adolescents. Int J Dermatol, 39(5): 354 357.
- Gupta MA and Gupta AK (1998) Depression and suicidal ideation in dermatology patients with acne, alopecia areata, atopic dermatitis and psoriasis. Br J Dermatol 139(5): 846-850.
- Dunn LK, O Neill J and Feldman SR (2011) Acne in adolescents: quality of life, self-esteem, mood and psychological disorders. Dermatol Online J 17(1): 1.
- Stern RS (2000) Medication and medical service utilization for acne 1995-1998. J Am Acad Dermatol, 43(6): 1042-1048.
- Stern RS (1992) The prevalence of acne on the basis of physical examination. J Am Acad Dermatol 26(6): 931-935.
- Stern RS (1996) Acne therapy. Medication use and sources of care in office-based practice. Arch Dermatol 132(7): 776-780.
- Goulden V, Stables GI and Cunliffe WJ (1999) Prevalence of facial acne in adults. J Am Acad Dermatol 41(4): 577-580.
- Poli F, Dreno B and Verschoore M (2001) An epidemiological study of acne in female adults: results of a survey conducted in France. J Eur Acad Dermatol Venereol 15(6): 54-545.
- Perkins AC, Maglione J, Hillebrand GG, Miyamoto K and Kimball AB (2012) Acne vulgaris in women: prevalence across the life span. J Womens Health 21(2): 223-230.
- Mallon E, Newton JN, Klassen A, Steward Brown SL, Ryan TJ, et al. (1999) The quality of life in acne: a comparison with general medical conditions using generic questionnaires. Br J Dermatol 140(4): 672-676.
- Tanghetti EA, Kawata AK, Daniels SR, Yeomans K, Burk CT, et al. (2014) Understanding the Burden of Adult Female Acne. J Clin Aesthet Dermatol 7(2): 22-30.
- Tan J, Beissert S, Cook Bolden F, Chavda R, Harper J, et al. (2022) Impact of facial atrophic acne scars on quality of life: a multi‐country population‐based survey. Am J Clin Dermatol 23(1): 115‐
- Darji K, Varade R, West D, Armbrecht ES and Guo MA (2017) Psychosocial impact of postinflammatory hyperpigmentation in patients with acne vulgaris. J Clin Aesthet Dermatol 10(5): 18-23.
- Barnes LE, Levender MM, Fleischer AB and Feldman SR (2012) Quality of life measures for acne patients. Dermatol Clin 30(2): 293-300.
- Dreno B (2006) Assessing quality of life in patients with acne vulgaris: implications for treatment. Am J Clin Dermatol 7(2): 99-106.
- Smith JA (2001) The impact of skin disease on the quality of life of adolescents. Adolesc Med 12(2): 343-353.
- Lewis Jones MS and Finlay A (1995) The Children's Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol 132(6): 942-949.
- Finlay AY and Khan GK (1994) Dermatology Life Quality Index (DLQI)-a simple practical measure for routine clinical use. Clin Exp Dermatol 19(3): 210-216.
- Walker N and Lewis Jones MS (2006) Quality of life and acne in Scottish adolescent children: use of the Children's Dermatology Life Quality Index (CDLQI) and the Cardiff Acne Disability Index (CADI) J Eur Acad Dermatol Venereol 20(1): 45-50.
- Pawin H, Chivot M, Beylot C, Faure M, Poli F, et al. (2007) Living with acne. A study of adolescents' personal experiences. Dermatology, 215(4): 308-314.
- Abdel Hafez K, Mahran AM, Hofny ER, Mohammed KA, Darweesh AM, et al. (2009) The impact of acne vulgaris on the quality of life and psychologic status in patients from upper Egypt. Int J Dermatol 48(3):280-285.
- Hanisah A, Omar K and Shah SA (2009) Prevalence of acne and its impact on the quality of life in school-aged adolescents in Malaysia. J Prim Health Care 1(1): 20-25.
- Gupta MA and Gupta AK (2003) Psychiatric and psychological co-morbidity in patients with dermatologic disorders: epidemiology and management. Am J Clin Dermatol 4(12): 833-842.
- Williams HC, Dellavalle RP and Garner S (2012) Acne vulgaris. Lancet 379(9813): 361-372.
- Zaenglein AL, Pathy AL, Schlosser BJ, Alikhan A, Baldwin HE, et al. (2016) Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol 74(5): 945-973.
- Fabbrocini G, Annunziata MC, D Arco V, Vita V, Lodi G, et al. (2010) Acne scars: pathogenesis, classification and treatment. Dermatol Res Pract 893080.
- Strauss JS, Krowchuk DP, Leyden JJ, Lucky AW, Shalita AR, et al. (2007) Guidelines of care for acne vulgaris management. J Am Acad Dermatol 56(4): 651‐
- Wu N, Sun H, Sun Q, Cong L, Liu C, et al. (2021) A meta-analysis of fractional CO2 laser combined with PRP in the treatment of acne scar. Lasers Med Sci 36(1): 1-12.
- Addor FAS and Schalka S (2010) Acne in adult women: Epidemiological, diagnostic and therapeutic aspects. An Bras Dermatol 85(6): 789-795.
- Holzmann R and Shakery K (2014) Postadolescent acne in females. Skin Pharmacol Physiol, 27(Suppl 1): 3-8.
- Rocha MA and Bagatin E (2018) Adult-onset acne: Prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol 11: 59-69.
- Kamangar F and Shinkai K (2012) Acne in the adult female patient: a practical approach. Int J Dermatol 51(10): 1162-1174.
- Ramos-e-Silva M, Ramos-e-Silva S and Carneiro S (2015) Acne in women. Br J Dermatol 172(Suppl 1), 20-26.
- Capitanio B, Sinagra JL, Bordignon V, Cordiali Fei P, Picardo M, et al. (2010) Underestimated clinical features of postadolescent acne. J Am Acad Dermatol 63(5): 782-788.
- Preneau S and Dreno B (2012) Female acne-a different subtype of teenager acne? J Eur Acad Dermatol Venereol 26(3): 277-282.
- Harper JC (2008) Evaluating hyperandrogenism: a challenge in acne management. J Drugs Dermatol 7(6): 527-530.
- Lucky AW, Biro FM, Huster GA, Leach AD, Morrison JA, et al. (1994) Acne vulgaris in premenarchal girls. An early sign of puberty associated with rising levels of dehydroepiandrosterone. Arch Dermatol 130(3): 308-314.
- Lucky AW, Biro FM, Simbartl LA, Morrison JA and Sorg NW (1997) Predictors of severity of acne vulgaris in young adolescent girls: results of a five-year longitudinal study. J Pediatr 130(1): 30-39.
- Lolis MS, Bowe WP and Shalita AR (2009) Acne and systemic disease. Med Clin North Am 93(6): 1161-1181.
- Carmina E, Dreno B, Lucky WA, Agak WG, Dokras A, et al. (2022) Female Adult Acne and Androgen Excess: A Report From the Multidisciplinary Androgen Excess and PCOS Committee. J Endocr Soc 6(3): bvac003.
- Imperato McGinley J, Gautier T, Cai LQ, Yee B, Epstein J, et al. (1993) The androgen control of sebum production. Studies of subjects with dihydrotestosterone deficiency and complete androgen insensitivity. J Clin Endocrinol Metab 76(2): 524-528.
- Thiboutot D (2004) Acne: hormonal concepts and therapy. Clin Dermatol 22(5): 419-428.
- Chen MJ, Chen CD, Yang JH, Chen CL, Ho HN, et al. (2011) High serum dehydroepiandrosterone sulfate is associated with phenotypic acne and a reduced risk of abdominal obesity in women with polycystic ovary syndrome. Hum Reprod 26(1): 227-234.
- Kharrati K (2023, November 10) Global Acne Treatment Market Size Likely to Surpass at a CAGR of 6.5% by 2033. Custom Market Insights. Press Release. Sandy USA.
- Fortune Business Insights (Report ID: FBI 103361) 150.
- Acne Treatment Market Size to Surpass USD 15.70 Billion by 2030, exhibiting a CAGR of 5.1% (GlobeNewswire) Fortune Business Insights.
- The Brainy Insights (Report ID: TBI-13845) December, 2023. 239.
- Gotadki R (2021, August) Acne Treatment Market. Market Research Future (Report ID: MRFR/HC/4220-CR) 153 pages.
- Acne Medication Market Size To Reach USD 19.17 Bn By 2033. Precedence Research (Report Code 3924) March 2024. 150.
- The Brainy Insights (Report ID: TBI-13265) January 2023. 236.
- Fortune Business Insights (Report ID: FBI 106565) July 2023.
