Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Multiple Versus Single Repeats of the Phenotypically Varying P270 Immunogen of Two Naturally-Occurring Isolate Types of Trichomonas vaginalis
*Corresponding author: JF Alderete, School of Molecular Biosciences, MC7520, College of Veterinary Medicine, Washington State University, Pullman, WA 99164, USA.
Received: May 31, 2024; Published: June 05, 2024
DOI: 10.34297/AJBSR.2024.22.003009
Abstract
Trichomonas vaginalis causes the number one, non-viral sexually transmitted infection (STI), trichomoniasis. There exists two naturally-occurring T. vaginalis isolates defined by the presence or absence of the dsRNA virus (TVV). TVV-positive trichomonal isolates (TVV+) are Type II and undergo phenotypic variation based on surface and cytoplasmic placement of a high Mr immunogenic protein, P270. TVV-negative isolates are Type I and only express P270 in the cytoplasm. P270 is encoded by a single-copy gene and present in all isolates that have been examined. P270 has a single, immunodominant tandemly-repeated epitope (RE). More recently, analysis of a TVV+ and TVVˉ T. vaginalis isolates found that the P270 gene and protein were highly conserved except for the number of repeat elements (REs). It was important to expand this earlier study and examine and quantitate the fresh TVV+ and TVVˉ isolates for multiple and single REs. Thirty (30) Type I isolates and 34 Type II isolates were analyzed. Of the 30 Type I isolates 16 had one RE and 14 had multiple REs for a ratio of ∼1:1. For the 34 Type II isolates 7 had one RE and 27 had multiple REs for a ratio of ∼1:4. Importantly, fluorescence experiments using a monoclonal antibody (MAb C20A3) to the DREGRD immunodominant epitope within the REs, all of the multiple and single RE Type II isolates had heterogeneous populations of trichomonads with surface or cytoplasmic p270. Type I isolate organisms only expressed P270 in the cytoplasm regardless of the number of REs. Finally, both Type I and Type II isolate trichomonads with cytoplasmic P270 cytoadhered to HeLa and vaginal epithelial cells. Type II isolate organisms were found to agglutinate erythrocytes at higher levels than Type I parasites, although both isolate Types readily lysed erythrocytes. This study shows the predominance of isolates with multiple REs among the Type II TVV+ trichomonads and that both Type I and Type II isolates had p270 with single REs
Keywords: dsRNA virus (TVV), monoclonal antibody (MAb), phenotypic variation (PV), phenotypically varying immunogen (P270), repeat element (RE), dsRNA virus-negative (Type I TVVˉ), dsRNA virus-positive (Type II TVV+), vaginal epithelial cells (VECs)
Introduction
Trichomonas vaginalis causes trichomoniasis, the number one, non-viral sexually transmitted infection (STI) worldwide and is considered one of the most neglected, curable STIs [1]. It is appreciated that infection and persistence of T. vaginalis in patients are complex and multifactorial [2-4]. For example, it is conceivable that the interactions of trichomonads with mucin [5] and vaginal epithelial cells (VECs) [6] fluctuate, especially given the constantly-changing urogenital environment of women. Therefore, it is important to continue to define and understand the mechanisms that mediate successful host parasitism.
Some T. vaginalis isolates are infected with double-stranded RNA virus (TVV) [7]. Fresh clinical isolates with TVV-negative (TVVˉ) and TVV-positive (TVV+) organisms are referred to as Type I and Type II isolates, respectively, and represent two naturally-occurring isolates [7,8]. Agar clones of isolate trichomonads derived from individual organisms of Type I and Type II isolates revealed that 100 percent of parasites of both isolate types were either positive or negative for TVV [9]. Indirect immunofluorescence of live Type II TVV+-positive organisms using a monoclonal antibody (MAb) C20A3 to a high Mr immunogenic surface protein called P270 (270-kDa) revealed heterogeneous populations of fluorescent (surface P270) and non-fluorescent (cytoplasmic P270) trichomonads [9,10]. It was then shown that batch culture of TVV+ isolate organisms resulted in spontaneous loss of TVV, and organisms became Type I-like TVVˉ progeny [9,10]. Fluorescence-activated cell sorting of fluorescent and non-fluorescent trichomonads of Type II isolates showed that, upon daily passage, purified organisms reverted to the opposite phenotype [9,11]. Importantly, only Type I and Type II isolate parasites with cytoplasmic P270 cytoadhered and lysed the host epithelial cells [6,9], and attachment to host cells was mediated by adhesin proteins [6,12].
