Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Prognostic Factors Influencing the Survival of Patients with Metastatic Prostate Cancer in The Moroccan Population: Retrospective Study Of 553 Cases
*Corresponding author: Khadija Hinaje, Department of Medical Oncology, Hassan II University Hospital, Faculty of Medicine, Pharmacy and Dental Medicine of Fez, University Sidi Mohamed Ben Abdellah, Fez, Morocco.
Received: March 31, 2024; Published: April 18, 2024
DOI: 10.34297/AJBSR.2024.22.002932
Abstract
Introduction: Prostate cancer is the most common urological cancer. Despite the therapeutic advances, its prognosis is very reserved. The aim of our study is to determine the different prognostic factors of patients with metastatic prostate cancer and their correlation with patient survival.
Materials and Methods: A retrospective study was conducted at the medical oncology department of Hassan II University Hospital in Fez, collecting 553 patients during a period of 10 years, from January 2014 to December 2023. The statistical analysis of the results was done by the software SPSS version 23, the survival was calculated by the Kaplan-Meier method. The Cox method was used to study prognostic factors.
Results: The average age of our patients was 72 years with a standard deviation of 8.9 years. In multivariate analysis, we found a significant correlation between the deterioration of survival and an age greater than 75 years (p<0.001), a general degraded state or PS >2 (p=0.046), an experiencing bone pain (p=0.039), a PSA >100 ng/ml (p=0.045), a Gleason score >6 (p<0.001), more than six bone metastases (p=0.041), and the presence of bone metastases associated with visceral metastases (p< 0.001). In addition, no significant association was found in patients with comorbidities or toxic habits or presenting a biological abnormality.
Conclusion: We propose to validate all the factors identified by retrospective studies by prospective studies in order to guarantee the best chances of survival for patients.
Keywords: Metastatic prostate cancer, Prognostic, Survival
Introduction
Prostate cancer is considered the 4th most common cancer in both sexes and the 2nd overall in men, after lung cancer. The highest incidence is observed in North America, with an incidence rate of 73.5 per 100,000, followed by Australia/New Zealand and Western Europe with incidence rates of 71.9 and 59.9 per 100,000 respectively [1]. In Morocco, prostate cancer is ranked 2th among cancers, accounting for 16.1% of incident cases of all cancers in men, which is 4,935 new cases [2]. It’s representing the 2nd leading cause of cancer mortality, with a rate of 59.6% per 100,000 habitants, accounting for 2030 cases according to The Global Cancer Observatory Morocco 2022[3]. Regarding the incidence and mortality of this cancer, there has been a significant increase in new cases over the years, especially advanced forms in developing countries, which makes the prognosis poor.
Prognostic factors are defined as things that can predict a patient’s prognosis before treatment is started. Several publications have been made in this direction, taking these factors into account is of great importance, more particularly in the choice of treatment. In this article, we will study these different factors and their influence on survival.
Materials and Methods
A descriptive retrospective study was carried out in the medical oncology department of the Hassan II University Hospital in Fez, collecting 553 patients with metastatic prostate cancer over a period of 10 years, from January 2014 to December 2023. The inclusion criteria were patients with histologically proven prostate adenocarcinoma, classified as stage 4. The study excluded patients with: incomplete or unusable medical records, non-metastatic prostate cancer (localized or locally advanced), other associated cancer, and histological types other than adenocarcinoma such as sarcoma, lymphoma or others. The data entry was performed using Excel, and the analysis was conducted using SPSS version 23 software. The data analysis employed descriptive analysis, which involved calculating percentages for qualitative variables and measures of central tendency (mean, median) and dispersion (standard deviation, minimum, maximum) for quantitative variables. Analytical study was based on statistical tests comparing frequencies or means, namely the Chi-square test and Fisher's exact test. Overall survival and progression-free survival were calculated using the Kaplan-Meier method. The log-rank test was used to examine the significance of survival differences between group distributions. Analysis of various independent prognostic factors was conducted through univariate and multivariate analyses using the Cox proportional hazards model. In general, a p-value <0.05 was considered statistically significant for all analyses.
