Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Novel Methods to Preserve Mesenchymal Stem Cells at low Temperatures
*Corresponding author: Jonathan R T Lakey, Department of Surgery, University of California Irvine, Irvine, California, USA, 92868 and Department of Biomedical Engineering, University of California Irvine, Irvine, California, USA, 92868.
Received: April 12, 2024; Published: April 16, 2024
DOI: 10.34297/AJBSR.2024.22.002926
Abstract
In recent years, stem cell therapy has been applied to the treatment of many diseases. Stem cells are extensively researched for their potential to replace diseased tissues in conditions such as degenerative diseases, tumors, trauma, and others, collectively known as regenerative therapy. However, as age advances, the number and quality of stem cells decreases; by the age of 50, a person has less than 3% of the stem cells they had at birth. Although stem cells can be preserved in vitro for long periods of time, their activity after cryopreservation and recovery does not reach the level expected under current technological conditions. Therefore, it is particularly important to find a more suitable freezing method. In this paper, we present a novel method for freezing stem cells that ensures high cellular activity after recovery. In this study we validate a combinational approach of using two permeating cryoprotective agents, at lower than normal concentrations resulting in higher recovery post cryopreservation of human mesenchymal stem cells.
Keywords: Stem cell therapy, Mesenchymal stem cell, Cryopreservation
Introduction
Stem cells are undifferentiated cells that are capable of self-renewal, regeneration, and differentiation into specific cell types to regenerate and repair damaged tissues. In recent years, stem cell therapy has become increasingly popular. Stem cells and the vesicles they secrete play an indispensable role in the treatment of human diseases, attracting significant interest in basic experiments, clinical trials, and clinical applications. The therapeutic effect of stem cells has been demonstrated in diabetes, osteoarthritis, liver cirrhosis, Crohn's disease, and other diseases [1-6].
Stem cells fall into two main categories: embryonic stem cells (ESCs), which are derived from cell masses within blastocysts, and adult stem cells, which are found in adult tissues [7]. Regardless of the source, cryopreservation of stem cells is necessary to ensure long-term viability.
Cryopreservation is currently the most effective method of stem cell preservation. Stem cells are cryopreserved at temperatures below -140°C after isolation. To ensure survival during cryopreservation, it is necessary to use specific reagents, including dimethyl sulfoxide (DMSO), sucrose, and ethylene glycol (EG), which is considered less toxic than DMSO and maintains cell pluripotency better than propylene glycol or glycerol. Additionally, it is essential to freeze/thaw the cells under controlled conditions to ensure their viability after thawing [8]. Cell freezing cryoprotectants can be divided into two main groups, penetrating and non- penetrating. Penetrating agents include DMSO, glycerin, ethylene glycol and propylene glycol, which can prevent the damaging effect of water from forming ice crystals by penetrates cell membranes, making it easier for the intracellular water to be replaced by cryoprotectants that promote vitrification [9,10]. Non- penetrating agents are macromolecular compounds such as sucrose, polyethylene glycol and so on, which cannot penetrate into the cell by absorbing extracellular water due to the osmotic gradient created, but they can also induce vitrification extracellularly to a lesser extent [10]. Both types of cryoprotectants are potentially toxic to cell viability when used at higher concentrations [11].
The most conventional freezing method involves mixing 90% FBS with 10% DMSO, followed by slow freezing and storage in liquid nitrogen. However, the cell recovery rate obtained using conventional cryoprotectants has gradually failed to meet researchers’ requirements and is being replaced, necessitating the development of new or improved techniques for more effective stem cell therapy applications. In this paper, we introduce a cryoprotectant combination strategy for mesenchymal stem cells , involving the mixture of DMSO and EG with 2% serial sucrose dilution of the cryoprotectants after thawing, which results in a much higher cell recovery rate after thawing. We believe the combination of lower than normal cryoprotectants and added in a stepwise manner will result in less damage to the cells after freezing and result in higher recovery post cryopreservation.
