Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Oral Supplementation with a Novel Collagen Tripeptide: Assessing Skin Health Improvements in a Single-Center, Open Label Pilot Study
*Corresponding author: Chunyue Zhang, Lithy Research Institute, Lithy One Health Group, Shanghai 200235, China.
Received: April 23, 2024; Published: April 29, 2024
DOI: 10.34297/AJBSR.2024.22.002947
Abstract
With the evolution of the oral beauty market from collagen protein to collagen peptides with smaller molecular weights and higher absorption rates. The application prospects of Collagen Tripeptides (CTP), known for superior absorption, are promising. In a single-center open-label trial involving 31 participants over 28 days of continuous oral administration of a CTP - DailyGlow® and 33 participants without product consumption, instrumental testing was employed. Results revealed that DailyGlow® CTP exhibited potent anti-wrinkle effects, improving various skin parameters significantly within 14 and 28 days. Moreover, the product showcased remarkable moisturizing capabilities, contributing to enhanced skin hydration and reduced transepidermal water loss. Notably, DailyGlow® CTP effectively promoted skin firmness and elasticity, suggesting its potential in skin-tightening applications. In conclusion, oral administration of DailyGlow® CTP for 28 days demonstrated substantial improvements in skin conditions, showcasing robust skincare efficacy.
Keywords: Collagen Tripeptide, Oral Beauty, Skin Care
Abbreviations: CTP: Collagen Tripeptides; AGEs: Glycation End Products; TEWL: Transepidermal Water Loss; CML: Carboxymethyllysine; AQP3: Aquaporin-3; MMPs: Matrix Metalloproteinases
Introduction
The global market for orally administered beauty products, commonly referred to as nutricosmetics, has witnessed a consistent and robust growth in recent years. These products, which range from tablets to drinks and sprays, are designed to enhance general well-being with specific benefits for skin health, hair care, and weight management. According to recent market analyses, the nutricosmetics sector is projected to reach a valuation of $9.22 billion by 2031, expanding at a compound annual growth rate of 11.4% [1]. Collagen, a key component in anti-aging products, remains a standout in the oral beauty domain, witnessing novel developmental trends. Its application extends from oral supplementation to topical use, playing a pivotal role in mitigating or decelerating the aging process [2,3]. The global demand for collagen, particularly in supplements and sports nutrition, has surged, exhibiting an impressive average annual growth of 17% [4]. Recent studies underscore the efficacy of orally ingested collagen peptides in enhancing skin hydration, elasticity, and reducing desquamation and wrinkling, thereby underscoring its potential in skin health [5]. These evolving market trends and consumer insights indicate a heightened interest in collagen-based products for both oral and topical applications. This interest is fueled by growing awareness of collagen’s benefits for skin health, driving significant advancements and innovations in collagen-based oral beauty products.
Collagen Tripeptide (CTP), distinguishing itself from conventional collagen supplements, emerges as a uniquely promising ingredient in the realm of oral beauty and skincare. The key distinctions lie in its molecular structure, bioavailability, and specific health benefits. Firstly, CTP's molecular composition, consisting of three amino acids, enables direct absorption by the intestinal tract, a stark contrast to conventional collagen supplements that require digestion by gastrointestinal enzymes for absorption [6]. This feature enhances its solubility and bioavailability while reducing allergenic properties, rendering CTP particularly suitable for food supplements and cosmeceutical skincare applications [6]. Regarding skin health, CTP has been demonstrated to significantly improve skin hydration, elasticity, desquamation, and wrinkling, both in oral and topical applications [7-9]. Additionally, it plays a role in inhibiting Advanced Glycation End products (AGEs) in the skin, which are implicated in the aging process [6]. Beyond these skin-centric benefits, CTP exhibits a range of biological effects, including improving dry skin, aiding wound healing, enhancing bone fracture healing, and offering protection against UVB-induced skin damage [6,9]. Furthermore, CTP's efficient absorption leads to higher serum levels of peptide metabolites compared to conventional collagen peptides, amplifying its efficacy [7]. Collectively, these attributes position CTP as a notably advanced and effective component in oral beauty and skincare formulations. In this single-center, open label pilot study, we assessed the dermatological effects of CTP through a controlled trial. Over a 28-day period, 31 subjects orally consumed CTP, while a control group of 33 abstained. Employing quantitative instrumental analysis, we aimed to rigorously evaluate the impact of CTP on skin attributes, specifically focusing on anti-wrinkle properties, hydration, elasticity, whitening, and its potential in anti-carbonylation and anti-glycation.