- Custom Market Insights (Report Code CMI 34688) Global Acne Treatment Market 2024-2033. 220.
- Hay RJ, Johns NE, Williams HC, Bolliger IW, Dellavalle RP, et al. (2014) The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol 134(6): 1527‐
- Collier CN, Harper JC, Cafardi JA, Cantrell WC, Wang W, et al. (2007) The prevalence of acne in adults 20 years and older. J Am Acad Dermatol 58(1): 56‐
- Ekore RI and Ekore JO (2021) Excoriation (skin‐picking) disorder among adolescents and young adults with acne‐induced postinflammatory hyperpigmentation and scars. Int J Dermatol 60(12): 1488-
- Layton AM, Thiboutot D and Tan J (2021) Reviewing the global burden of acne: how could we improve care to reduce the burden? Br J Dermatol 184(2): 219-225.
- Bhate K and Williams HC (2013) Epidemiology of acne vulgaris. Br J Dermatol 168(3): 474-485.
- Ghodsi SZ, Orawa H and Zouboulis CC (2009) Prevalence, severity, and severity risk factors of acne in high school pupils: A community-based study. J Invest Dermatol 129(9): 2136-2141.
- Habeshian KA and Cohen BA (2020) Current Issues in the Treatment of Acne Vulgaris. Pediatrics, 145(Suppl 2): S225-S230.
- Rea JN, Newhouse ML and Halil T (1976) Skin disease in Lambeth. A community study of prevalence and use of medical care. Br J Prev Soc Med 30(2): 107-114.
- Wolkenstein P, Grob JJ, Bastuji Garin S, Ruszczynski S, Roujeau JC, et al. (2003) French people and skin diseases: results of a survey using a representative sample. Arch Dermatol 139(12): 1614-1619.
- Johnson MT and Roberts J (1978) Skin conditions and related need for medical care among persons 1-74 years. United States 1971-1974. Vital Health Stat 212(4): 1-72.
- Cordain L, Lindeberg S, Hurtado M, Hill K, Eaton SB, et al. (2002) Acne vulgaris: a disease of Western civilization. Arch Dermatol 138(12): 1584-
- Wei B, Pang Y, Zhu H, Qu L, Xiao T, et al. (2010) The epidemiology of adolescent acne in North East China. J Eur Acad Dermatol Venereol 24(8): 953-957.
- Seattle WI (2013) GBD Compare. Seattle: University of Washington.
- Jacob CI, Dover JS and Kaminer MS (2001) Acne scarring: a classification system and review of treatment options. J Am Acad Dermatol 45(1): 109-117.
- Zaenglein AL (2018) Acne vulgaris. N Engl J Med 379(14): 1343-1352.
- Khunger N and Kumar C (2012) A clinico-epidemiological study of adult acne: is it different from adolescent acne? Indian J Dermatol Venereol Leprol 78(3): 335-341.
- Lynn DD, Umari T, Dunnick CA and Dellavalle RP (2016) The epidemiology of acne vulgaris in late adolescence. Adolesc Health Med Ther 7: 13-25.
- Bataille V, Snieder H, MacGregor AJ, Sasieni P and Spector TD (2002) The influence of genetics and environmental factors in the pathogenesis of acne: a twin study of acne in women. J Invest Dermatol 119(6): 1317-1322.
- Ismail NH, Manaf ZA and Azizan NZ (2012) High glycemic load diet, milk and ice cream consumption are related to acne vulgaris in Malaysian young adults: a case control study. BMC Dermatol 12: 13.
- Kwon HH, Yoon JY, Hong JS, Jung JY, Park MS, et al. (2012) Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol 92(3): 241-246.
- Smith RN, Mann NJ, Braue A, Makelainen H and Varigos GA (2007) A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr 86(1): 107-115.
- Smith RN, Mann NJ, Braue A, Makelainen H, and Varigos GA (2007) The effect of a high-protein, low glycemic-load diet versus a conventional, high glycemic-load diet on biochemical parameters associated with acne vulgaris: a randomized, investigator-masked, controlled trial. J Am Acad Dermatol 57(2): 247-256.
- Smith R, Mann N, Makelainen H, Roper J, Braue A, et al. (2008) A pilot study to determine the short-term effects of a low glycemic load diet on hormonal markers of acne: A nonrandomized, parallel, controlled feeding trial. Mol Nutr Food Res 52(6): 718-726.
- Magin P, Pond D, Smith W and Watson A (2005) A systematic review of the evidence for 'myths and misconceptions' in acne management: diet, face-washing and sunlight. Fam Pract 22(1): 62-70.
- Adebamowo CA, Spiegelman D, Berkey CS, Danby FW, Rockett HH, et al. (2006) Milk consumption and acne in adolescent girls. Dermatol Online J 12(4): 1.
- Adebamowo CA, Spiegelman D, Berkey CS, Danby FW, Rockett HH, et al. (2008) Milk consumption and acne in teenaged boys. J Am Acad Dermatol 58(5): 787-793.
- Di Landro A, Cazzaniga S, Parazzini F, Ingordo V, Cusano F, et al. (2012) Family history, body mass index, selected dietary factors, menstrual history, and risk of moderate to severe acne in adolescents and young adults. J Am Acad Dermatol 67(6): 1129-1135.
- Klaz I, Kochba I, Shohat T, Zarka S and Brenner S (2006) Severe acne vulgaris and tobacco smoking in young men. J Invest Dermatol 126(8): 1749-1752.
- Schäfer T, Nienhaus A, Vieluf D, Berger J and Ring J (2001) Epidemiology of acne in the general population: The risk of smoking. Br J Dermatol 145(1): 100-104.
- Plewig G, Fulton JE and Kligman AM (1970) Pomade acne. Arch Dermatol 101(5): 580-584.
- Tucker S B (1983) Occupational tropical acne. Cutis 31(1): 79-81.
- Albuquerque RG, Rocha MA, Bagatin E, Tufik S and Andersen ML (2014) Could adult female acne be associated with modern life? Arch Dermatol Res 306(8): 683-688.
- Do JE, Cho SM, In SI, Lim KY, Lee S, et al. (2009) Psychosocial aspects of acne vulgaris: A community-based study with Korean adolescents. Ann Dermatol 21(2): 125-129.
- Knaggs HE, Wood EJ, Rizer RL and Mills OH (2004) Post-adolescent acne. Int J Cosmet Sci 26(3): 129-138.
- Krutmann J, Moyal D, Liu W, Kandahari S, Lee GS, et al. (2017) Pollution and acne: Is there a link? Clin Cosmet Investig Dermatol 10: 199-204.
- Skroza N, Tolino E, Mambrin A, Zuber S, Balduzzi V, et al. (2018) Adult acne versus adolescent acne: A retrospective study of 1167 Patients. J Clin Aesthet Dermatol 11(1): 21-25.
- Tan J, Kang S and Leyden J (2017) Prevalence and risk factors of acne scarring among patients consulting dermatologists in the USA. J Drugs Dermatol 16(2): 97-102.
- Yentzer BA, Hick J, Reese EL, Uhas A, Feldman SR, et al. (2010) Acne vulgaris in the United States: a descriptive epidemiology. Cutis 86(2): 94-99.
- American Academy of Dermatology/Milliman. Burden of Skin Disease. 2017.
- Patel P, Lin HC, Feldman SR, Fleischer AB, Nahata MC, et al. (2011) Medication choice and associated health care outcomes and costs for patients with acne and acne-related conditions in the United States. J Drugs Dermatol 10(7): 766-771.
- Bickers DR, Lim HW, Margolis D, Weinstock MA, Goodman C, et al. (2006) The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol 55(3): 490-500.
- Hazarika N and Archana M (2016) The Psychosocial Impact of Acne Vulgaris. Indian J Dermatol 61(5): 515-520.
- Reich A, Trybucka K, Tracinska A, Samotij D, Jasiuk B, et al. (2008) Acne itch: Do acne patients suffer from itching? Acta Derm Venereol, 88(1): 38-42.
- Tasoula E, Gregoriou S, Chalikias J, Lazarou D, Danopoulou I, et al. (2012) The impact of acne vulgaris on quality of life and psychic health in young adolescents in Greece. Results of a population survey. An Bras Dermatol 87(6): 862-869.
- Yolaç Yarpuz A, Demirci Saadet E, Erdi Sanli H and Devrimci Ozgüven H (2008) Social anxiety level in acne vulgaris patients and its relationship to clinical variables. Turk Psikiyatri Derg 19(1): 29-37.
- Fried RG and Wechsler A (2006) Psychological problems in the acne patient. Dermatol Ther 19(4): 237-240.
- Magin P, Adams J, Heading G, Pond D and Smith W (2006) Psychological sequelae of acne vulgaris: Results of a qualitative study. Can Fam Physician 52(8): 978-979.