T. vaginalis isolate NYH 286 organisms synthesize P270 within which are 19 tandemly repeated elements (REs) [13]. Each RE is 333-bp that encodes a protein of 11,770.90 daltons [13]. Further, the tandemly REs have a dominant DREGRD epitope detected by the MAb C20A3 [10,13]. The p270 gene is single copy and partial restriction using HindIII gives a ladder pattern expected of tandemly REs [13,14]. It became important to investigate at the molecular level the p270 gene of representative Type I and Type II fresh clinical isolates. It was first revealed that the large P270 protein was related and highly conserved among various T. vaginalis isolates [15]. A more recent study demonstrated that for a Type I T016 isolate, the p270 gene varied in size and had only one RE [16]. This study was performed to investigate and quantitate using many trichomonal isolates the percentage of Type I and Type II isolates with either single or multiple REs within p270. This report presents data on 30 Type I and 34 Type II isolates analyzed for single and multiple REs. Approximately equal numbers of the TVVˉ Type I isolates (∼50%) had single and multiple REs. On the other hand 80% of the TVV+ Type II isolates had with multiple REs.
Methods
vaginalis Organisms
Details for growth and multiplication of fresh clinical T. vaginalis isolates in Tables 1 and 2 have been detailed on previous publications [6,12,17,18]. Organisms were grown to mid-logarithmic phase in trypticase-yeast extract-maltose medium with 10% heat-inactivated horse serum. The isolates NYH 286, JH31A, T068-II, AL20W, IR-78 and RU375 have been used before [8-12,17,18]. The isolates with the T0 and 94 designations were all from the San Antonio, TX USA area. The isolates labeled JHH were from the Baltimore area.
Extraction and Analysis of dsRNA for Type I TVVˉ and Type II TVV+ Designations of Isolates
The dsRNA was extracted as described previously [19-21] from total nucleic acid. Trichomonads (109) were lysed in lysis buffer (4M guanidine isothiocyanate, 25mM sodium citrate, pH 7.0, 0.1M β-mercaptoethanol, and 0.5% laurylsarcosine). The lysate was cleared by low-speed centrifugation at 10,000 x g before being loaded onto a 5.7M CsCl gradient for centrifugation at 100,000 x g for 24h. The fraction containing the dsRNA was diluted in lysis buffer, and the cycle was repeated. The dsRNA was removed and then dialyzed in diethylpyrocarbonate treated with distilled water. Finally, the dsRNA was precipitated with 3 vol of ethanol.
Agarose Gel Electrophoresis for dsRNA Detection
The dsRNA was analyzed by agarose gel electrophoresis, as before [19-21]. Briefly, electrophoresis was performed in 1% agarose gels in buffer containing 89mM Tris base, 89mM boric acid, and 2mM ethylenediaminetetraacetic acid (EDTA), pH 8.0. For routine examination of dsRNA, electrophoresis was done at 80V for 2h. The gel was stained in the same buffer with 0.5µg/ml ethidium bromide.
vaginalis DNA Isolation
Trichomonad genomic DNA was isolated by standard procedures as previously detailed [13,14,16,22]. Briefly, 108 organisms were lysed with 0.2% sodium dodecylsulfate (SDS) in buffer consisting of 100mM NaCl, 10mM EDTA and 10mM Tris-HCl, pH 8.0 before extracting four times using phenol-chloroform mixture. After two additional phenol-chloroform extractions, the DNA was precipitated by ethanol. The DNA pellet was suspended in TE buffer (1mM EDTA and 10mM Tris-HCl, pH 8.0) containing RNase A and incubated for 10 min at 37°C. DNA was phenol-chloroform extracted and precipitated again by ethanol, and the DNA dissolved in TE buffer for storage at 4°C.
Analysis of Multiple and Single REs of the vaginalis P270 Among Type I and Type II Isolates
The generation of multiple, equidistant, higher-sized PCR products of the tandemly REs versus a single RE within the p270 gene was done exactly as before [16]. In this earlier report it was found that isolate T016 had only one RE compared with the tandemly REs of the other isolates examined. Another published report shows an alternative approach that permits analysis of isolates for REs [13]. This approach is based on the findings of the cDNA encoding the RE within the p270 gene.