Materials and Methods
Testing Sample
The test samples consisted of a solid drink product, characterized by a favored taste profile. The core ingredient DailyGlow® CTP was integrated with fruit powder and flavoring agents to create a palatable formulation. Each serving of the product was precisely formulated to contain a total weight of 6 grams. The production of the testing supplement was undertaken by the Shanghai Powell Research and Development team. The manufacturing process was carried out in a cleanroom environment with a 100K magnitude classification.
Intervention Design
The study was structured as a single-center, open label pilot study, involving 31 participants who orally consumed a CTP solid drink for 28 consecutive days. A control group of 33 individuals did not consume the product. This setup was aimed at evaluating the product's efficacy in skin health improvements, specifically targeting anti-wrinkle, moisturizing, elasticity, whitening, anti-carbonylation, and anti-glycation properties. Efficacy assessments were conducted at the start (day 0), midpoint (day 14), and end (day 28) of the intervention period. During the trial, participants maintained their regular diet and lifestyle without significant changes and were prohibited from taking any other skin improvement supplements or functional foods. They reported compliance daily, and any adverse events were immediately communicated to professional physicians. This rigorous approach ensured a controlled environment for accurately assessing the test product's impact on skin health.
Participants
Informed Consent: Prior to participation in the study, all subjects signed an informed consent form. This process involved both oral and written consent, in line with regulatory standards. The informed consent clearly outlined the nature, purpose, and potential risks involved in the study. It emphasized the voluntary nature of participation, allowing subjects to withdraw from the study at any time, for any reason. Adequate time was provided for subjects to ask questions and consider their participation before signing the consent forms, ensuring that all consents were obtained before the start of the study.
Inclusion Criteria: The study included healthy normal men and women, aged between 30-50 years, with a balanced distribution across this age range. Eligible participants had visible wrinkles around the eyes and were able to maintain regularity in their lifestyle throughout the study period. They were required to be capable of reading and comprehending the contents of the informed consent form and voluntarily agree to its terms. Participants agreed not to use any cosmetics, drugs, or health products that could potentially influence the study results during the trial period. Additional inclusion criteria were applied as detailed in the study documentation.
Exclusion Criteria: The exclusion criteria for the study were extensive to ensure the safety and integrity of the results. These included recent use of antihistamines or immunosuppressants, application of anti-inflammatory drugs on the test site, history of skin diseases like psoriasis, eczema, or skin cancer, insulin-dependent diabetes, ongoing treatment for chronic respiratory diseases, recent anti-cancer chemotherapy, immune deficiencies, pregnancy or lactation, and recent surgeries such as bilateral mastectomy. Additionally, participants with skin conditions that could affect the test results, such as scars, pigmentation, or atrophy, were excluded. The study also excluded individuals who had used specific medications like retinoids, alpha-hydroxy acids, salicylic acid, hydroquinone, and certain prescription drugs in the recent months, as well as those on oral contraceptives unless consistently used for the past six months.
Skin Condition Assessment
Testing Conditions, Instruments and Parameters: Before the measurement, subjects first cleaned their facial skin using dry facial tissue, then rested in a constant temperature (21±1℃) and humidity (50±10% RH) controlled environment for 30 minutes. These measurements were recorded at the baseline and then reassessed at 14 and 28 days into the study. Testing Instruments, parameters, and testing parts are organized in Table 1.
Anti-Wrinkle Assessment: The anti-wrinkle assessment involved a meticulous preparation and measurement process. The Antera 3D was used to capture images of facial wrinkles, including nasolabial folds, crow's feet, and forehead lines, allowing for the quantification of wrinkle number, area, and volume. The VISIA-CR system complemented this by photographing wrinkles around the eyes, calculating both the area of wrinkles and their proportion to the total facial area. Antera 3D system is an advanced handheld camera employs structured light technology, wherein a network of light fringes is projected onto the skin. These fringes deform based on the skin's relief, creating detailed three-dimensional color images of the skin surface. This method enables precise and accurate analysis of skin wrinkles, texture, pigmentation, and other characteristics [10].