- Loney T, Standage M and Lewis S (2008) Not just ‘skin deep’: Psychosocial effects of dermatological-related social anxiety in a sample of acne patients. J Health Psychol 13(1): 47-54.
- Jowett S and Ryan T (1985) Skin disease and handicap: An analysis of the impact of skin conditions. Soc Sci Med 20(4): 425-429.
- Tan JK (2004) Psychosocial impact of acne vulgaris: Evaluating the evidence. Skin Therapy Lett 9(7): 1-3.
- Purvis D, Robinson E, Merry S and Watson P (2006) Acne, anxiety, depression and suicide in teenagers: A cross-sectional survey of New Zealand secondary school students. J Paediatr Child Health 42(12): 793-796.
- Pruthi GK and Babu N (2012) Physical and psychosocial impact of acne in adult females. Indian J Dermatol 57(1): 26-29.
- Cunliffe WJ (1986) Acne and unemployment. Br J Dermatol 115(3): 386.
- Feingold A (1992) Good-looking people are not what we think. Psychological Bulletin 111(2): 304-341.
- Motley RJ and Finlay AY (1989) How much disability is caused by acne? Clin Exp Dermatol 14(3): 194-198.
- Uzun O, Başoğlu C, Akar A, Cansever A, Ozşahin A, et al. (2003) Body dysmorphic disorder in patients with acne. Compr Psychiatry 44(5): 415-419.
- Tedechi A, Dall Oglio F, Micali G, Schwartz RA and Janniger CK (2007) Corrective camouflage in pediatric dermatology. Cutis 79(2): 110-112.
- Girman CJ, Hartmaier S, Thiboutot D, Johnson J, Barber B, et al. (1996) Evaluating health-related quality of life in patients with facial acne: Development of a self-administered questionnaire for clinical trials. Qual Life Res 5(5): 131-138.
- Matsuoka Y, Yoneda K, Sadahira C, Katsuura J, Moriue T and Kubota Y (2006) Effects of skin care and makeup under instructions from dermatologists on the quality of life of female patients with acne vulgaris. J Dermatol 33(11): 745-752.
- Lowe JG (1993) The stigma of acne. Br J Hosp Med 49(11): 809-812.
- Singam V, Rastogi S, Patel KR, Lee HH and Silverberg JI (2019) The mental health burden in acne vulgaris and rosacea: an analysis of the US National Inpatient Sample. Clin Exp Dermatol 44(7): 766-772.
- Gollnick H, Cunliffe W, Berson D, Dreno B, Finlay A, et al. (2003) Management of acne: a report from a Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol 49(Suppl 1): S1-S37.
- Yazici K, Baz K, Yazici AE, Köktürk A, Tot S, et al. (2004) Disease-specific quality of life is associated with anxiety and depression in patients with acne. J Eur Acad Dermatol Venereol 18(4): 435-439.
- Sayar K, Ugurad I, Kural Y and Acar B (2000) The psychometric assessment of acne vulgaris patients. Dermatology Psychosomatics 1(2): 62-65.
- Khan MZ, Naeem A and Mufti KA (2001) Prevalence of mental health problems in acne patients. J Ayub Med Coll Abbottabad 13(4): 7-8.
- Picardi A, Mazzotti E and Pasquini P (2006) Prevalence and correlates of suicidal ideation among patients with skin disease. J Am Acad Dermatol 54(3): 420-426.
- van Der Meeren HL, van Der Schaar WW and van Der Hurk CM (1985) The psychological impact of severe acne. Cutis 36(1): 84-86.
- Wu SF, Kinder BN, Trunnell TN and Fulton JE (1998) Role of anxiety and anger in acne patients: a relationship with severity of the disorder. J Am Acad Dermatol 18(2 Pt 1): 325-333.
- Hassan J, Grogan S, Clark Carter D, Richards H and Yates VM (2009) The individual health burden of acne: appearance-related distress in male and female adolescents and adults with back, chest and facial acne. J Health Psychol 14(8): 1105-1118.
- Lasek RJ and Chren MM (1998) Acne vulgaris and the quality of life of adult dermatology patients. Arch Dermatol 134(4): 454-458.
- Smithard A, Glazebrook C and Williams HC (2001) Acne prevalence, knowledge about acne and psychological morbidity in mid-adolescence: a community-based study. Br J Dermatol 145(2): 274-279.
- Finlay AY (1997) Quality of life measurement in dermatology: a practical guide. Br J Dermatol 136(3): 305-314.
- Hahn HB, Melfi CA, Chuang TY, Lewis CW, Gonin R, et al. (2001) Use of the Dermatology Life Quality Index (DLQI) in a Midwestern US urban clinic. J Am Acad Dermatol 45(1): 44-48.
- Tan JK, Vasey K, Fung KY (2001) Beliefs and perceptions of patients with acne. J Am Acad Dermatol 44(3): 439-445.
- Dalgard F, Gieler U, Holm JO, Bjertness E and Hauser S (2008) Self-esteem and body satisfaction among adolescents with acne: Results from a population survey. J Am Acad Dermatol 59(5): 746-751.
- Feton Danou N (2010) Psychological impact of acne vulgaris. Ann Dermatol Venereol, 137(Suppl 2), S62-S65.
- Gupta MA, Gupta AK, Schork NJ, Ellis CN and Voorhees JJ (1990) Psychiatric aspects of the treatment of mild to moderate facial acne. Some preliminary observations. Int J Dermatol 29(10): 719-721.
- Schulpis K, Georgala S, Papakonstantinou ED and Michas T (1999) Psychological and sympatho-adrenal status in patients with cystic acne. J Eur Acad Dermatol Venereol 13(1): 24-22.
- Chiu A, Chon SY and Kimball AB (2003) The response of skin disease to stress: changes in the severity of acne vulgaris as affected by examination stress. Arch Dermatol 139(7): 897-900.
- Malinowska S, Jaguś D, Woźniak W and Mlosek RK (2021) Usefulness of high‐frequency ultrasound in the monitoring of laser treatment of acne scars. J Ultrason 20(83): e279-
- Park SY, Park MY, Suh DH, Kwon HH, Min S et al. (2016) Cross‐sectional survey of awareness and behavioral pattern regarding acne and acne scar based on smartphone application. Int J Dermatol 55(6): 645-
- Guo R, Xuan W, He X and Xu K (2022) Clinical efficacy and safety of pulsed dye laser combined with pingyangmycin on hyperplastic scar after acne. Mediators Inflamm 28: 3305107.
- Chun Q, Zhi Yong W, Fei S and Xi Qiao W (2016) Dynamic biological changes in fibroblasts during hypertrophic scar formation and regression. Int Wound J 13(2): 257-262.
- Xu X, Lai L, Zhang X, Chen J, Chen J, et al. (2018) Autologous chyle fat grafting for the treatment of hypertrophic scars and scar‐related conditions. Stem Cell Res Ther 9(1): 64.
- Goodman GJ (2000) Postacne scarring: A review of its pathophysiology and treatment. Dermatol Surg 26(9): 857-871.
- Liu L, Xue Y, Chen Y, Chen T, Zhong J, et al. (2023) Prevalence and risk factors of acne scars in patients with acne vulgaris. Skin Res Technol 29(6): e13386.
- Connolly D, Vu HL, Mariwalla K, and Saedi N (2017) Acne scarring-pathogenesis, evaluation, and treatment options. J Clin Aesthet Dermatol 10(9): 12-23.
- Rivera AE (2008) Acne scarring: A review and current treatment modalities. J Am Acad Dermatol, 59(4): 659-676.
- Fife D (2011) Practical evaluation and management of atrophic acne scars: Tips for the general dermatologist. J Clin Aesthet Dermatol 4(8): 50-57.
- Thiboutot D and Gollnick H (2009) New insights into the management of acne: An update from the Global Alliance to Improve Outcomes in Acne Group. J Am Acad Dermatol 60(5 Suppl): S1-S50.
- Fife D and Zachary CB (2012) Combining techniques for treating acne scars. Curr Dermatol Rep 1: 82-88.
- LaTowsky B, MacGregor JL, Dover JS and Arndt KA (2012) Prevention and treatment of scars. In M. Alam (Ed.), Evidence-Based Procedural Dermatology 149-177.
- Bhatia N, David CV, Hazany S and Samrao A (2014) Acne scarring. In J. A. Zeichner (Ed.), Acneiform Eruptions in Dermatology: A Differential Diagnosis 237-243.
- Beasley K, Dai JM, Brown P, Lenz B and Hivnor CM (2013) Ablative fractional versus nonablative fractional lasers-where are we and how do we compare differing products? Curr Dermatol Rep 2: 135-143.