Adherence and Erythrocyte Binding and Lysis Assays
Adherence and cytotoxicity assays of T. vaginalis organisms to HeLa or vaginal epithelial cells (VECs) were performed as has been detailed using established procedures [6,12,23,24]. HeLa cells were cultured as monolayers in 96-well microtiter plates. Immortalized human VECs were utilized as before [6]. Contact- dependent cytotoxicity of HeLa cells was determined using a quantitative colorimetric assay [23,24]. Briefly, for both assays 5x104 trichomonads at mid-logarithmic phase of growth were added to confluent monolayers with 5x104 cells in microtiter well plates and incubated for 18h. The wells were then washed with PBS and remaining host cells were fixed to the wells with 2% formaldehyde. After 10 min the wells were washed and stained with crystal violet, and stained cells solubilized in 1% sodium dodecyl sulfate. Intensity of color was measured by an ELISA reader at 570nm. For quantitative cytotoxicity measurements the experimental samples with parasites were compared with controls, as before [23,24]. The trichomonad-erythrocyte agglutination was visualized by brightfield and darkfield microscopy, as before [25]. Organisms grown as above were washed in PBS, and 2x106 in 1ml PBS-0.5% maltose (PBS-M) were aliquoted into siliconized microfuge tubes with 50µl of a 10% suspension of erythrocytes and incubated for different times at 37°C. The percent lysis was determined by counting using a hemocytometer. The extent of agglutination was determined by attaching erythrocytes to microtiter wells coated with polylysine. Wells were then washed with PBS-1% BSA followed by addition of 50µl PBS-0.5% maltose with radiolabeled trichomonads. After incubation at 37°C for different times, wells were counted by scintillation spectroscopy, as detailed before [25]. Finally, to demonstrate the role of parasite cysteine proteinases mediating hemolysis, experiments were also performed using individually 1mM leupeptin, 1mM TLCK (N-α-p-tosyl-L-lysine chloromethyl ketone), and 1mM TPCK (L-1-tosylamide-2-phenylethyl chloromethyl ketone) [17].
Results
Examination of vaginalis Isolates for the Presence or Absence of the dsRNA Virus
Some T. vaginalis isolate trichomonads were found to be infected with TVV [7]. Therefore, it was important to assess a collection of isolates for the presence (TVV+) or absence (TVVˉ) of the virus dsRNA (Materials and Methods). The TVVˉ and TVV+ isolates are designated as Type I and Type II, respectfully [9]. Tables 1 and 2 list the representative thirty Type I isolates and the thirty-four Type II isolates utilized for determination of the number of repeat elements (REs) within the p270 gene that encodes the immunogenic, phenotypically varying P270 protein [13].
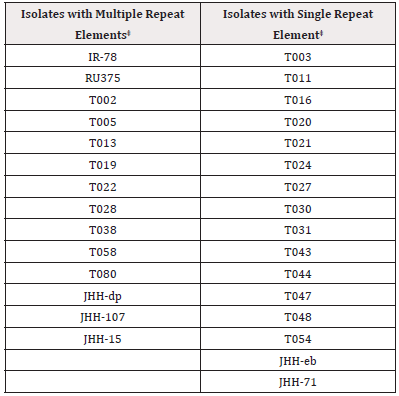
Table 1: Total Type I TVV ¯¯ Trichomonas vaginalis Isolates* with Multiple versus Single P270 Repeat Elements‡.
*Note: *T. vaginalis organisms were tested for absence of dsRNA that encodes the TVV (Materials and Methods). ‡Multiple versus single repeat elements were determined by agarose gel electrophoresis (Materials and Methods) as shown for Figure 1.

Table 2: Total Type II TVV+ Trichomonas vaginalis Isolates* with Multiple versus Single P270 Repeat Elements‡.
*Note: *T. vaginalis organisms were tested for presence of dsRNA virus as described for Table 1. ‡As described in Table 1.