Moisturization Testing: The Corneometer CM825 measured the moisture content of the stratum corneum, providing vital data on skin hydration levels. The Tewameter TM300 was utilized to measure Trans Epidermal Water Loss (TEWL), a key indicator of the skin's moisture retention capability and barrier function integrity. TEWL quantifies water passage from the body through the epidermis to the atmosphere, signifying the skin's health in terms of hydration and barrier quality [11].
Elasticity and Firmness Testing: Elasticity and firmness testing of the skin was conducted using the Cutometer Dual MPA 580. The Cutometer Dual MPA 580 operates on a suction method, creating negative pressure that mechanically deforms the skin. This method involves drawing the skin into the probe's aperture and then releasing it after a set time. An internal optical measuring system within the probe, comprising a light source, light receptor, and facing prisms, measures the penetration depth of the skin drawn into the probe. This system accurately assesses the skin's resistance to negative pressure (firmness, F4) and its ability to revert to its original shape (elasticity, R2), displaying these parameters as real-time curves of penetration depth over time.
Whitening Testing: For the assessment of skin whitening effects, the Colorimeter CL400 was employed to measure skin color changes on the right cheek of the subjects. The measurement values were expressed as coordinates in the L*a*b* color space, a standard in colorimetry, representing lightness and color chromaticity. The device's operation involves the emission of white LED light in a circular arrangement around the probe to ensure uniform skin illumination. When this light is scattered upon striking the skin, the probe measures the reflected light, capturing precise color values. This method provides accurate data on melanin and erythema levels in the skin, crucial for assessing the effectiveness of whitening products.
Anti-Carbonylation and Anti-Glycation Testing: To assess anti-carbonylation and anti-glycation effects, the study utilized D-Squame® cuticle sampling tape to collect stratum corneum samples. The sampling process involved adhering a transparent, adhesive disc to the skin's surface for 30 seconds, effectively gathering corneocytes from the outermost skin layer. These collected cells were then analyzed using a graded scale or Quantisquam® software, providing a detailed assessment at the cellular level. The degree of carbonylation in the skin was determined by measuring the fluorescence intensity of carbonylation products. This method reflects the extent of protein carbonylation, a process that can indicate oxidative stress in the skin. Additionally, the study employed ELISA (Enzyme-Linked Immunosorbent Assay) to detect CML (Carboxymethyllysine), a non-fluorescent, non-crosslinking Advanced Glycation End Product (AGE) produced from lysine via the intermediate glyoxal. CML levels serve as an indicator of skin glycation, a key factor in skin aging and collagen degradation. These comprehensive measurements provided valuable insights into the effectiveness of the tested product in mitigating skin carbonylation and glycation processes, key factors in skin aging and health.
Data Processing and Statistical Analysis
The collected data, including images and quantitative measurements from tests around the subjects' eyes, were analyzed to evaluate the test product's effects on anti-wrinkle, moisturizing, elasticity, firmness, whitening, anti-carbonylation, and anti-glycation properties. Statistical analysis was conducted using SPSS software, with results expressed as mean ±standard deviation. A normal distribution test was first performed; for data following a normal distribution, the t-test was applied to examine changes in skin parameters before and after product use. This analysis, using a two-sided test with an alpha level of 0.05, determined the effectiveness of the test product. Conclusions about the product’s efficacy were drawn from these statistical results, ensuring a scientifically valid assessment of its impact on skin health.
Results
Anti-Wrinkle Effects
In subjects from the sample group and the control group, periocular wrinkle area and percentage, nasolabial fold (laugh lines), crow's feet, as well as the number, area, and volume of forehead lines, were assessed at various time points after oral administration of DailyGlow® CTP.
Periocular Wrinkles: In subjects from the sample group, as shown in Figure 1a and 1b, after 14 days of oral administration of DailyGlow® CTP, there was a significant reduction of 9.95% in periocular wrinkle area (p<0.001).and a significant decrease of 9.91% in the percentage of periocular wrinkle area (p=0.003). After 28 days, a noteworthy reduction of 13.36% was observed in periocular wrinkle area (p<0.001), along with a significant decrease of 14.06% in the percentage of periocular wrinkle area (p=0.002). Within 28 days, there were no significant changes observed in periocular wrinkles in the control group subjects. From image analysis, it is evident that there is a noticeable reduction in periocular wrinkles in subjects after 14 days of consuming the sample (Figure 1c). The effect becomes even more pronounced after 4 weeks (Figure 1a-1d).