Results
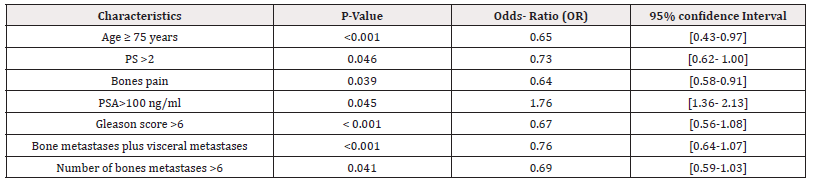
Between January 2014 and December 2023, we included 553 patients with metastatic prostate cancer in the medical oncology department of CHU Hassan II in Fez. The average age of our patients was 72 years with a standard deviation of 8.9 years. The percentage of patients with an age greater than or equal to 70 years was 36%. The most common antecedents were active smoking (28.2%), followed by hypertension (22.2%), and then diabetes (16.8%). The most common symptoms were urinary signs suggestive of obstruction (38.3%), followed by irritative signs (21.4%). Other extra-urinary signs related to metastatic spread were described, mainly bone pain (18.2%) notably in the spine (36%) and pelvis (34%), as well as neurological signs (7.3%). 67.5% of patients were in good general condition (Performance Status (PS) 0 to 1) at the time of diagnosis. PSA testing was performed on all patients, with levels ranging between 10 and 2000 ng/ml, with an average of 189.5 ng/ml. All our patients had histological type adenocarcinoma. The predominant Gleason score was 7 (36.4%), followed by a score of 8 (31.8%). According to the TNM classification, 27.1% of patients were classified as T4 and lymph node invasion N1 was found in 38.1%. All our patients were metastatic, with 71% had de novo metastases, also known as synchronous, and 29% had metachronous metastases. 54.7% of patients had isolated bone metastases, while 45.3% had associated visceral metastases, of which 26% were hepatic and 13.8% were pulmonary. Regarding the number of bone metastases, 57% of patients had more than six bone metastases; while 43% had less than six. All patients performed a biological workup including a count blood, an assay of the level of albumin, calcium level, Alkaline Phosphatase (ALP), testosterone, liver and kidney function. This assessment had demonstrated anemia in 29.8% of patients, hyperleukocytosis in 15.9% of cases, thrombocytosis in 9.8% of cases, hypoalbuminemia in 26% of cases, hypercalcemia in 25% of cases, an increase in the level of ALP in 34.3% of cases, liver function impairment was observed in 11% of cases, predominantly cytolytic, renal insufficiency, primarily obstructive, was found in 20% of cases. All our patients underwent castration, with 17.4% of cases being surgical and 82.6% being medical using LH-RH analogues. At the hormone-naive stage, 31.9% received castration alone, 9.1% received abiraterone acetate plus prednisone in addition to castration, while 59% received docetaxel-based chemotherapy plus prednisone alongside castration. The median Progression-Free Survival (PFS) was 14 months (95% CI: [13.7; 14.2]), 32.6 months (95% CI: [30.5; 41.6]), and 20 months (95% CI: [19.8; 20.18]) respectively. 74% of these patients became resistant to castration. The median Overall Survival (OS) was 54 months (Figure 1). In multivariate analysis, we found a significant correlation between the deterioration of survival and an age greater than 75 years (p< 0.001), a deteriorated general condition PS >2 (p=0.046), experiencing bone pain (p=0.039), PSA>100 ng/ml (p=0.045), a Gleason score >6 (p<0.001), more than six bone metastases (p=0.041), and the presence of bone metastases associated with visceral metastases (p<0.001). Furthermore, no significant association was found in patients with comorbidities or toxic habits or presenting a biological abnormality (Table 2 and Table 3).
