Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Prognostic Factors of Metastatic renal cell carcinoma: analysis of 95 patients
*Corresponding author: Oumaima Talbi, Department of medical oncology, Hassan II University Hospital, University Sidi Mohamed Ben Abdellah, Fez, Morocco.
Received: April 27, 2024; Published: May 1, 2024
DOI: 10.34297/AJBSR.2024.22.002953
Abstract
Introduction: Renal cell carcinoma (RCC) accounts for approximately 2 to 3% of all malignant tumors in adults. 20 to 25% of patients still have metastatic disease at the time of diagnosis. Several patient- or tumor-related parameters have been identified as prognostic factors for survival in metastatic renal cell carcinoma (MRCC).
Objectives: We evaluated the role of several prognostic factors in predicting death and/or progressive disease in patients with metastatic renal cell carcinoma.
Materials and methods: Between 2016 and 2023, 95 consecutive patients who were presented with metastatic MRCC in our department were included in this analysis. All patients were treated with either surgery, targeted therapy or immunotherapy. The parameters analyzed to determine their impact on prognosis included laboratory parameters, treatment-related factors, tumor-related factors, and patient-related factors.
Results: Variables significantly associated with death and/or disease progression in univariate analysis were histological subtype (p<0.003), type of surgery ( p<0,002 ) , vascular invasion (p=0.003), IMDC score, Furhman grade (p=0,005). However, in multivariate analysis only the histological subtype,type of surgey, IMDC score, vascular invasion as well as the patient's WHO retained statistical significance and were associated with a lower survival rate. The median overall survival of patients with 3 or 4 prognostic factors, 2 factors, and 0 or 1 factor was 10 months, 32 months, and 61 months, respectively.
Conclusion: Clinical and pathological parameters have an impact on the prognosis of CRC. The most consistent prognostic factors may be histological subtype, vascular invasion, type of surgery.
Keywords: metastatic renal cell carcinoma, prognosis, targeted therapies, IMDC score
Introduction
Kidney cancer accounts for 3% of all cancers. It ranks third among urological cancers after prostate and bladder cancer [1]. There are several histological types of kidney cancer, the most common being renal cell carcinoma (RCC), which represents over 85% of all kidney cancers and is the ninth most common cancer in developed countries [2].
Kidney cancer is twice as common in men, with a peak incidence between 60 and 70 years old [3]. The incidence of kidney cancer is up to 10 times higher in North America and Europe than in Asia and Africa.
In Morocco, kidney cancer represented 32.7% of urinary system cancers, which in turn represented 2.4% of all cancers [4].
Patients with advanced stage RCC have an extremely grim prognosis due to the limited efficacy of treatment (5*). Immunotherapy can induce an objective response rate (ORR) was 42% versus 27% and complete response rate (CRR) was 11% (6). Nephrectomy in patients with metastatic disease mainly has cytoreductive and palliative effects on local and systemic symptoms.
Prognostic factors serve as markers for disease progression, and precise identification of these factors is crucial for assessing and managing patients with RCC. Various prognostic factors of RCC have been discussed in the literature, including clinical, anatomical, and molecular parameters, but none have been successfully validated thus far [5-7].
The aim of this study was to determine and establish associations between the histopathological characteristics of RCC and their impact on survival and metastasis.
Materials and Methods
This is a retrospective study of patients with metastatic RCC admitted to the Department of Medical Oncology at Hassan II University Hospital in Fez from January 2016 to January 2024. The study included all cases meeting the following criteria: kidneys affected by any subtype of RCC (including clear cell RCC, papillary RCC, chromophobe RCC, and collecting duct carcinoma) that underwent surgery or not and received either TKI or immunotherapy.