Figure 1: Changes in periocular wrinkles at Day 0, 14 and 28 of DailyGlow® CTP supplementation. (a)Periocular wrinkle area change rate, (b)Periocular wrinkle area percentage change rate, (c)Image analysis of the periocular wrinkles of a subject.
Nasolabial Folds: The analysis of nasolabial folds indicates that, in subjects from the sample group, after 14 days of oral administration of the product, there was a significant decrease of 28.00% in the number of nasolabial folds (Figure 2a), a reduction of 33.19% in nasolabial fold area (p=0.035) (Figure 2b), and a 26.41% decrease in nasolabial fold volume (p=0.001) (Figure 2c). After 28 days of oral administration, there was a remarkable reduction of 62.00% in the number of nasolabial folds (p=0.006) (Figure 2a), a 64.00% decrease in nasolabial fold area (p<0.001) (Figure 2b), and a 38.45% decrease in nasolabial fold volume (p=0.002) (Figure 2c). Similarly, there were no significant changes in nasolabial folds within 28 days for subjects in the control group (Figure 2a, 2b, 2c). Image analysis also shows that as subjects orally consume CTPs, there is a noticeable lightening of nasolabial folds (Figure 2a-2d).

Figure 2: Changes in nasolabial folds at Day 0, 14 and 28 of DailyGlow® CTP supplementation. (a)Nasolabial fold wrinkle count change rate, (b)Nasolabial fold wrinkle area change rate, (c)Nasolabial fold wrinkle volume change rate, (d)Image analysis of the nasolabial folds of a subject.
Crow's Feet: The analysis of crow's feet indicates that, in subjects from the sample group, after 14 days of oral administration of the product, there was a reduction of 20.79% in the number of crow's feet (Figure 3a), a decrease of 15.99% in crow's feet area (Figure 3b), and an 8.57% decrease in crow's feet volume (Figure 3c). After 28 days of oral administration, there was a significant reduction of 27.72% in the number of crow's feet (p=0.026) (Figure 3a), a 23.65% decrease in crow's feet area (Figure 3b), and a 13.84% decrease in crow's feet volume (Figure 3c). No significant changes were noted in crow's feet within 28 days for control group subjects (Figure 3a, 3b, 3c). Image analysis indicates that there is a significant improvement in crow's feet within 14 days of oral administration of CTPs (Figure 3a-3d).
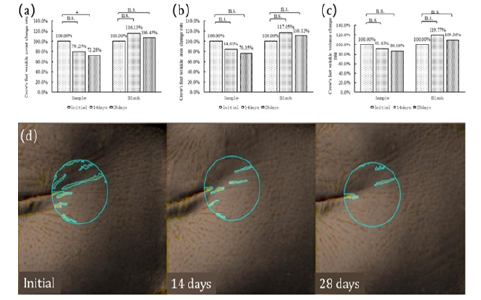
Figure 3: Changes in crow’s feet at Day 0, 14 and 28 of DailyGlow® CTP supplementation. (a)Crow’s feet wrinkle count change rate, (b)Crow’s feet wrinkle area change rate, (c)Crow’s feet wrinkle volume change rate, (d)Image analysis of the crow’s feet of a subject.
Forehead Wrinkles: The analysis of forehead wrinkles indicates that, in subjects from the sample group, after 14 days of oral administration of the product, there was a significant reduction of 29.21% in the number of forehead wrinkles (p=0.01) (Figure 4a), an 18.18% decrease in forehead wrinkles area (Figure 4b), and a 19.99% decrease in forehead wrinkles volume (Figure 4c). After 28 days of oral administration, there was a substantial reduction of 48.31% in the number of forehead wrinkles (p<0.001) (Figure 4a), a 36.14% decrease in forehead wrinkles area (p=0.004) (Figure 4b), and a 45.28% decrease in forehead wrinkles volume (p=0.001) (Figure 4c). Likewise, there were no significant changes observed in forehead wrinkles within 28 days for subjects in the control group (Figure 4a, 4b, 4c). Image analysis also indicates as the testing progresses, there is a noticeable lightening of forehead wrinkles in the subjects (Figure 4a-4d).