Discussion
Prostate cancer is considered one of the leading causes of cancer-related deaths. According to the most recent epidemiological studies, it ranks as the 5th cause of mortality worldwide after lung cancer, liver cancer, and digestive system cancers, including stomach, colon, and rectum cancers. In 2022, it was noted that the mortality rate related to prostate cancer was 7.3% per 100,000 inhabitants, accounting for 397,430 cases [4]. Many studies have been conducted to identify various clinical, pathological, radiographic and biochemical prognostic factors that can influence survival in these patients. Age has been mentioned as a prognostic indicator [5]. It has been concluded that, generally, younger patients have a more aggressive tumor and therefore a poorer prognosis than older patients [6]. However, this has not been confirmed by the results of other studies [7-9]. In our series, older age ≥ 75 years was a significant prognostic factor (p<0.001), and these patients had a higher risk of shorter survival compared to those under 75 years old. Another clinical parameter is the functional status, mainly categorized by the Karnofsky performance status. De Voogt and al have shown that this is the most important prognostic indicator, and an increasing number of investigators have confirmed this finding [10]. A poor performance status is generally associated with shorter survival [10-11]. This parameter is related to the advanced stage of the disease and it is useful in therapeutic decision-making. The performance status as a prognostic factor may be more important when used in combination with the pain score [11]. These various studies are consistent with our results; a performance status >2 was a significant prognostic factor (p= 0.046). Bone pain is a commonly described clinical sign in patients with metastatic prostate cancer. Multiple studies show that pain is an important predictive factor in men with metastatic prostate cancer. However, the presence of pain has not been integrated into prognostic models, and few studies have evaluated it in subgroup analysis [12]. For example, in an analysis of 85 patients with CRPC, Berry et al. identified severe bone pain as predictive of shorter overall survival [5]. Due to the limited sample size, the analysis was based on a univariate model. More recently, using data from the TAX 327 trial, Armstrong et al. identified pain as a statistically significant prognostic factor for overall survival. The adjusted hazard ratio for men who had pain at baseline was 1.48 (95% CI, 1.23 to 1.79) and was among the strongest predictors in the multivariate model [13]. The results of our study align with those found in the literature since the presence of bone pain was a statistically significant prognostic factor (p=0.039) correlated with a reduction in survival rates.
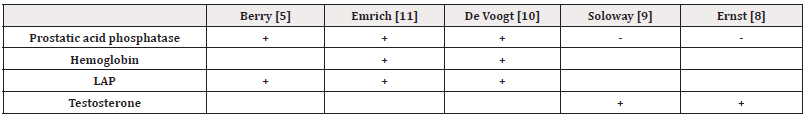
PSA is important for diagnosing, staging, and monitoring prostate cancer [14,15]. However, there are divergent opinions in the literature regarding its evaluation as a prognostic factor. Killian, et al. assert that overall survival is shorter in patients with a high PSA level [16]. Similarly, Zagars, et al. mention that PSA is an independent prognostic indicator, outside of stage and grade [17]. According to Schubert, et al. PSA can be useful for monitoring the patient's response to treatment and for postoperative monitoring of tumor residues and relapses [18]. Cooper et al. showed that the prognosis is good in patients with a very low PSA level (<10 ng/ml) six months after the start of hormonal treatment, but they used this factor in combination with bone scintigraphy (<15 lesions) to achieve significant results [19]. Conversely, Kelly et al. argue that the PSA value before treatment is not prognostically significant [20]. In our study, it was found that the PSA value before treatment was statistically significant in determining the overall survival of patients. We noticed that a PSA level >100 ng/ml significantly influenced this survival (p=0.045). According to this result, in accordance with literature, use of PSA for assessing the reaction of the patients with metastatic prostate cancer to the treatment is more appropriate. Other biological parameters are less studied. Soloway et al. described the value of pre-treatment testosterone levels. A low testosterone level before treatment initiation was associated with shorter survival. In a multivariate analysis, this factor was used in combination with functional status and the number of bone lesions to obtain significant prognostic groups [9]. Other investigations have also shown that patients with low pre-treatment testosterone levels were less responsive to androgen deprivation [8]. We summarize the most frequently used prognostic parameters in disseminated prostate cancer (Table 4).