- Common JEA, Barker JN and van Steensel MAM (2019) What does acne genetics teach us about disease pathogenesis? Br J Dermatol 181(4): 665-676.
- Carlavan I, Bertino B, Rivier M, Martel P, Bourdes V, et al. (2018) Atrophic scar formation in patients with acne involves long-acting immune responses with plasma cells and alteration of sebaceous glands. Br J Dermatol 179(4): 906-917.
- Madu P and Kundu RV (2014) Follicular and scarring disorders in skin of color: Presentation and management. Am J Clin Dermatol 15(4): 307-321.
- Brown JJ, Ollier W, Arscott G, Ke X, Lamb J, et al. (2008) Genetic susceptibility to keloid scarring: SMAD gene SNP frequencies in Afro-Caribbeans. Exp Dermatol 17(7): 610-613.
- Brown JJ and Bayat A (2009) Genetic susceptibility to raised dermal scarring. Br J Dermatol 161(1): 8-18.
- Ju Q, Tao T, Hu T, Karadag AS, Al Khuzaei S, et al. (2017) Sex hormones and acne. Clin Dermatol 35(2): 130‐
- Sugawara T, Nakagawa N, Shimizu N, Hirai N, Saijo Y, et al. (2019) Gender- and age-related differences in facial sebaceous glands in Asian skin, as observed by non-invasive analysis using three-dimensional ultrasound microscopy. Skin Res Technol 25(3): 347‐
- Tan J, Thiboutot D, Gollnick H, Kang S, Layton A, et al. (2017) Development of an atrophic acne scar risk assessment tool. J Eur Acad Dermatol Venereol 31(9): 1547‐
- Xu Y and Deng Y (2018) Ablative fractional CO2 laser for facial atrophic acne scars. Facial Plast Surg 34(2): 205-219.
- Magnani LR and Schweiger ES (2014) Fractional CO2 lasers for the treatment of atrophic acne scars: A review of the literature. J Cosmet Laser Ther 16(2): 48-56.
- Fang F, Yang H, Liu X, Ding H, Yang Y, et al. (2022) Treatment of acne scars with fractional carbon dioxide laser in Asians: A retrospective study to search for predicting factors associated with efficacy. Lasers Med Sci 37(6): 2623-2627.
- Galal O, Tawfik AA, Abdalla N and Soliman M (2019) Fractional CO2 laser versus combined platelet‐rich plasma and fractional CO2 laser in treatment of acne scars: Image analysis system evaluation. J Cosmet Dermatol 18(6): 1665-
- Zhou BR, Zhang T, Bin Jameel AA, Xu Y, et al. (2016) The efficacy of conditioned media of adipose‐derived stem cells combined with ablative carbon dioxide fractional resurfacing for atrophic acne scars and skin rejuvenation. J Cosmet Laser Ther 18(3): 138-
- Abdelwahab , Omar GAB and Hamdino M (2022) A combined subcision approach with either fractional CO2 laser (10,600 nm) or cross-linked hyaluronic acid versus subcision alone in atrophic post-acne scar treatment. Lasers Med Sci 38(1): 20.
- Cho SB, Lee SJ, Cho S, Oh SH, Chung WS, et al. (2010) Non-ablative 1550-nm erbium-glass and ablative 10,600-nm carbon dioxide fractional lasers for acne scars: A randomized split-face study with blinded response evaluation. J Eur Acad Dermatol Venereol 24(8): 921-925.
- Chan NP, Ho SG, Yeung CK, Shek SY and Chan HH (2010) Fractional ablative carbon dioxide laser resurfacing for skin rejuvenation and acne scars in Asians. Lasers Surg Med 42(9): 615-623.
- Katz B (2010) Efficacy of a new fractional CO2 laser in the treatment of photodamage and acne scarring. Dermatol Ther 23(4): 403-406.
- Chilicka K, Rusztowicz M, Szyguła R and Nowicka D (2022) Methods for the improvement of acne scars used in dermatology and cosmetology: A review J Clin Med 11(10): 2744.
- Cannarozzo G, Bennardo L, Zingoni T, Pieri L, Duca ED et al. (2021) Histological skin changes after treatment with 675 nm laser. Photobiomodul Photomed Laser Surg 39(9): 617-621.
- Cannarozzo G, Silvestri M, Tamburi F, Sicilia C and Duca ED (2021) A new 675-nm laser device in the treatment of acne scars: An observational study. Lasers Med Sci 36(1): 227-231.
- Rogachefsky AS, Hussain M and Goldberg DJ (2003) Atrophic and a mixed pattern of acne scars improved with a 1320-nm Nd:YAG laser. Dermatol Surg 29(9): 904-908.
- Sadick NS and Schecter AK (2004) A preliminary study of utilization of the 1320‐nm Nd:YAG laser for the treatment of acne scarring. Dermatol Surg 30(7); 995-
- Lee SR and Cho S (2023) Clinical factors affecting the effectiveness of 1550‐nm erbium‐doped fractional photothermolysis laser for individual atrophic acne scar types. Dermatol Ther 13(2): 609-
- Tam C, Khong J, Tam K, Vasilev R and Wu W (2022) A Comprehensive Review of Non-Energy-Based Treatments for Atrophic Acne Scarring. Clin Cosmet Investig Dermatol 15: 455-469.
- de Pinho Tavares J, Pires Oliveira CAC, Prado Torres R and Bahmad F (2017) Facial thread lifting with suture suspension. Braz J Otorhinolaryngol 83(6): 712-719.
- Savoia A, Accardo C, Vannini F, Di Pasquale B and Baldi A (2014) Outcomes in Thread Lift for Facial Rejuvenation: A Study Performed with Happy Lift™ Revitalizing. Dermatol Ther 4(1): 103-114.
- Donnarumma M, Vastarella M, Ferrillo M, Cantelli M, D Andrea M, et al. (2021) An innovative treatment for acne scars with thread-lift technique: our experience. Ital J Dermatol Venerol 156(Suppl 1 to No. 6): 40-41.
- Ramaut L, Hoeksema H, Pirayesh A, Stillaert F and Monstrey S (2018) Microneedling: Where do we stand now? A systematic review of the literature. J Plast Reconstr Aesthet Surg 71(1): 1-14.
- Kwon HH, Park HY, Choi SC, Bae Y, Jung JY, et al. (2018) Novel device‐based acne treatments: Comparison of a 1450‐nm diode laser and microneedling radiofrequency on mild‐to‐moderate acne vulgaris and seborrhoea in Korean patients through a 20‐week prospective, randomized, split-face study. J Eur Acad Dermatol Venereol 32(4): 639-
- Osman MA, Shokeir HA, Fawzy MM (2017) Fractional erbium-doped yttrium aluminum garnet laser versus microneedling in treatment of atrophic acne scars: A randomized split-face clinical study. Dermatol Surg 43(Suppl 1): S47-S56.
- Juhasz MLW and Cohen JL (2020) Microneedling for the treatment of scars: An update for clinicians. Clin Cosmet Investig Dermatol 13: 997-1003.
- Bandral MR, Padgavankar PH, Japatti SR, Gir PJ, Siddegowda CY, et al. (2019) Clinical evaluation of microneedling therapy in the management of facial scar: A prospective randomized study. J Maxillofac Oral Surg 18(4): 572-578.
- Rana S, Mendiratta V and Chander R (2017) Efficacy of microneedling with 70% glycolic acid peel vs microneedling alone in treatment of atrophic acne scars: A randomized controlled trial. J Cosmet Dermatol, 16(4): 454-459.
- Ali B, ElMahdy N and Elfar NN (2019) Microneedling (Dermapen) and Jessner's solution peeling in treatment of atrophic acne scars: A comparative randomized clinical study. J Cosmet Laser Ther¸21(6): 357-363.
- Gupta A, Kaur M, Patra S, Khunger N and Gupta S (2020) Evidence-based surgical management of post-acne scarring in skin of color. J Cutan Aesthet Surg 13(2): 124-141.
- El Domyati M, Abdel Wahab H and Hossam A (2018) Microneedling combined with platelet-rich plasma or trichloroacetic acid peeling for management of acne scarring: A split-face clinical and histologic comparison. J Cosmet Dermatol 17(1): 73-83.
- Porwal S, Chahar YS and Singh PK (2018) A comparative study of combined dermaroller and platelet-rich plasma versus dermaroller alone in acne scars and assessment of quality of life before and after treatment. Indian J Dermatol 63(5): 403-408.
- Ebrahimi Z, Alimohamadi Y, Janani M, Hejazi P, Kamali M, et al. (2022) Platelet-rich plasma in the treatment of scars, to suggest or not to suggest? A systematic review and meta-analysis. J Tissue Eng Regen Med 16(10): 875-899.