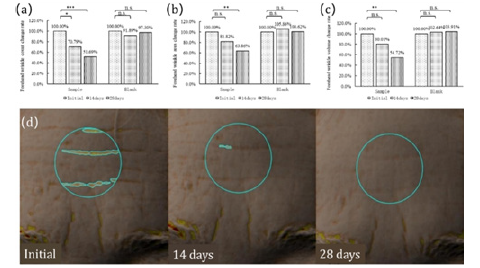
Figure 4: Changes in forehead wrinkles at Day 0, 14 and 28 of DailyGlow® CTP supplementation. (a)Forehead wrinkle count change rate, (b)Forehead wrinkle area change rate, (c)Forehead wrinkle volume change rate, (d)Image analysis of the forehead wrinkles of a subject.
Moisturizing Effects
Subjects from the sample group and the control group were tested for skin stratum corneum hydration and transepidermal water loss at various times after oral administration of DailyGlow® CTP. The results indicate that, in subjects from the sample group, after 14 days of oral administration of the product, there was a significant increase of 7.25% in skin stratum corneum hydration (p=0.002) (Figure 5a). After 28 days, the hydration further increased by 9.66%, and the difference was statistically significant (p<0.001) (Figure 5a). In terms of trans epidermal water loss, there was a non-significant reduction of 2.82% after 14 days (p=0.335), but after 28 days, there was a significant decrease of 21.12% (p<0.001) (Figure 5b). No significant changes were observed in skin stratum corneum hydration and transepidermal water loss in subjects from the control group during the testing period (Figure 5a, 5b).

Figure 5: Changes in stratum corneum hydration change rate (a)and transepidermal water loss change rate, (b)at Day 0, 14 and 28 of DailyGlow® CTP supplementation.
Elasticity and Firmness Effects
Subjects from both the sample group and the control group underwent tests for skin elasticity R2 and firmness F4. The results indicate that, in subjects from the sample group, after 14 days of oral administration of the product, there was a significant increase of 10.40% in skin elasticity R2 (p<0.001) (Figure 6a). After 28 days, the skin elasticity R2 further increased by 16.77% (p<0.001) (Figure 6a). In terms of skin firmness F4, there was a significant improvement of 22.29% after 14 days (p<0.001), after 28 days, the improvement reached 30.83%, and the difference was statistically significant (p<0.001) (Figure 6b). No significant changes were observed in skin elasticity R2 and skin firmness F4 in subjects from the control group during the testing period (Figure 6a, 6b).

Figure 6: Changes in skin elasticity (R2) change rate (a)and skin firmness (F4) change rate, (b)at Day 0, 14 and 28 of DailyGlow® CTP supplementation.
Whitening Effects
Subjects from both the sample group and the control group were tested for facial skin brightness (L value), yellowness (B value), and glossiness (ITA). In subjects from the sample group, after 14 days of oral administration of Daily Glow® CTP, there was an increase of 0.52% in facial skin brightness (L value) (Figure 7a), a decrease of 1.52% in yellowness (B value) (Figure 7b), and an increase of 1.76% in glossiness (ITA) (Figure 7c). After 28 days, the facial skin brightness (L value) increased by 2.62%, and the difference was statistically significant (p<0.001) (Figure 7a). Yellowness (B value) decreased by 2.23%, but the difference was not statistically significant (p=0.202) (Figure 7b). Glossiness (ITA) increased by 7.50%, and the difference was statistically significant (p<0.001) (Figure 7c). No significant changes were observed in facial skin brightness (L value), yellowness (B value), and glossiness (ITA) in subjects from the control group during the testing period (Figure 7a, 7b, 7c). From the images, it is evident that there is an enhancement in facial skin glossiness in the subjects (Figure 7d).
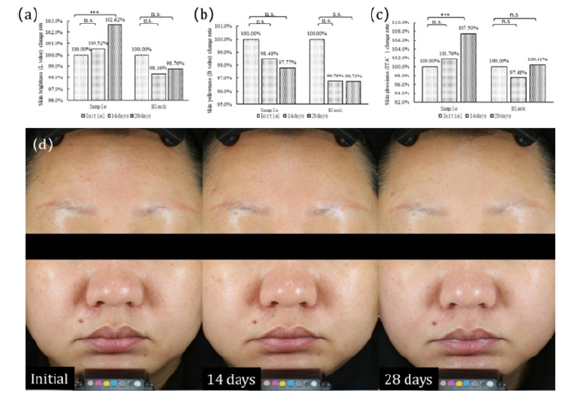
Figure 7: Changes in facial skin whitening at Day 0, 14 and 28 of DailyGlow® CTP supplementation. (a)Skin brightness (L value) change rate, (b)Skin yellowness (B value) change rate, (c)Skin glossiness (ITA°) change rate, (d)Image analysis of the facial skin whitening of a subject.