Methods
Stem Cell Sources and Culture Methods
Human mesenchymal stem cells were derived from cells isolated from umbilical cords collected from screened donors after safe delivery of infants after social and medical consent was completed by the mother as per defined protocols. A consent for research protocol was submitted and approved by the University of California Irvine Institutional Review Board (IRB) committee. Collected specimens were washed three times with phosphate-buffered saline (PBS) and cut into 1-cubic-millimeter tissue blocks, which were then inoculated into cell culture flasks containing Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) in a humidified environment of 5% CO2 and 95% air at 37°C [12]. Tissue culture media (50%) were replaced every 2-3 days as per published protocols. When cells reached 80-90% confluency in tissue culture, they were passaged with 0.25% trypsin and split into new culture plates and culture media. Groups of umbilical cords derived MSC stem cells were cultured for multiple passages in tissue culture using previously defined protocols. Viability was assessed after each passage using FDA/PI [13].
Freezing Methods
After normal in vitro culture at 37C, the cells were collected and allocated into groups of approximately 10,000 human MSC stem cells per vial were placed in different cryoprotectant groups:
1. 1 M DMSO,
2. Combination of 0.75 M DMSO/0.75 M EG,
3. Combination of 0.5 M DMSO/0.5 M EG, and
4. No cryoprotectant.
The cryoprotectant mixed was added to labelled vials in a stepwise manner following previously published methods of adding the cryoprotectant solution in a stepwise manner until the final dose was achieved [14,15] The vials containing the MSC cells were frozen using a modified cryopreservation protocol where the vials from each group were supercooled to -8oC for a period of 10 minutes, manually nucleated using an external supercooled rod and after time for the release of the latent heat of fusion, slow cooled frozen to -40°C at 0.75°C/min, after which and the vials were then immersed in liquid nitrogen for long-term storage (-196oC) [10]. Vials were stored for periods of 1-3 months before thawing and post thawing assessment of recovery and viability.
Cell Recovery and Viability Assays
After a period of time in long term storage in liquid nitrogen, the frozen vials were rapidly thawed in a water bath at 37°C for approximately 50 seconds, to the ice ball stage, and then placed in an ice bath before the cryoprotectant was slowly removed using the difference in permeability between extracellular fluid and intracellular water, and DMSO and EG were slowly removed using a stepwise dilution method. After washing the frozen thawed cells were collected and examined by FDA/PI cell viability assay [13].
Statistical analysis
Quantitative values were presented as mean ± standard deviation. Statistical analysis was conducted using SPSS Statistics software. A t-test was used to compare differences between the treatment and the control groups at specific time points. For categorical data, the Chi-Square test was employed to assess differences between groups. A value of p<0.05 was considered statistically significant.
Results
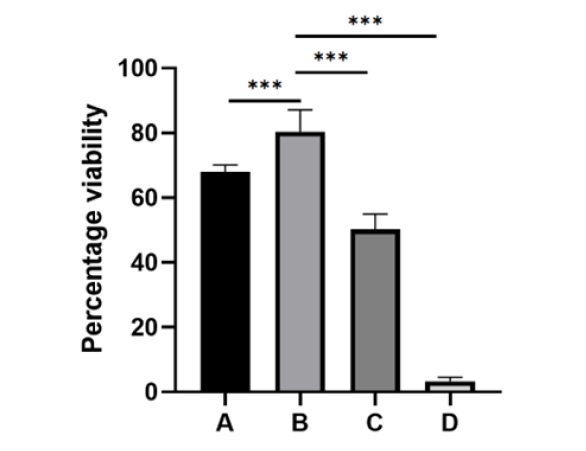
This study focused on the cryopreservation of among the four groups of human MSC cells, using a lower than normal concentrations and a combination of two permeating cryoprotectants, DMSO and EG. The groups were compared to control group of cells that were frozen using the same freezing protocol without the addition of any cryoprotectants. The final cell viability was determined immediately after thawing and removal of the cryoprotectant which was performed using stepwise methods using sucrose from a series of 6 separate vials of cells from each group was 67.9±2.2%(mean +/- SEM) in group A, 80.4±6.7 in group B, 50.2 ± 4.7 in group C, and dropped significantly to 3.3±1.2 in group D, the group of cells that were frozen and thawed without any cryoprotectant (Figure 1). The cell recovery rate of group B was significantly higher compared to the other three groups (P < 0.01, ANOVA).