- Meghe S, Saoji V, Madke B, and Singh A (2024) Efficacy of Microneedling and CO2 Laser for Acne Scar Remodelling: A Comprehensive Review. Cureus 16(2): e55092.
- Gowda A, Healey B, Ezaldein H and Merati M (2021) A systematic review examining the potential adverse effects of microneedling. J Clin Aesthet Dermatol 14(1):45-54.
- Gold MH and Sadick NS (2018) Optimizing outcomes with polymethylmethacrylate fillers. J Cosmet Dermatol 17(3): 298-304.
- Wollina U and Goldman A (2011) Hyaluronic acid dermal fillers: Safety and efficacy for the treatment of wrinkles, aging skin, body sculpturing and medical conditions. Clin Med Rev Ther 3: 107-121.
- Buhren BA, Schrumpf H, Bölke E, Kammers K and Gerber PA (2018) Standardized in vitro analysis of the degradability of hyaluronic acid fillers by hyaluronidase. Eur J Med Res 23(1): 37.
- Hussain SN, Goodman GJ and Rahman E (2017) Treatment of a traumatic atrophic depressed scar with hyaluronic acid fillers: A case report. Clin Cosmet Investig Dermatol 10: 285-287.
- Turlier V, Delalleau A, Casas C, Rouquier A, Bianchi P, et al. (2013) Association between collagen production and mechanical stretching in dermal extracellular matrix: In vivo effect of cross-linked hyaluronic acid filler. A randomised, placebo-controlled study. J Dermatol Sci 69(3): 187-194.
- Prasetyo AD, Prager W, Rubin MG, Moretti EA and Nikolis A (2016) Hyaluronic acid fillers with cohesive polydensified matrix for soft-tissue augmentation and rejuvenation: A literature review. Clin Cosmet Investig Dermatol 9: 257-280.
- Fitzgerald R, Bass LM, Goldberg DJ, Graivier MH and Lorenc ZP (2018) Physiochemical Characteristics of Poly-L-Lactic Acid (PLLA) Aesthet Surg J 38(suppl_1): S13-S17.
- Kim SA, Kim HS, Jung JW, Suh SI and Ryoo YW (2019) Poly-L-lactic acid increases collagen gene expression and synthesis in cultured dermal fibroblast (Hs68) through the p38 MAPK pathway. Ann Dermatol, 31(1): 97-100.
- Kadouch JA (2017) Calcium hydroxylapatite: A review on safety and complications. J Cosmet Dermatol 16(2): 152-161.
- Da Costa A, Biccigo Dgz, De Souza Weimann Et, Mercadante Lm, Et al. (2017) Durability Of Three Different Types Of Hyaluronic Acid Fillers In Skin: Are There Differences Among Biphasic, Monophasic Monodensified, And Monophasic Polydensified Products? Aesthet Surg J 37(5): 573-581.
- Beer K (2007) A single-center, open-label study on the use of injectable poly-L-lactic acid for the treatment of moderate to severe scarring from acne or varicella. Dermatol Surg 33(Suppl 2): 159-167.
- Joseph JH, Shamban A, Eaton L, Lehman A, Cohen S, et al. (2019) Polymethylmethacrylate collagen gel-injectable dermal filler for full face atrophic acne scar correction. Dermatol Surg 45(12): 1558-1566.
- Soleymani T, Lanoue J and Rahman Z (2018) A practical approach to chemical peels: A review of fundamentals and step-by-step algorithmic protocol for treatment. J Clin Aesthet Dermatol 11(8): 21-28.
- Rendon MI, Berson DS, Cohen JL, Roberts WE, Starker I and Wang B (2010) Evidence and considerations in the application of chemical peels in skin disorders and aesthetic resurfacing. J Clin Aesthet Dermatol 3(7): 32-43.
- Chandrashekar BS, Ashwini KR, Vasanth V and Navale S (2015) Retinoic acid and glycolic acid combination in the treatment of acne scars. Indian Dermatol Online J 6(2): 84-88.
- Puri N (2015) Efficacy of modified Jessner's Peel and 20% TCA versus 20% TCA peel alone for the treatment of acne scars. J Cutan Aesthet Surg 8(1): 42-45.
- Gozali MV and ZhouB (2015) Effective treatments of atrophic acne scars. J Clin Aesthet Dermatol 8(5): 33-40.
- Boen M and Jacob C (2019) A review and update of treatment options using the acne scar classification system. Dermatol Surg 45(3): 411-422.
- Khunger N, Bhardwaj D and Khunger M (2011) Evaluation of CROSS technique with 100% TCA in the management of ice pick acne scars in darker skin types. J Cosmet Dermatol 10(1): 51-57.
- Al Hamamy HR, Al Dhalimi MA and Abtan AF (2021) Evaluation of treatment of acne scars with 25% trichloroacetic acid chemical peel followed by manual dermasanding. J Cosmet Dermatol 20(6): 1750-1755.
- Fabbrocini G, Cacciapuoti S, Fardella N, Pastore F and Monfrecola G (2008) CROSS technique: chemical reconstruction of skin scars method. Dermatol Ther 21(Suppl 3): S29-S32.
- Chung HJ, Al Janahi S, Cho SB and Chang YC (2021) Chemical reconstruction of skin scars (CROSS) method for atrophic scars: A comprehensive review. J Cosmet Dermatol 20(1): 18-27.
- Hevia O, Nemeth AJ and Taylor JR (1991) Tretinoin accelerates healing after trichloroacetic acid chemical peel. Arch Dermatol 127(5): 678-682.
- Levy LL and Zeichner JA (2012) Management of acne scarring, part II: A comparative review of non-laser-based, minimally invasive approaches. Am J Clin Dermatol 13(5): 331-340.
- Goodman GJ (2003) Post acne scarring: A review. J Cosmet Laser Ther 5(2): 77-95.
- Aronsson A, Eriksson T, Jacobsson S and Salemark L (1997) Effects of dermabrasion on acne scarring: A review and a study of 25 cases. Acta Derm Venereol 77(1): 39-42.
- Hirsch RJ and Lewis AB (2001) Treatment of acne scarring. Semin Cutan Med Surg 20(3): 190-198.
- Fernandes M, Pinheiro NM, Crema VO and Mendonca AC (2014) Effects of microdermabrasion on skin rejuvenation. J Cosmet Laser Ther 16(1): 26-31.
- Bhargava S, Cunha PR, Lee J and Kroumpouzos G (2018) Acne scarring management: Systematic review and evaluation of the evidence. Am J Clin Dermatol 19(4): 459-477.
- Faghihi G, Nouraei S, Asilian A, Keyvan S, Abtahi Naeini B, et al. (2015) Efficacy of punch elevation combined with fractional carbon dioxide laser resurfacing in facial atrophic acne scarring: A randomized split-face clinical study. Indian J Dermatol 60(5): 473.
- Kim HS, Cho EJ, Park YM, Kim HO and Lee JY (2011) Punch excision combined with erbium: yAG fractional laser: Its application on different types of scars in Asian patients (a pilot study) J Cosmet Laser Ther 13(4): 196-199.
- Grevelink JM and White VR (1998) Concurrent use of laser skin resurfacing and punch excision in the treatment of facial acne scarring. Dermatol Surg 24(5): 527-530.
- Johnson WC (1986) Treatment of pitted scars: Punch transplant technique. J Dermatol Surg Oncol 12(3): 260-265.
- Nilforoushzadeh MA, Lotfi E, Heidari Kharaji M, Nickhah N, Alavi S, et al. (2020) Comparing cannula-based subcision with the common needle method: A clinical trial. Skin Res Technol 26(1): 39-44.
- Ramadan SA, El Komy MH, Bassiouny DA and El Tobshy SA (2011) Subcision versus 100% trichloroacetic acid in the treatment of rolling acne scars. Dermatol Surg 37(5): 626-633.
- Bhargava S, Kumar U and Varma K (2019) Subcision and microneedling as an inexpensive and safe combination to treat atrophic acne scars in dark skin: A prospective study of 45 patients at a tertiary care center. J Clin Aesthet Dermatol 12(8): 18-22.
- Barikbin B, Akbari Z, Yousefi M and Dowlati Y (2017) Blunt blade subcision: An evolution in the treatment of atrophic acne scars. Dermatol Surg 43(Suppl 1): S57-S63.
- Dadkhahfar S, Robati RM, Gheisari M and Moravvej H (2020) Subcision: Indications, adverse reactions, and pearls. J Cosmet Dermatol 19(5): 1029-1038.