Type I TVVˉ and Type II TVV+ vaginalis Isolates Analyzed for Single Versus Multiple REs Within the p270 Gene
An earlier report found that a representative T. vaginalis isolate T016 had only one RE compared with the multiple REs of the other isolates [16]. Therefore, it was important to undertake a more detailed examination for single REs and multiple REs of the Type I TVVˉ and Type II TVV+ isolates. Table 1 lists the total of 30 Type I with 16 isolates possessing single REs and 14 having multiple REs for a ratio of ∼1:1. In contrast Table 2 shows only 7 isolates with single REs and 27 isolates with multiple REs for a ratio of 1:3.8 (∼1:4). Prior to analyzing the isolates for REs, it was important to reproduce the earlier report using the T016 isolate with a single RE and the T068-II isolate with multiple REs within the p270 gene [16]. As shown in Figure 1, a single product was again obtained for isolate T016 while multiple REs were seen for isolate T068-II. This then permitted the testing of the 64 total Type I and Type II isolates for REs. Figure 2 presents representative results of 34 isolates. A total of 30 Type I isolates were examined, and among these isolates 16 had a single RE and 14 had multiple REs. Likewise, 34 Type II isolates were tested, and 7 gave single REs and 27 had multiple REs. As with the data summarized in Tables 1 and 2, the agarose gel data show that both isolate types can have either single or multiple REs within the p270 gene encoding P270.
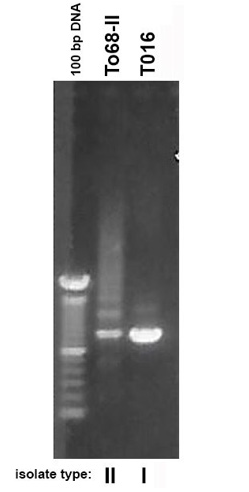
Figure 1: Ethidium bromide-stained agarose gels after electrophoresis in 1.2% agarose of amplified DNA of the single repeat element (RE) within the p270 gene of Type I TVVˉ T016 isolate trichomonads and the Type II TVV+ T068-II organisms showing the multiple REs, as before [13,16] and described in Materials and Methods. The size markers of 100 bp bands are included for comparison and note the expected intense 600 bp marker obtained for T016, as before [16].
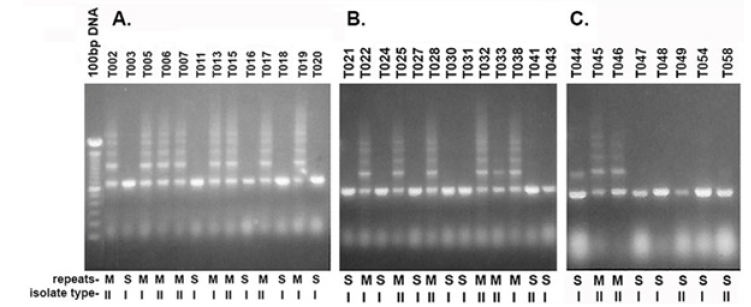
Figure 2: Ethidium bromide-stained agarose gels after electrophoresis in 1.2% agarose of amplified DNA as in Figure 1. A through C show the representative Type I and Type II isolates designated as I and II below the figures. M and S refer to multiple repeat elements and single repeat element bands, respectively.
Adherence and Erythrocyte Binding and Hemolysis by Trichomonal Isolates with Single and Multiple REs
Consistent with earlier work, the Type I and Type II trichomonads with cytoplasmic P270 and surface adhesins [6,12] readily cytoadhered to HeLa cells and vaginal epithelial cells in monolayer cultures (data not shown). Not unexpectedly, these adherent parasites then resulted in cytotoxicity to the host cells [23]. When examining isolates with either single or multiple RE for the property of hemagglutination and hemolysis [25], Type I organisms were less effective at associating with erythrocytes compared with Type II trichomonads (data not shown). Regardless of the number REs, both isolate parasites readily lysed erythrocytes, and lysis was abrogated with the addition of cysteine proteinase inhibitor (Materials and Methods), showing the role of proteinases in hemolysis [17].
Discussion
This study confirms and extends an earlier report [16] and shows that both isolate types have p270 with single or multiple REs. Interestingly, most Type II TVV+ isolates (27 of 34) had multiple tandemly REs within the p270 gene (Table 2). These data indicate that isolates with single REs are mostly within the Type I isolates (Table 1). In all experiments the parasites of Type I and Type II subpopulation with cytoplasmic P270 [26], and surface adhesin proteins [6,12] cytoadhered to both HeLa and vaginal epithelial cells. Although the Type II isolate organisms, regardless of the number of REs, bound higher numbers of erythrocytes than Type I trichomonads, both isolate type cells readily hemolyzed erythrocytes as a result of released cysteine proteinases (data not shown). Nonetheless, these observations do not allow for conclusions on the loss of REs from P270 protein on other important aspects of virulence and mechanisms of pathogenesis.