The Gleason score is identified as a major prognostic factor in several studies. Fijikawa, et al. revealed that the Gleason score was a good prognostic factor in their study involving 195 prostate cancer patients [21]. Epstein et al. explained that the Gleason score was the best prognostic factor in prostate cancer [22]. In our study as well, it was found that the Gleason score was an important prognostic factor. In the group with a Gleason score of 6 or higher, it was significant that the survival rate was reduced in these patients (p<0.001). According to the study conducted by Soloway, et al. the overall survival is shorter for patients with bone metastases with more than 6 foci compared to patients with bone metastases with fewer foci [9]. Ernst, et al. found a survival rate of 75% for the group with fewer than 6 metastases and 55% for the group with more than 6 metastases [8]. They concluded that the most important prognostic factor was the extent of disease on bone scan. In our study, it was observed that the number of bone metastases was a prognostic factor consistent with the literature. The survival rate was 90% for patients with 6 or fewer metastases and 58% for patients with more than 6 metastases.
Guijarro, et al. found that patients with visceral involvement had a poorer prognosis compared to those with exclusive nodal metastases, exclusive bone metastases, or nodal plus bone metastases, which was consistent with Mazzone's results [23, 24]. Additionally, Mazzone, et al. also suggested that the prognosis of patients with bone-only involvement was inferior to those with nodal-only involvement [24]. Similarly, Gandaglia, et al. showed that overall and prostate cancer-specific survival of patients with nodal metastases was higher than those with bone metastases, which was higher than those with visceral metastases [25]. However, they did not study the impact of different visceral metastatic sites on survival. On the other hand, N Xu, et al. reported the impact of specific visceral metastatic sites on overall and prostate cancer-specific survival in metastatic prostate cancer. In this study, lung-only metastases had the best overall survival outcomes compared to liver-only metastases and brain-only metastases. Brain-only metastases had the worst overall survival outcomes compared to liver-only metastases and lung-only metastases [26]. In Guijarro's study visceral metastases with corresponding bone involvement conferred poorer survival outcomes compared to visceral metastases alone, which was consistent with Mazzone's study [23,24]. These results also agree with those of our series, where the combination of visceral metastases plus bone involvement was significantly associated with reduced survival (p<0.001).
Conclusion
Through this retrospective study, the presence of these following factors (age ≥ 75 years, PS > 2, bone pain, PSA >100ng/ml, Gleason score >6; bones metastases > 6, association of bone metastases and visceral metastases) were associated with a poor prognosis, these factors must be taken into consideration to define optimal management and guide the clinician in the choice of the best therapeutic protocol. We propose as perspectives to validate all the factors identified in retrospective studies by prospective work in order to guarantee the patient the best chances of survival.
Acknowledgements
None.
Conflicts of Interest
None.
References
- (2022) Cancer TODAY, Incidence in the world 2022.
- (2022) Cancer TODAY, Incidence in MOROCCO 2022.
- (2022) Cancer TODAY, Mortality in MOROCCO 2022.
- (2022) Cancer TODAY, Mortality in the world 2022.
- Berry W R, Laszlo J, Cox E, A Walker, D Paulson (1979) Prognostic factors in metastatic and hormonally unresponsive carcinoma of the prostate. Cancer 44(2): 763-765.
- Wilson JM, Kemp IW, Stein GJ (1984) Cancer of the prostate. Do younger men have a poorer survival rate. Br J Urol 56(4): 391-396.
- Harrison GSM (1983) The prognosis of prostatic cancer in the younger man. Br J Urol 55(3): 315-320.
- Ernst DS, Hanson J, Venner PM (1991) Uro-oncology group of northern Alberta: Analysis of prognostic factors in men with metastatic prostate cancer. J Urol 146(2): 372-376.
- Soloway MS (1990) The importance of prognostic factors in advanced prostate cancer. Cancer 66(5 Suppl): 1017-1021.
- De Voogt HJ, Suciu S, Sylvester R, Pavone Macaluso M, Smith PH, et al. (1989) Multivariate analysis of prognostic factors in patients with advanced prostatic cancer. Results from 2 European Organization for research on treatment of Cancer trials. J Urol 141(4): 883-888.