- Bhargava S, Kroumpouzos G, Varma K and Kumar U (2019) Combination therapy using subcision, needling, and platelet-rich plasma in the management of grade 4 atrophic acne scars: A pilot study. J Cosmet Dermatol 18(4): 1092-1097.
- Deshmukh NS and Belgaumkar VA (2019) Platelet-rich plasma augments subcision in atrophic acne scars: A split-face comparative study. Dermatol Surg 45(1): 90-98.
- Alser OH and Goutos I (2018) The evidence behind the use of platelet-rich plasma (PRP) in scar management: A literature review. Scars Burn Heal 4: 2059513118808773.
- Lee JW, Kim BJ, Kim MN and Mun SK (2011) The efficacy of autologous platelet rich plasma combined with ablative carbon dioxide fractional resurfacing for acne scars: A simultaneous split-face trial. Dermatol Surg 37(7): 931-938.
- Chawla S (2014) Split face comparative study of microneedling with PRP versus microneedling with vitamin C in treating atrophic post acne scars. J Cutan Aesthet Surg 7(4): 209-212.
- English RS and Shenefelt PD (1999) Keloids and hypertrophic scars. Dermatol Surg 25(8): 631-638.
- Leyden J, Stein Gold L and Weiss J (2017) Why topical retinoids are mainstay of therapy for acne. Dermatol Ther 7(3): 293-304.
- Leyden JJ, Shalita A, Thiboutot D, Washenik K and Webster G. (2005) Topical retinoids in inflammatory acne: A retrospective, investigator-blinded, vehicle-controlled, photographic assessment. Clin Ther 27(2): 216-224.
- Dréno B, Bissonnette R, Gagné Henley A, Barankin B, Lynde C, et al. (2018) Prevention and reduction of atrophic acne scars with adapalene 0.3%/benzoyl peroxide 2.5% gel in subjects with moderate or severe facial acne: Results of a 6-month randomized, vehicle-controlled trial using intra-individual comparison. Am J Clin Dermatol 19(2): 275-286.
- Thiboutot DM, Dréno B, Abanmi A, Alexis AF, Araviiskaia E, et al. (2018) Practical management of acne for clinicians: An international consensus from the Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol 78(2 Suppl 1): S1-
- Amichai B, Shemer A and Grunwald MH (2006) Low-dose isotretinoin in the treatment of acne vulgaris. J Am Acad Dermatol 54(4): 644-646.
- Chivot M and Midoun H (1990) Isotretinoin and acne--a study of relapses. Dermatologica 180(4): 240-243.
- Peck GL, Olsen TG, Butkus D, Pandya M, Arnaud Battandier J, et al. (1982) Isotretinoin versus placebo in the treatment of cystic acne: A randomized double-blind study. J Am Acad Dermatol 6(4 Pt 2 Suppl): 735-745.
- Goldstein JA, Socha Szott A, Thomsen RJ, Pochi PE, Shalita AR, et al. (1982) Comparative effect of isotretinoin and etretinate on acne and sebaceous gland secretion. J Am Acad Dermatol 6(4 Pt 2 Suppl): 760-765.
- Goulden V, Clark SM and Cunliffe WJ (1997) Post-adolescent acne: A review of clinical features. Br J Dermatol 136(1): 66-70.
- Goulden V, Clark SM, McGeown C and Cunliffe WJ (1997) Treatment of acne with intermittent isotretinoin. Br J Dermatol 137(1): 106-108.
- Stainforth JM, Layton AM, Taylor JP and Cunliffe WJ (1993) Isotretinoin for the treatment of acne vulgaris: Which factors may predict the need for more than one course? Br J Dermatol 129(3): 297-301.
- Rubinow DR, Peck GL, Squillace KM and Gantt GG (1987) Reduced anxiety and depression in cystic acne patients after successful treatment with oral isotretinoin. J Am Acad Dermatol 17(1): 25-32.
- Strauss JS, Rapini RP, Shalita AR, Konecky E, Pochi PE, et al. (1984) Isotretinoin therapy for acne: Results of a multicenter dose-response study. J Am Acad Dermatol 10(3): 490-496.
- Jones DH, King K, Miller AJ and Cunliffe WJ (1983) A dose-response study of 13-cis-retinoic acid in acne vulgaris. Br J Dermatol 108(3): 333-343.
- King K, Jones DH, Daltrey DC and Cunliffe WJ (1982) A double-blind study of the effects of 13-cis-retinoic acid on acne, sebum excretion rate and microbial population. Br J Dermatol 107(5): 583-590.
- Strauss JS and Stranieri AM (1982) Changes in long-term sebum production from isotretinoin therapy. J Am Acad Dermatol 6(4 Pt 2 Suppl): 751-756.
- Lehucher Ceyrac D and Weber Buisset MJ (1993) Isotretinoin and acne in practice: A prospective analysis of 188 cases over 9 years. Dermatology 186(2); 123-128.
- Layton AM, Knaggs H, Taylor J and Cunliffe WJ (1993) Isotretinoin for acne vulgaris--10 years later: A safe and successful treatment. Br J Dermatol 129(3): 292-296.
- Lester RS, Schachter GD and Light MJ (1985) Isotretinoin and tetracycline in the management of severe nodulocystic acne. Int J Dermatol 24(4): 252-257.
- Huang YC and Cheng YC (2017) Isotretinoin treatment for acne and risk of depression: A systematic review and meta-analysis. J Am Acad Dermatol 76(6): 1068-1076.
- Costa CS, Bagatin E, Martimbianco ALC, da Silva EM, Lucio MM, et al. (2018) Oral isotretinoin for acne. Cochrane Database Syst Rev 11(11): CD009435.
- Tkachenko E, Singer S, Sharma P, Barbieri J and Mostaghimi A (2019) US Food and Drug Administration reports of pregnancy and pregnancy-related adverse events associated with isotretinoin. JAMA Dermatol 155(10): 1175-1179.
- Lammer EJ, Chen DT, Hoar RM, Agnish ND, Benke PJ, et al. (1985) Retinoic acid embryopathy. N Engl J Med 313(14): 837-841.
- Tan AU, Schlosser BJ and Paller AS (2018) A review of diagnosis and treatment of acne in adult female patients. Int J Womens Dermatol 4(2): 56-71.
- Kong YL and Tey HL (2013) Treatment of acne vulgaris during pregnancy and lactation. Drugs 73(8): 779-787.
- Nagler AR (2019) Early strides for necessary data-driven improvement in iPLEDGE. JAMA Dermatol 155(10): 1111-1112.
- Collins MK, Moreau JF, Opel D, Swan J, Prevost N, et al. (2014) Compliance with pregnancy prevention measures during isotretinoin therapy. J Am Acad Dermatol 70(1): 55-59.
- Cunliffe WJ, Holland KT, Bojar R and Levy SF (2002) A randomized, double-blind comparison of a clindamycin phosphate/benzoyl peroxide gel formulation and a matching clindamycin gel with respect to microbiologic activity and clinical efficacy in the topical treatment of acne vulgaris. Clin Ther 24(7): 1117-1133.
- Ross JI, Snelling AM, Carnegie E, Coates P, Cunliffe WJ, et al. (2003) Antibiotic-resistant acne: Lessons from Europe. Br J Dermatol 148(3): 467-478.
- Fanelli M, Kupperman E, Lautenbach E, Edelstein PH and Margolis DJ (2011) Antibiotics, acne, and Staphylococcus aureus colonization. Arch Dermatol 147(8): 917-921.
- Simonart T and Dramaix M (2005) Treatment of acne with topical antibiotics: Lessons from clinical studies. Br J Dermatol 153(2): 395-403.
- Walsh TR, Efthimiou J and Dréno B (2016) Systematic review of antibiotic resistance in acne: An increasing topical and oral threat. Lancet Infect Dis 16(3): e23-e33.
- Barbieri JS, Spaccarelli N, Margolis DJ and James WD (2019) Approaches to limit systemic antibiotic use in acne: Systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments. J Am Acad Dermatol 80(2): 538-549.
- Adler BL, Kornmehl H and Armstrong AW (2017) Antibiotic resistance in acne treatment. JAMA Dermatol 153(8): 810-811.
- Dutil M (2010) Benzoyl peroxide: Enhancing antibiotic efficacy in acne management. Skin Therapy Lett 15(10): 5-7.
- Hale EK and Pomeranz MK (2002) Dermatologic agents during pregnancy and lactation: An update and clinical review. Int J Dermatol 41(4): 197-203.
- Rothman KF and Pochi PE (1988) Use of oral and topical agents for acne in pregnancy. J Am Acad Dermatol 19(3): 431-442.