An important feature of P270 regardless of the number of REs is the stability of the p270 gene and the conservation of the DREGRD immunodominant epitope within the RE [14,15,17]. Trichomonal isolates cultured in vitro for longer than 20 years have shown no divergence in nucleotide and protein amino acid sequence within the RE portion of P270. This lack of sequence divergence may indicate an important biological function within the parasite as has been determined for other parasites. For example, Trypanosoma brucei possesses a microtubule-associated protein with tandemly repeated sequences [27]. That the sera of patients has antibody to P270 that is directed entirely to the DREGRD epitope may indicate a role of P270 in host immune surveillance during infection. This may be similar to that described for tandemly-repeated sequences of proteins of Plasmodium knowlesi, such as the circumsporozoite protein that is responsible for 95% of the antibody response [28].
Conclusion
This study emphasizes that basic biological research should take seriously the fact that there are two types of naturally-occurring isolates of T. vaginalis. In this scenario the use of either Type I TVVˉ or Type II TVV+
isolates may influence interpretations of research findings that are not generalizable to the other isolate type. For example, heterogeneous populations of surface and cytoplasmic P270 protein are found within the Type II TVV+ isolates, which undergo phenotypic variation for P270 surface and cytoplasmic expression, and this property is regulated by iron [29]. Thus, the iron status of trichomonads cultivated in batch culture is another issue to consider. This is significant in the study of certain virulence properties in addition to phenotypic variation, such as cytoadherence mediated by specific proteins where synthesis, and surface expression of these proteins are also regulated by iron [12,29]. In this case only the organisms with cytoplasmic P270 [26] express the adhesins on the parasite surface [6,12,26]. This further shows that T. vaginalis undergoes orchestrated surface and non-surface placement of repertoires of proteins, including P270, adhesins and immunogenic proteins [11,30]. Furthermore, investigations with Type II TVV+ showed the loss of TVV during batch culture [9] yielding TVVˉ progeny trichomonads that may be different from the parental population depending on the biological property being studied.
Acknowledgements
This study was supported by Public Health Service grants AI-39803 from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health. As per instructions by the International Committee of Medical Journal Editors on “Who Is an Author?,” (http://www.icmje.org/recommendations/browse/roles-and-responsibilities/defining-the-role-of-authors-and-contributors.html), I want to acknowledge Oxana Musatovova who generated the data under my supervision in my laboratory while at The University of Texas Health Science Center at San Antonio, Texas.
Conflict of Interest
I declare that there are no conflicts of interest. I alone was responsible in the collection, analyses, and interpretation of data, in the writing of the manuscript, and in the decision to publish the results.
References
- Muzny CA (2018) Why does Trichomonas vaginalis continue to be a “neglected” sexually transmitted infection? Clin Infect Dis 67(2): 218-220.
- Peterman TA, Tian LH, Metcalf CA, Malotte CK, Paul SM, et al. (2009) Persistent, undetected Trichomonas vaginalis infections? Clin Infect Dis 28(2): 259-260.
- Rogers SM, Turner CF, Hobbs M, Miller WC, Tan S, et al. (2014) Epidemiology of undiagnosed trichomoniasis in a probability sample of urban young adults. PLOS One 9(3): e90548.
- Lazenby GB, Thompson L, Powell AM, Soper DE (2019) Unexpected high rates of persistent Trichomonas vaginalis infection in a retrospective cohort of treated pregnant women. Sex Trans Dis 46(1): 2-8.
- Lehker MW, Sweeney D (1999) Trichomonad invasion of the mucous layer requires adhesins, mucinases, and motility. Sex Transm Inf 75(4): 231-238.
- Arroyo R, Engbring J, Alderete JF (1992) Molecular basis of host epithelial cell recognition by Trichomonas vaginalis. Mol Microbiol 6(7): 853-862.
- Wang AL, Wang CC (1986) The double-stranded RNA in Trichomonas vaginalis may be originated from virus-like particles. Proc Natl Acad Sci USA 83(20): 7956-7960.
- Wang A, Wang CC, Alderete JF (1987) Trichomonas vaginalis phenotypic variation occurs only among trichomonads infected with the double-stranded RNA virus. J Exp Med 166(1): 142-150.