- Emrich LJ, Priore RL, Murphy GP, Brady MF (1985) Prognostic factors in patients with advanced stage prostate cancer. Cancer Res 45(10): 5173-5179.
- Berthold DR, Pond G, De Wit R, Freidele Soban, Mario Eisenberger, et al. (2006) Association of pain and quality of life (QOL) response with PSA response and survival of patients (pts) with metastatic hormone refractory prostate cancer (mHRPC) treated with docetaxel or mitoxantrone in the TAX-327 study. J Clin Oncol 24: 221s (suppl, abstr 4516).
- Armstrong A, Garrett Mayer ES, Ou Yang YC, Ronald de Wit, Ian F Tannock, et al. (2007) A contemporary prognostic nomogram for men with hormone-refractory metastatic prostate cancer: A TAX 327 study analysis. Clin Cancer Res 13(21): 6396-6403.
- Kuriyama M, Wang MC, Lee CI, Papsidero LD, Killian CS, et al. (1981) Use of human prostate specific antigen in monitoring prostate cancer. Cancer Res 41(10): 3874-3876.
- Polascik TJ, Oesterling JE, Partin AW (1999) Prostate specific antigen: A decade of discovery-what we have learned and where we are going. J Urol 162(2): 293-306.
- Killian CS, Yang N, Emrich LJ, Vargas FP, Kuriyama M, et al. (1985) Prognostic importance of prostate specific antigen for monitoring patients with stage B2 to D1 prostate cancer. Cancer Res 45(2): 886-891.
- Zagars GK, Pollack A, Kavadi VS, A C von Eschenbach (1995) Prostate-specific antigen and radiation therapy for clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 32(2): 293-306.
- Schubert J, Kowalik S (1994) The value of prognostic factors in prostatic cancer. Int Urol Nephrol 26(5): 541-547.
- Cooper EH, Armitage TG, Robinson MRH, Newling DW, Richards BR, et al. (1990) Prostatic specific antigen and the prediction of prognosis in metastatic prostatic cancer. Cancer 66(5 Suppl): 1025-1028.
- Kelly WK, Scher HI, Mazumdar M, V Vlamis, M Schwartz, et al. (1993) Prostate specific antigen as a measure of disease outcome in metastatic hormone refractory prostate cancer. J Clin Oncol 11(4): 607-615.
- Fijikawa K, Sasaki M, Arai Y, H Yamabe, O Ogawa, et al. (1997) Prognostic criteria in patients with prostate cancer: Gleason Score vs. volume weighted mean nuclear volume. Clin Cancer Res 3(4): 613-618.
- Epstein JI, Allsbrook WC, Amin MB, Lars L Egevad (2005) The 2005 International Society of Urological Pathology (ISUP). Consensus on Gleason grading of prostatic carcinoma. Am J Surg Pathol 29(9): 1228-1242.
- Guijarro A, Hernandez V, De la Morena JM, Jiménez Valladolid I, Pérez Fernández E, et al. (2017) Influence of the location and number of metastases in the survival of metastatic prostatic cancer patients. Actas Urol Esp 41(4): 226-233.
- Mazzone E, Preisser F, Nazzani S, Zhe Tian, Marco Bandini, et al. (2018) Location of Metastases in Contemporary Prostate Cancer Patients Affects Cancer-Specific Mortality. Clin Genitourin Cancer 16(5): 376-384.e1.
- Gandaglia G, Karakiewicz PI, Briganti A, Niccolò Maria Passoni, Jonas Schiffmann, et al. (2015) Impact of the Site of Metastases on Survival in Patients with Metastatic Prostate Cancer. Eur Urol 68(2): 325-334.
- N XU, YP Wu, ZB Ke, XD L, Ying Chun Liang, et al. (2019) Risk factors of developing visceral metastases at diagnosis in prostate cancer patients Transl Cancer Res 8(3): 928-938.






 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.