Frozen/thawed MSC cells were then collected and then returned back to tissue culture for an additional period of 48 hours before reassessment of the cell viability. After 48 hours of post cryopreservation culture, viability was maintained in groups A, B and C, however, the cells cultured from Group D were not recovered for analysis (n =ns).
Discussion
Stem cells can be extracted from blood, bone marrow, umbilical cords and adipose tissue [16,17], but cryopreservation is necessary for long-lasting banking and use in the future. In cryopreservation, cryoprotectants are an important part of the freezing solution. Cryoprotectants can be categorized into penetrating and non- penetrating types, and the main difference between them is their ability to penetrate cells [10]. DMSO and EG are representatives of osmotic cryoprotectants, of which DMSO is considered to be the most preferred cytoprotectant in penetrating vitrification cryopreservation solution, with an typical concentration of 10% W/V [18].
Currently, DMSO and EG as cryoprotectants have been applied to stem cells, hepatocytes, pancreatic islets and germ cells [10,19-23]. Our previous studies on pancreatic islet have shown that although DMSO and EG have excellent effects in islet cryopreservation, the high concentrations that maintain their inhibition of ice crystal formation make them inevitably toxic, and slow cooling and vitrification help to mitigate toxicity [24]. However, as the concentration of DMSO decreases, its cytotoxicity gradually diminishes, but so does its protective effect on the cells. Conversely, high DMSO concentration can lead to excessive cytotoxicity [25]. These factors result in decreased cell activity after recovery.
Cryoprotectant options
Even with the most optimal cryoprotectant, a decrease in cell viability after thawing is inevitable. Consequently, researchers have been searching for new cryoprotectants to mitigate this decrease. Both penetrating and non-penetrating cryoprotectants have some degree of toxicity. However, non-penetrating cryoprotectants are generally considered less toxic, while penetrating cryoprotectants show a superior cryoprotective effect [26]. Valentin, et al. demonstrated that penetrating cryoprotectants retained epithelial cell viability better than non-penetrating ones by cryopreservation of thymus tissue samples [27]. The same phenomenon was observed by Rose, et al. through cryopreservation of frog spermatozoa [28]. For penetrating cryoprotectants (mainly DMSO), cytotoxicity is the most northern point of criticism. To address the toxicity associated with DMSO, efforts have been made to explore strategies that involve reducing its concentration. A meta-analysis showed that decreasing the concentration of DMSO from 10% to 5% during deep cryopreservation of autologous peripheral blood stem cells enhanced cell viability [29]. Sung, et al. found that the addition of 10% ethylene glycol to 5% DMSO + 50% FBS significantly improved the viability rate during cryopreservation of human embryonic stem cells [30]. Katkov, et al. demonstrated that during cryopreservation of induced pluripotent stem cells, EG was significantly less toxic than DMSO [31]. DMSO can be completely replaced. Arutyunyan, et al. achieved good cryopreservation using 1.5 M EG and 20% glycerol without using DMSO [32]. Some researchers have replaced DMSO entirely with other cryoprotectants [33], however, their efficiency still requires further evaluation.
Based on the above research, in this paper, we used a combination of DMSO and EG as cryoprotectants to achieve a higher recovery rate compared to the traditional DMSO cryopreservation method. Our results are consistent with previous reports that a cryoprotectant based on 0.75 M DMSO/0.75 M EG results in better activity of the recovered cells.
Temperature Control During Cryopreservation
Donaldson, et al. demonstrated that increasing the cooling rate from 1 degree Celsius to 10 degrees Celsius per minute significantly reduced the recovery rate of human cord blood [34]. Djuwantono, et al. compared rapid freezing with slow freezing in CD34+ enumeration of human cord blood mononuclear cells and found that although rapid freezing resulted in higher cellular activity, their CD34 expression was lower than that of the slow freezing group [35]. This led to the suggestion that rapid freezing may induce cell differentiation. Therefore, in our experiments, we chose to freeze at 0.5-1.0°C/min to -40°C and then place in liquid nitrogen to preserve stem cell function.