- Wenk RE, Gebhardt FC, Bhagavan BS, Lustgarten JA and McCarthy EF (1981) Tetracycline-associated fatty liver of pregnancy, including possible pregnancy risk after chronic dermatologic use of tetracycline. J Reprod Med 26(3): 135-141.
- Spencer JP, Gonzalez 3rd LS and Barnhart DJ (2001) Medications in the breast-feeding mother. Am Fam Physician 64(1): 119-126.
- Fleischer JrABJr, Dinehart S, Stough D, Plott RT, Solodyn Phase2 & 3 Study Groups (2006) Safety and efficacy of a new extended-release formulation of minocycline. Cutis 78(4 Suppls): 21-31.
- Margolis DJ, Fanelli M, Hoffstad O and Lewis JD (2010) Potential association between the oral tetracycline class of antimicrobials used to treat acne and inflammatory bowel disease. Am J Gastroenterol 105(12) 2610-2616.
- Bartlett JG, Chang TW, Gurwith M, Gorbach SL and Onderdonk AB (1978) Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med 298(10): 531-534.
- Carroll KC and Bartlett JG (2011) Biology of Clostridium difficile: Implications for epidemiology and diagnosis. Annu Rev Microbiol 65: 501-521.
- Hernandez Diaz S, Werler MM, Walker AM and Mitchell AA (2000) Folic acid antagonists during pregnancy and the risk of birth defects. N Engl J Med 343(22): 1608-1614.
- Koren G, Pastuszak A and Ito S (1998) Drugs in pregnancy. N Engl J Med 338(16): 1128-1137.
- Kallen BA, Otterblad Olausson P and Danielsson BR (2005) Is erythromycin therapy teratogenic in humans? Reprod Toxicol 20(2): 209-214.
- McCormack WM, George H, Donner A, Kodgis LF, Alpert S, et al. (1977) Hepatotoxicity of erythromycin estolate during pregnancy. Antimicrob Agents Chemother 12(5): 630-635.
- Hoffmann K, George A, Heschl L, Leifheit AK and Maier M (2015) Oral contraceptives and antibiotics: A cross-sectional study about patients' knowledge in general practice. Reprod Health 12: 43.
- Guengerich FP (1990) Inhibition of oral contraceptive steroid-metabolizing enzymes by steroids and drugs. Am J Obstet Gynecol 163(6 Pt 2): 2159-2163.
- Back DJ, Breckenridge AM, Crawford FE, Hall JM, MacIver M, et al. (1980) The effect of rifampicin on the pharmacokinetics of ethynylestradiol in women. Contraception 21(2): 135-143.
- Bainton R (1986) Interaction between antibiotic therapy and contraceptive medication. Oral Surg Oral Med Oral Pathol 61(5): 453-455.
- Fazio A (1991) Oral contraceptive drug interactions: Important considerations. South Med J 84(8): 997-1002.
- Skolnick JL, Stoler BS, Katz DB and Anderson WH (1976) Rifampin, oral contraceptives, and pregnancy. JAMA 236(12): 1382.
- Andriessen A and Lynde CW (2014) Antibiotic resistance: Shifting the paradigm in topical acne treatment. J Drugs Dermatol 13(11) 1358-1364.
- Blasiak RC, Stamey CR, Burkhart CN, Lugo Somolinos A and Morrell DS (2013) High-dose isotretinoin treatment and the rate of retrial, relapse, and adverse effects in patients with acne vulgaris. JAMA Dermatol 149(12): 1392-1398.
- Rademaker M (2016) Making sense of the effects of the cumulative dose of isotretinoin in acne vulgaris. Int J Dermatol 55(5): 518-523.
- Schrom K, Nagy T and Mostow E (2016) Depression screening using health questionnaires in patients receiving oral isotretinoin for acne vulgaris. J Am Acad Dermatol 75(1); 237-239.
- Tan JKL, Jones E, Allen E, Pripotnev S, Raza A and Wolfe B (2013) Evaluation of essential clinical components and features of current acne global grading scales. J Am Acad Dermatol 69(5): 754-761.
- Abdel Maguid EM, Awad SM, Hassan YS, El Mokhtar MA, El Deek HE et al. (2019) Efficacy of stem cell-conditioned medium vs. platelet-rich plasma as an adjuvant to ablative fractional CO2 laser resurfacing for atrophic post-acne scars: A split-face clinical trial. J Dermatolog Treat 32(2); 242-249.
- El Domyati M, Moftah NH, Nasif GA, Ragaie MH, Ibrahim MR, et al. (2019) Amniotic fluid-derived mesenchymal stem cell products combined with microneedling for acne scars: A split-face clinical, histological, and histometric study. J Cosmet Dermatol 18(5): 1300-1306.
- Park CS, Park JH, Kim CR and Lee JH (2021) Objective analysis of volume restoration in atrophic acne scars and skin pores: A split study using human stem cell-conditioned media. J Dermatolog Treat 32(1): 73-77.
- Prakoeswa CRS, Natallya FR, Harnindya D, Thohiroh A, Oktaviyanti RN, et al. (2018) The efficacy of topical human amniotic membrane-mesenchymal stem cell-conditioned medium (hAMMSC-CM) and a mixture of topical hAMMSC-CM + vitamin C and hAMMSC-CM + vitamin E on chronic plantar ulcers in leprosy: A randomized control trial. J Dermatolog Treat 29(8): 835-840.
- Aryan A, Bayat M, Bonakdar S, Taheri S, Haghparast N, et al. (2018) Human bone marrow mesenchymal stem cell conditioned medium promotes wound healing in deep second-degree burns in male rats. Cells Tissues Organs 206(6): 317-329.
- Li JY, Ren KK, Zhang WJ, Xiao L, Wu HY, et al. (2019) Human amniotic mesenchymal stem cells and their paracrine factors promote wound healing by inhibiting heat stress-induced skin cell apoptosis and enhancing their proliferation through activating PI3K/AKT signaling pathway. Stem Cell Res Ther 10(1): 247.
- Zhou P, Li X, Zhang B, Shi Q, Li D, et al. (2019) A human umbilical cord mesenchymal stem cell-conditioned medium/chitosan/collagen/β-glycerophosphate thermosensitive hydrogel promotes burn injury healing in mice. Biomed Res Int 5768285.
- Sun J, Zhang Y, Song X, Zhu J and Zhu Q (2019) The healing effects of conditioned medium derived from mesenchymal stem cells on radiation-induced skin wounds in rats. Cell Transplant 28(1): 105-115.
- Fridoni M, Kouhkheil R, Abdollhifar MA, Amini A, Ghatrehsamani M, et al. (2019) Improvement in infected wound healing in type 1 diabetic rat by the synergistic effect of photobiomodulation therapy and conditioned medium. J Cell Biochem 120(6): 9906-9916.
- Kouhkheil R, Fridoni M, Abdollhifar MA, Amini A, Bayat S, et al. (2019) Impact of photobiomodulation and conditioned medium on mast cell counts, degranulation, and wound strength in infected skin wound healing of diabetic rats. Photobiomodul Photomed Laser Surg, 37(11): 706-714.
- Seetharaman R, Mahmood A, Kshatriya P, Patel D and Srivastava A (2019) Mesenchymal stem cell conditioned media ameliorate psoriasis vulgaris: A case study. Case Rep Dermatol Med 2019: 8309103.
- Gunawardena TNA, Rahman MT, Abdullah BJJ and Abu Kasim NH (2019) Conditioned media derived from mesenchymal stem cell cultures: The next generation for regenerative medicine. J Tissue Eng Regen Med 13(4): 569-586.
- Maguire G (2013) Stem cell therapy without the cells. Commun Integr Biol 6(6): e26631.
- Ratajczak MZ, Kucia M, Jadczyk T, Greco NJ, Wojakowski W, et al. (2012) Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: Can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? Leukemia 26(6): 1166-1173.
- Deng H, Sun C, Sun Y, Li H, Yang L, et al. (2018) Lipid, protein, and microRNA composition within mesenchymal stem cell-derived exosomes. Cell Reprogram 20(3): 178-186.
- Pawitan JA. (2014) Prospect of stem cell conditioned medium in regenerative medicine. Biomed Res Int 2014: 965849.
- Mizukami H and Yagihashi S (2016) Is stem cell transplantation ready for prime time in diabetic polyneuropathy? Curr Diab Rep 16(9): 86.
- Chen L, Tredget EE, Wu PYG, Wu Y and Wu Y (2008) Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 3(4): e1886.
- Shohara R, Yamamoto A, Takikawa S, Iwase A, Hibi H, et al. (2012) Mesenchymal stromal cells of human umbilical cord Wharton's jelly accelerate wound healing by paracrine mechanisms. Cytotherapy, 14(10): 1171-1181.