Anti-Carbonylation and Anti-Glycation Effects
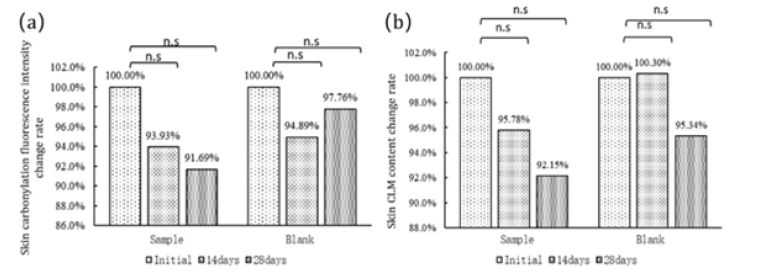
Five subjects from the sample group and five subjects from the control group were tested at various time points after oral administration of the product for the fluorescence intensity of D-squame films on facial stratum corneum samples and the content of CML (carboxymethyl lysine) in facial skin. The results indicate that, in subjects from the sample group, after 14 days of oral administration of Daily Glow® CTP, there was a non-significant reduction of 6.07% in skin carbonylation (p=0.488) (Figure 8a). After 28 days, the reduction in skin carbonylation increased to 8.31%, but the difference was still not statistically significant (p=0.365) (Figure 8a). In terms of skin CML (carboxymethyl lysine) content, there was a non-significant reduction of 4.22% after 14 days (p=0.464), and after 28 days, the reduction increased to 7.85%, with no statistically significant difference (p=0.368) (Figure 8b).
Discussion
DailyGlow® CTP is derived from the enzymatic hydrolysis of Hainan tilapia, which is known for its fibril-forming ability, biodegradability, and high purity. Tilapia fish collagen is ideal for wound healing and skin repair [12-14]. Daily Glow® CTP, with a molecular weight of 250-500 Da, features a short linear structure. It contains crucial CTPs G-X-Y (≥40%), G-P-X (≥20%), and G-P-H (≥3.5%). These tripeptides, including G-X-Y, G-P-X, and G-P-H, are essential for collagen's triple-helical structure. This structure provides structural integrity to connective tissues by forming fibrils and fibers [15]. Peptides enhance thermal stability and contribute to collagen's role in supporting tissue integrity [16], and are crucial for collagen's unique structure, vital for providing support to various tissues in the body [17]. The findings of this study reveal a significant improvement in various facial wrinkles following four weeks of oral administration of Daily Glow® CTP. CTP exerts its wrinkle-reducing effects through diverse mechanisms. Existing research suggests its capacity to enhance skin elasticity, density, and barrier function, thereby mitigating skin wrinkling [18,19]. Additionally, anti-inflammatory effects observed in CTP contribute to its efficacy in reducing wrinkles [19]. Moreover, the oral application of collagen-building peptides, including CTP, has demonstrated increased collagen production, providing both immediate and long-term benefits in diminishing facial lines and wrinkles [20].
CTP is integral to skin hydration, interacting with Aquaporin-3 (AQP3) water channels crucial for moisturization. AQP3, prevalent in the basal and spinous layers of the epidermis, regulates intracellular and intercellular water flow [21]. CTP enhances skin hydration, expediting collagen repair and minimizing tissue damage [22]. It regulates AQP3 levels, essential for skin aquation, and contributes to intracellular and intercellular water flow, maintaining skin elasticity and aiding wound healing [22]. Additionally, CTP facilitates glycerol transport within the epidermis, impacting epidermal hydration [23]. CTP exhibits a multifaceted impact on skin health and aging-related gene expressions, elucidating molecular mechanisms that influence diverse aspects of skin physiology. Although specific insights into the effects of CTP on LOX, FBN2, and MMP1 are not explicitly addressed in current literature, a compilation of findings sheds light on its interactions with other genes and proteins. Collagen peptides, through SMAD and AP-1 dependent signaling pathways, stimulate the synthesis of various proteins, emphasizing their regulatory role in cellular processes [24]. Additionally, their interaction with redox signaling pathways, crucial for the profibrotic effects of TGF-β3, underscores their involvement in cellular responses and skin health [24]. CTP further demonstrates inhibition of Matrix Metalloproteinases (MMPs), including collagenase and gelatinase, essential for mitigating collagen degradation in the extracellular matrix [6]. Moreover, CTP's upregulation of collagen genes via the p38 Mitogen-Activated Protein Kinase (MAPK)/SKN-1 pathway suggests potential contributions to increased lifespan and enhanced skin health [25]. Furthermore, its ability to restore gene expression, suppressed by oxidative stress in human aortic endothelial cells, highlights its potential in mitigating reactive oxygen species-induced transcriptional repression [23].