- Alderete JF, Kasmala L, Metcalfe, Garza GE (1986) Phenotypic variation and diversity among Trichomonas vaginalis isolates and correlation of phenotype with trichomonal virulence determinants. Infect Immun 53(2): 285-293.
- Alderete JF, Suprun-Brown L, Kasmala L (1986) Monoclonal antibody to a major surface glycoprotein immunogen differentiates isolates and subpopulations of Trichomonas vaginalis. Infect Immun 52(1): 70-75.
- Alderete JF (1988) Alternating phenotypic expression of two classes of Trichomonas vaginalis surface markers. Rev Infect Dis 10(S2): S408-S412.
- Lehker MW, Arroyo R, Alderete JF (1991) The regulation by iron of the synthesis of adhesins and cytoadherence levels in the protozoan Trichomonas vaginalis. J Exp Med 174(2): 311-318.
- Dailey DC, Alderete JF (1991) The phenotypically variable surface protein of Trichomonas vaginalis has a single, tandemly repeated immunodominant epitope. Infect Immun 59(6): 2083-2088.
- Mustovova O, Alderete JF (1998) Molecular analysis of the gene encoding the immunodominant phenotypically varying P270 protein of Trichomonas vaginalis. Microb Pathogen 24(4): 223-239.
- Alderete JF, Neale (1989) Relatedness of structures of a major immunogen in Trichomonas vaginalis Infect Immun 57(6): 1849-1853.
- Musatovova O, Alderete JF. (1999) The Trichomonas vaginalis phenotypically varying P270 immunogen is highly conserved except for the numbers of repeated elements. Microbial Pathogen 27(2): 93-104.
- Neale KA, Alderete JF (1990) Analysis of the proteinases of representative Trichomonas vaginalis Infect Immun 58(1): 157-162.
- Peterson KM, Alderete JF (1982) Host plasma proteins on the surface of pathogenic Trichomonas vaginalis. Infect Immun 37(2): 755-762.
- Khoshnan A, Provenzano D, Alderete JF (1994) Unique double-stranded RNAs associated with the Trichomonas vaginalis virus are synthesized by viral RNA-dependent RNA polymerase. J Virol 68(11): 7108-7114.
- Chirgwin JM, Przybyla E, MacDonald RJ, Rutter WJ (1979) Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochem 18(24): 5294-5299.
- Khoshnan A, Alderete JF (1993) Multiple double-stranded RNA segments are associated with virus particles infecting Trichomonas vaginalis. J Virol 67(12): 6950-6955.
- Alderete JF, O’Brien JL, Arroyo R, Engbring JA, Musatovova O, et al. (1995) Cloning and molecular characterization of two genes encoding adhesion proteins involved in Trichomonas vaginalis Mol Microbiol 17(1): 69-83.
- Alderete JF, Pearlman E (1984) Pathogenic Trichomonas vaginalis cytotoxicity to cell culture monolayers. Br J Vener Dis 60(2): 99-105.
- Alderete JF, Garza (1985) Specific nature of Trichomonas vaginalis parasitism of host cell surfaces. Infect Immun 50(3): 701-708.
- Lehker MW, Chang TH, Dailey DC, Alderete JF (1990) Specific erythrocyte binding is an additional nutrient acquisition system for Trichomonas vaginalis. J Exp Med 171(6): 2165-2170.
- Alderete JF (2021) Localization of the phenotypically varying P270 protein on dsRNA virus-positive and negative Trichomonas vaginalis Am J Biomed Sci Res 14(2): 199-209.
- Schneider A, Hemphill A, Wyler T, Seebeck T (1988) Large microtubule-associated protein of T. brucei has tandemly repeated, near identical sequences. Science 241(4864): 459-462.
- Zavala F, Cochrane AH, Nardin EH, Nussenzweig RS, Nussenzweig V (1983) Circumsporozoite proteins of malaria parasites contain a single immunodominant region with two or more identical epitopes. J Exp Med 157(6): 1947-1957.
- Alderete JF (1999) Iron modulates phenotypic variation and phosphorylation of P270 in double- stranded RNA virus-infected Trichomonas vaginalis. Infect Immun 67(8): 4298-4302.
- Alderete JF (1987) Trichomonas vaginalis NYH 286 phenotypic variation may be coordinated for a repertoire of trichomonad surface immunogens. Infect Immun 55(9): 1957-1962.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.