Conclusions
We have introduced an innovative stem cell freezing method that preserves high activity and function of stem cells upon recovery, surpassing the efficacy of traditional cryoprotectants. This advancement promises enhanced therapeutic outcomes from these cells. Next steps are to further study these beneficial effects of this observation that a combination of using two permeating cryoprotectants that have been used solely in the past. The results of these initial studies could provide improved methods to freeze and thawed human MSC. Further studies will focus on continued efforts to better under the beneficial effects of using a combination of two permeating cryoprotectants to allow improved recovery of frozen thawed human MSC.
References
- Hogrebe NJ, Ishahak M and Millman JR (2023) Developments in stem cell-derived islet replacement therapy for treating type 1 diabetes. Cell Stem Cell 30(5):530-548.
- Lakey JRT, Wang Y, Alexander M, Chan MKS, Wong MBF, et al (2023) Exosomes; a Potential Source of Biomarkers, Therapy, and Cure for Type-1 Diabetes. Int J Mol Sci 24(21): 15713.
- Yu H, Huang Y and Yang L (2022) Research progress in the use of mesenchymal stem cells and their derived exosomes in the treatment of osteoarthritis. Ageing Res Rev 80:101684.
- Trounson A McDonald C (2015) Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell 17(1):11-22.
- Temple S (2023) Advancing cell therapy for neurodegenerative diseases. Cell Stem Cell 30(5): 512-529.
- Wang R, Yao Q, Chen W, Gao F, Li P, et al (2021) Stem cell therapy for Crohn's disease: systematic review and meta-analysis of preclinical and clinical studies. Stem Cell Res Ther 12(1):463.
- Estrov Z (2009) Stem cells and somatic cells: reprogramming and plasticity. Clin Lymphoma Myeloma 9(Suppl 3): S319-328.
- Crowley CA, Smith WPW, Seah KTM, Lim SK and Khan WS (2021) Cryopreservation of Human Adipose Tissues and Adipose-Derived Stem Cells with DMSO and/or Trehalose: A Systematic Review. Cells 10(7): 1837.
- Lecchi L, Giovanelli S, Gagliardi B, Pezzali I and Ratti I, et al (2016) An update on methods for cryopreservation and thawing of hemopoietic stem cells. Transfus Apher 54(3): 324-336.
- Whaley D, Damyar K, Witek RP, Mendoza A, Alexander M, (2021) Cryopreservation: An Overview of Principles and Cell-Specific Considerations. Cell Transplant 30:963689721999617.
- Jahan S, Kaushal R, Pasha R and Pineault N (2021) Current and Future Perspectives for the Cryopreservation of Cord Blood Stem Cells. Transfus Med Rev 35(2):95-102.
- Yang YK, Ogando CR, Wang See C, Chang TY and Barabino GA (2018) Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res Ther 9(1):131.
- Herrero A, Souche R, Panaro F and Navarro F (2020) Endovascular balloon occlusion during reconstruction of portal vein injury. Langenbecks Arch Surg 405(3): 391-395.
- Mitrus I, Smagur A, Fidyk W, Czech M, Prokop M, et al (2018) Reduction of DMSO concentration in cryopreservation mixture from 10% to 7.5% and 5% has no impact on engraftment after autologous peripheral blood stem cell transplantation: results of a prospective, randomized study. Bone Marrow Transplant 53(3): 274-280.
- Lakey JR, Anderson TJ and Rajotte RV (2001) Novel approaches to cryopreservation of human pancreatic islets. Transplantation 72(6): 1005-1011.
- Zakrzewski W, Dobrzynski M, Szymonowicz M and Rybak Z (2019) Stem cells: past, present, and future. Stem Cell Res Ther 10(1):68.
- Bacakova L, Zarubova J, Travnickova M, Musilkova J, Pajorova J, et al (2018) Stem cells: their source, potency and use in regenerative therapies with focus on adipose-derived stem cells - a review. Biotechnol Adv 36(4): 1111-1126.
- Bakken AM, Bruserud O, Abrahamsen JF (2003) No differences in colony formation of peripheral blood stem cells frozen with 5% or 10% dimethyl sulfoxide. J Hematother Stem Cell Res 12(3): 351-358.