In our study, an intriguing observation emerged as oral administration of Daily Glow® CTP for four weeks significantly improved skin whitening effects in participants, contrasting with the non-significant reduction in results from the anti-carbonylation and anti-glycation test. This contrasts with the outcomes of a topical application study by [18], which showed notable reduced accumulation of AGEs after four weeks. The disparity in anti-glycation effects between oral and topical CTP administration suggests divergent mechanisms of action. Oral CTP may predominantly contribute to skin collagen synthesis and the regulation of associated genes and pathways, rather than exerting antioxidant and anti-glycation effects. Nevertheless, effective combinations of CTP with other functional substances can enhance its anti-glycation efficacy. For instance, research has proposed that carnosine, a naturally occurring dipeptide, can inhibit protein carbonyl group formation and reduce sugar-induced crosslinking, resulting in decreased levels of AGEs in the skin [26].
Conclusion
In this 28-day clinical study involving 31 participants, our investigation into the effects of an oral CTP Daily Glow® revealed significant skincare benefits. Notably, Daily Glow® CTP exhibited potent anti-wrinkle properties, with visible improvements in periorbital wrinkles, nasolabial folds, crow's feet, and forehead lines after both 14 and 28 days of consumption. Concurrently, the product demonstrated remarkable moisturizing capabilities, enhancing skin hydration, and reducing trans epidermal water loss. Daily Glow® CTP also proved effective in promoting skin firmness and elasticity, indicating potential contributions to skin tightening. Our findings underscore the positive impact of the CTP solid beverage on skin health, encompassing anti-wrinkle, moisturizing, and firming effects. While further research is warranted, these results contribute to the evolving understanding of CTPs as promising components in skincare regimens.
Acknowledgements
The authors wish to acknowledge Shanghai Huiwen Biotechnology Co., Ltd for the clinical experimentation.
Conflict of Interest
None.
References
- (2023) Analytic Global Beauty Ingestible Market Report.
- Al Atif H (2022) Collagen Supplements for Aging and Wrinkles: A Paradigm Shift in the Fields of Dermatology and Cosmetics. Dermatol Pract Concept 12(1): e2022018.
- Polonini H, E Dijkers, A O Ferreira (2021) Beauty from within: A Review of the Science behind YulivTM Collagen Drink: An Anti-Aging Nutraceutical. Journal of Cosmetics Dermatological Sciences and Applications 11(3): 263-278.
- (2023) Nutritioninsight. Trends in collagen type II launches for skin and joint well-being.
- Lee M, Eunjoung Kim, Hyunwoo Ahn, Seokjun Son, Hyunjun Lee (2023) Oral intake of collagen peptide NS improves hydration, elasticity, desquamation, and wrinkling in human skin: a randomized, double-blinded, placebo-controlled study. Food Funct 14(7): 3196-3207.
- Pyun HB, Minji Kim, Jieun Park, Yasuo Sakai, Noriaki Numata, et al. (2012) Effects of Collagen Tripeptide Supplement on Photoaging and Epidermal Skin Barrier in UVB-exposed Hairless Mice. Prev Nutr Food Sci 17(4): 245-53.
- Tomosugi N, Shoko Yamamoto, Masayoshi Takeuchi, Hideto Yonekura, Yasuhito Ishigaki, et al. (2017) Effect of Collagen Tripeptide on Atherosclerosis in Healthy Humans. J Atheroscler Thromb 24(5): 530-538.
- Tak YJ, Dae Keun Shin, Ae Hyang Kim, Jun Il Kim, Ye Li Lee, et al. (2021) Effect of Collagen Tripeptide and Adjusting for Climate Change on Skin Hydration in Middle-Aged Women: A Randomized, Double-Blind, Placebo-Controlled Trial. Front Med (Lausanne) 7: 608903.
- Lee YI, Sang Gyu Lee, Inhee Jung, Jangmi Suk, Mun Hoe Lee, et al. (2022) Effect of a Topical Collagen Tripeptide on Antiaging and Inhibition of Glycation of the Skin: A Pilot Study. Int J Mol Sci 23(3): 1101.