- Kim WS, Park BS and Sung JH (2009) Protective role of adipose-derived stem cells and their soluble factors in photoaging. Arch Dermatol Res 301(5): 329-336.
- Padeta I, Nugroho WS, Kusindarta DL, Fibrianto YH and Budipitojo T (2017) Mesenchymal stem cell-conditioned medium promotes the recovery of skin burn wound. AJAVA 12(3): 132-141.
- Tarcisia T, Damayanti L, Antarianto RD, Moenadjat Y and Pawitan JA (2017) Adipose-derived stem cell conditioned medium effect on the proliferation phase of wound healing in Sprague Dawley rat. Med J Indonesia 26(4): 239-245.
- Walter MNM, Wright KT, Fuller HR, MacNeil S and Johnson WEB (2010) Mesenchymal stem cell-conditioned medium accelerates skin wound healing: An in vitro study of fibroblast and keratinocyte scratch assays. Exp Cell Res 316(7): 1271-1281.
- De Gregorio C, Contador D, Diáz D, Cárcamo C, Santapau D, et al. (2020) Human adipose-derived mesenchymal stem cell-conditioned medium ameliorates polyneuropathy and foot ulceration in diabetic BKS db/db mice. Stem Cell Res Ther 11(1): 168.
- Kim YJ, Ahn HJ, Lee SH, Lee MH and Kang KS (2020) Effects of conditioned media from human umbilical cord blood-derived mesenchymal stem cells in the skin immune response. Biomed Pharmacother, 131: 110789.
- Sagaradze G, Grigorieva O, Nimiritsky P, Basalova N, Kalinina N, et al. (2019) Conditioned medium from human mesenchymal stromal cells: Towards clinical translation. Int J Mol Sci 20(7): 1656.
- Im GB, Kim YH, Kim YJ, Kim SW, Jung E, et al. (2019) Enhancing the wound healing effect of conditioned medium collected from mesenchymal stem cells with high passage number using bioreducible nanoparticles. Int J Mol Sci 20(19): 4835.
- Park BS, Kim WS, Choi JS, Kim HK, Won JH, et al. (2010) Hair growth stimulated by conditioned medium of adipose-derived stem cells is enhanced by hypoxia: Evidence of increased growth factor secretion. Biomed Res 31(1): 27-34.
- Du HC, Jiang L, Geng WX, Li J, Zhang R, et al. (2017) Growth factor-reinforced ECM fabricated from chemically hypoxic MSC sheet with improved in vivo wound repair activity. Biomed Res Int 2017: 2578017.
- Chen L, Xu Y, Zhao J, Zhang Z, Yang R, et al. (2014) Conditioned medium from hypoxic bone marrow-derived mesenchymal stem cells enhances wound healing in mice. PLoS One 9(4): e96161.
- Jun EK, Zhang Q, Yoon BS, Moon JH, Lee G, et al. (2014) Hypoxic conditioned medium from human amniotic fluid-derived mesenchymal stem cells accelerates skin wound healing through TGF-β/SMAD2 and PI3K/Akt pathways. Int J Mol Sci 15(1): 605-628.
- Du L, Lv R, Yang X, Cheng S, Ma T, et al. (2016) Hypoxic conditioned medium of placenta-derived mesenchymal stem cells protects against scar formation. Life Sci 149: 51-57.
- Joseph A, Baiju I, Bhat IA, Pandey S, Bharti M, et al. (2020) Mesenchymal stem cell-conditioned media: A novel alternative of stem cell therapy for quality wound healing. J Cell Physiol 235(7-8): 5555-5569.
- Sun B, Guo S, Xu F, Wang B, Liu X, et al. (2014) Concentrated hypoxia-preconditioned adipose mesenchymal stem cell-conditioned medium improves wounds healing in full-thickness skin defect model. Int Sch Res Notices 2014: 652713.
- Deng C, He Y, Feng J, Dong Z, Yao Y and Lu F (2019) Conditioned medium from 3D culture system of stromal vascular fraction cells accelerates wound healing in diabetic rats. Regen Med 14(10): 925-937.
- Rong X, Chu W, Zhang H, Wang Y, Qi X, et al. (2019) Antler stem cell-conditioned medium stimulates regenerative wound healing in rats. Stem Cell Res Ther 10(1): 326.
- Zhang Q, Liu LN, Yong Q, Deng JC and Cao WG (2015) Intralesional injection of adipose-derived stem cells reduces hypertrophic scarring in a rabbit ear model. Stem Cell Res Ther 6(1): 145.
- Hu CH, Tseng YW, Lee CW, Chiou CY, Chuang SS, et al. (2020) Combination of mesenchymal stem cell-conditioned medium and botulinum toxin type A for treating human hypertrophic scars. J Plast Reconstr Aesthet Surg 73(3): 516-527.
- Liu J, Ren J, Su L, Cheng S, Zhou J, et al. (2018) Human adipose tissue-derived stem cells inhibit the activity of keloid fibroblasts and fibrosis in a keloid model by paracrine signaling. Burns 44(2): 370-385.
- Zhou BR, Xu Y, Guo SL, Xu Y, Wang Y, et al. (2013) The effect of conditioned media of adipose-derived stem cells on wound healing after ablative fractional carbon dioxide laser resurfacing. Biomed Res Int 2013: 519126.
- Amini A, Pouriran R, Abdollahifar MA, Abbaszadeh HA, Ghoreishi SK, et al. (2018) Stereological and molecular studies on the combined effects of photobiomodulation and human bone marrow mesenchymal stem cell conditioned medium on wound healing in diabetic rats. J Photochem Photobiol B 182: 42-51.
- Bagheri M, Amini A, Abdollahifar MA, Ghoreishi SK, Piryaei A, et al. (2018) Effects of photobiomodulation on degranulation and number of mast cells and wound strength in skin wound healing of streptozotocin-induced diabetic rats. Photomed Laser Surg 36(8): 415-423.
- Pouriran R, Piryaei A, Mostafavinia A, Zandpazandi S, Hendudari F, et al. (2016) The effect of combined pulsed wave low-level laser therapy and human bone marrow mesenchymal stem cell-conditioned medium on open skin wound healing in diabetic rats. Photomed Laser Surg 34(8): 345-354.
- Phonchai R, Naigowit P, Ubonsaen B, Nilubol S, Brameld S, et al. (2020) Improvement of Atrophic Acne Scar and Skin Complexity by Combination of Aqueous Human Placenta Extract and Mesenchymal Stem Cell Mesotherapy. JCDSA 10(1): 1-7.
- Wu Y, Peng Y, Gao D, Feng C, Yuan X, et al. (2015) Mesenchymal stem cells suppress fibroblast proliferation and reduce skin fibrosis through a TGF-β3-dependent activation. Int J Low Extrem Wounds 14(1): 50-62.
- Lakey JRT, Whaley D, Alfiere T, Alexander M and Drummond III C (2024) Improvements in Diabetic Neuropathy with Topical Human Mesenchymal Stem Cell Conditioned Media AJBSR 21(1): 002792.
- Snyder S, Crandell I, Davis SA and Feldman SR (2014) Medical adherence to acne therapy: a systematic review. Am J Clin Dermatol 15(2): 87-94.
- Moradi Tuchai S, Makrantonaki E, Ganceviciene R, Dessinioti C, Feldman SR and Zouboulis CC (2015) Acne vulgaris Nat Rev Dis Primers 1: 15029.
- Iuga AO and McGuire MJ (2014) Adherence and health care costs. Risk Manag Healthc Policy 7: 35-44.

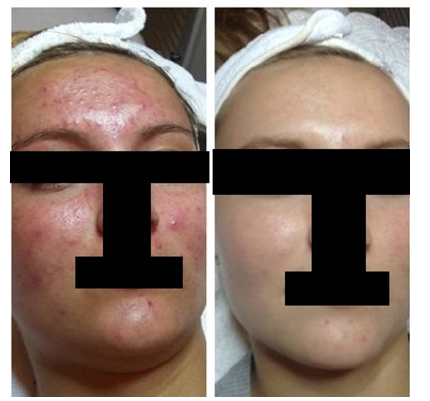
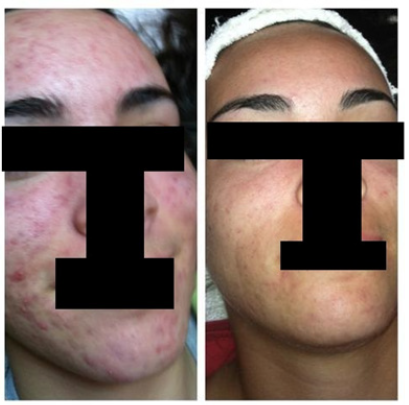


 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.