- William N and Acker JP (2020) Cryoprotectant-dependent control of intracellular ice recrystallization in hepatocytes using small molecule carbohydrate derivatives. Cryobiology 97:123-130.
- Silva AM, Pereira AG, Bezerra LGP, Jeronimo Moreira SS, Pereira AF, et al (2022) Cryopreservation of Testicular Tissue from Adult Red-Rumped Agoutis (Dasyprocta leporina Linnaeus, 1758). Animals (Basel) 12(6): 738.
- Kwan HCK (2023) Reconsideration of the safety and effectiveness of human oocyte cryopreservation. Reprod Biol Endocrinol 21(1): 22.
- Kojayan G, Whaley D, Alexander M, Rodriguez S, Lee S, et al (2019) Improved cryopreservation yield of pancreatic islets using combination of lower dose permeable cryoprotective agents. Cryobiology 88: 23-28.
- Tan FC, Lee KH, Gouk SS, Magalhaes R, Poonepalli A, et al (2007) Optimization of cryopreservation of stem cells cultured as neurospheres: comparison between vitrification, slow-cooling and rapid cooling freezing protocols. Cryo Letters 28(6): 445-460.
- Kojayan GG, Alexander M, Imagawa DK and Lakey JRT (2018) Systematic review of islet cryopreservation. Islets 10(1): 40-49.
- Li R, Johnson R, Yu G, McKenna DH, Hubel A (2019) Preservation of cell-based immunotherapies for clinical trials. Cytotherapy 21(9): 943-957.
- Sztein JM, Noble K, Farley JS and Mobraaten LE (2001) Comparison of permeating and nonpermeating cryoprotectants for mouse sperm cryopreservation. Cryobiology 42(1): 28-39.
- Shichkin VP, Gorbach OI, Zuieva OA, Grabchenko NI, Aksyonova IA, et al (2017) Effect of cryopreservation on viability and growth efficiency of stromal-epithelial cells derived from neonatal human thymus. Cryobiology 78:70-79.
- Upton R, Clulow S, Colyvas K, Mahony M and Clulow J (2023) Paradigm shift in frog sperm cryopreservation: reduced role for non-penetrating cryoprotectants. Reproduction 165(6): 583-592.
- Bennett B, Hanotaux J, Pasala AR, Hasan T, Hassan D, et al (2024) Impact of lower concentrations of dimethyl sulfoxide on cryopreservation of autologous hematopoietic stem cells: a systematic review and meta-analysis of controlled clinical studies. Cytotherapy 21: S1465-3249.
- Ha SY, Jee BC, Suh CS, Kim HS, Oh SK, et al (2005) Cryopreservation of human embryonic stem cells without the use of a programmable freezer. Hum Reprod 20(7): 1779-1785.
- Katkov, II, Kan NG, Cimadamore F, Nelson B, Snyder EY, et al (2011) DMSO-Free Programmed Cryopreservation of Fully Dissociated and Adherent Human Induced Pluripotent Stem Cells. Stem Cells Int 2011: 981606.
- Arutyunyan IV, Strokova Scapital O C, Makarov capital A C, Mullabaeva Scapital Em C, Elchaninov capital A C, et al (2018) DMSO-Free Cryopreservation of Human Umbilical Cord Tissue. Bull Exp Biol Med 166(1): 155-162.
- Yao X and Matosevic S (2021) Cryopreservation of NK and T Cells Without DMSO for Adoptive Cell-Based Immunotherapy. BioDrugs 35(5): 529-545.
- Donaldson C, Armitage WJ, Denning-Kendall PA, Nicol AJ, Bradley BA, et al (1996) Optimal cryopreservation of human umbilical cord blood. Bone Marrow Transplant 18(4): 725-731.
- Djuwantono T, Wirakusumah FF, Achmad TH, Sandra F, Halim D, et al (2011) A comparison of cryopreservation methods: Slow-cooling vs. rapid-cooling based on cell viability, oxidative stress, apoptosis, and CD34+ enumeration of human umbilical cord blood mononucleated 26(4): 371.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.