- Messaraa C, A Metois, M Walsh, S Hurley, L Doyle, et al. (2018) Wrinkle and roughness measurement by the Antera 3D and its application for evaluation of cosmetic products. Skin Res Technol 24(3): 359-366.
- Gardien KL, Dominique C Baas, Henrica C W de Vet, Esther Middelkoop (2016) Transepidermal water loss measured with the Tewameter TM300 in burn scars. Burns 42(7): 1455-1462.
- Hu Z, Ping Yang, Chunxia Zhou, Sidong Li, Pengzhi Hong (2017) Marine Collagen Peptides from the Skin of Nile Tilapia (Oreochromis niloticus): Characterization and Wound Healing Evaluation. Mar Drugs 15(4): 102.
- Lv K, Lei Wang, Xiaoli He, Wenjun Li, Lei Han, et al. (2021) Application of Tilapia Skin Acellular Dermal Matrix to Induce Acute Skin Wound Repair in Rats. Front Bioeng Biotechnol 9: 792344.
- Li D, Wendell Q Sun, Tong Wang, Yonglin Gao, Jinglei Wu, et al. (2021) Evaluation of a novel tilapia-skin acellular dermis matrix rationally processed for enhanced wound healing. Mater Sci Eng C Mater Biol Appl 127: 112202.
- Koide T (2007) Designed triple-helical peptides as tools for collagen biochemistry and matrix engineering. Philos Trans R Soc Lond B Biol Sci 362(1484): 1281-1291.
- Shoulders MD, RT Raines (2009) Collagen structure and stability. Annu Rev Biochem 78: 929-58.
- Karsdal M (2023) Biochemistry of collagens, laminins and elastin: structure, function and biomarkers.
- Lee YI, Sang Gyu Lee, Inhee Jung, Jangmi Suk, Mun Hoe Lee, et al. (2022) Effect of a Topical Collagen Tripeptide on Antiaging and Inhibition of Glycation of the Skin: A Pilot Study. Int J Mol Sci 23(3): 1101.
- Pyun HB, Minji Kim, Jieun Park, Yasuo Sakai, Noriaki Numata, et al. (2012) Effects of collagen tripeptide supplement on photoaging and epidermal skin barrier in UVB-exposed hairless mice. Prev Nutr Food Sci 17(4): 245-253.
- Trookman NS, Ronald L Rizer, Rosanne Ford, Elizabeth Ho, Vincent Gotz, et al. (2009) Immediate and Long-term Clinical Benefits of a Topical Treatment for Facial Lines and Wrinkles. J Clin Aesthet Dermatol 2(3): 38-43.
- Qin H, Xiangjian Zheng, Xiaofeng Zhong, Anita K Shetty, Peter M Elias, et al. (2011) Aquaporin-3 in keratinocytes and skin: its role and interaction with phospholipase D2. Arch Biochem Biophys 508(2): 138-43.
- Harris M, Johan Potgieter, Kashif Ishfaq, Muhammad Shahzad (2021) Developments for collagen hydrolysate in biological, biochemical, and biomedical domains: A comprehensive review. Materials (Basel) 14(11): 2806.
- Saito Takatsuji H, Yasuo Yoshitomi, Yasuhito Ishigaki, Shoko Yamamoto, Noriaki Numata, et al. (2021) Protective Effects of Collagen Tripeptides in Human Aortic Endothelial Cells by Restoring ROS-Induced Transcriptional Repression. Nutrients 13(7): 2226.
- Edgar S, Blake Hopley, Licia Genovese, Sara Sibilla, David Laight, et al. (2018) Effects of collagen-derived bioactive peptides and natural antioxidant compounds on proliferation and matrix protein synthesis by cultured normal human dermal fibroblasts. Scientific Reports 8(1): 10474.
- Morikiri Y, E Matsuta, H Inoue (2018) The collagen-derived compound collagen tripeptide induces collagen expression and extends lifespan via a conserved p38 mitogen-activated protein kinase cascade. Biochem Biophys Res Commun 505(4): 1168-1173.
- Zheng W, Huijuan Li, Yuyo Go, Xi Hui Chan, Qing Huang, et al. (2022) Research Advances on the Damage Mechanism of Skin Glycation and Related Inhibitors. Nutrients 14(21): 4588.




 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.