The data were categorized into four major groups, comprising epidemiologic information, clinical parameters, histopathological parameters (morphological subtype, grade, presence of necrosis, sarcomatous change, microvascular invasion, invasion of renal capsule, renal sinus, and tumor stage), and follow-up information (survival, recurrence, metastasis, and time and cause of death). Tumors were graded according to the Fuhrman and International Society of Urological Pathology (ISUP) scoring systems and classified using the American Joint Committee on Cancer (AJCC) and Tumor, Node, Metastasis (TNM) classification systems.
Statistical analysis was conducted using the SPSS software. A two-tailed P value equal to or less than 0.05 was considered indicative of significance in all tests.
Results
In total, 95 patients (males n=53, females n=42, median age: 65 years, range: 40 to 65 years) were included in our analysis. The most common metastatic site was the lung (46%), followed by the bones (23%) and the cerebral (3%). Out of the 95 samples, 63 (66,3%) were identified as clear cell carcinoma, 16 (16%) as papillary tumors, 7 (7%) as chromophobe tumors. A group of 2 patients exhibited sarcomatous features and were studied separately. Sarcomatous RCC, initially considered a distinct subtype, is now recognized as a high-grade transformation that can occur in any subtype of RCC.
Regarding the Fuhrman grade, 9 patients (9,5%) were classified as grade I, 37 (38,9%) as grade II, 33 (34,7%) as grade III, and 16(16.8%) as grade IV. Perinephric fat invasion, collecting system invasion, and vascular invasion were present in the samples of 25 ,12, and 24 (30.4%) patients, respectively.
Hemoglobin levels were found to be above 11.5 g/dL in 82% of patients. Lactate dehydrogenase levels were normal in 6.3% of patients.
Death and/or progressive disease results significantly correlated with histological subtype (p<0.003), Fuhrman grade (p=0.0005), vascular invasion (p=0.002), type of surgery (p<0,002), IMDC score (p<0,005), index of performance status. Presence of adrenal invasion or collecting system invasion was not significant (p=0.14 and p<0.083). Only histological subtype, tumor, vascular invasion, type of surgery, IMDC score, and index of performance status were significantly associated with overall survival (OS) on multivariate analysis.
The median overall survival was 13 months (range: 1 to 100 months), Sex and age did not influence survival using log-rank analysis (P=0.6 and p=0,2 respectively), Tumor size did not have a significant impact on survival in univariate analysis and multivariate regression analysis. Tumor grade had a significant impact on survival in univariate analysis and did not retain its individual significance when combined in multivariate regression analysis.
Survival analysis by tumor type revealed a significant difference between clear cell RCC, papillary RCC, and chromophobe RCC. The significant difference pertained to worse outcomes for the remaining histological types grouped compared to clear cell carcinoma (P=0.034) and chromophobe carcinoma (P <0.001).
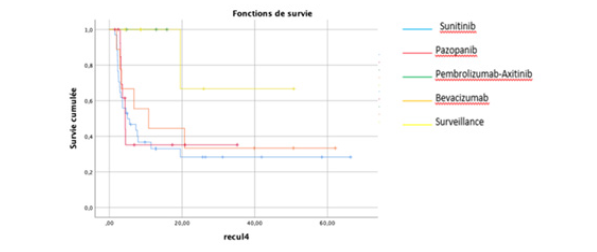
There was a statistically significant difference in survival between patients in the low- (P<0.001), medium- and high-risk groups: 25 months (range: 6.4-280), 12.1 months (range: 0.43-67) and 2.9 months (range: 1-39 months), respectively (Figures 1,2).
Discussion
The purpose of this analysis was to define the prognostic factors and survival outcomes in patients undergoing treatment for MRCC, either through surgery, TKI, immunotherapy, or a combination thereof.
Numerous stratification models for MRCC survival have been defined in the past, with KPS, CRP, nephrectomy, Hb, and nuclear grade being the most commonly identified risk factors.
RCC accounts for approximately 85% of renal malignancies, making it the most common renal tumor. However, conventional histopathological grading provides limited insight into tumorogenesis and predicting the clinical behavior of the disease [9]. Over the last few decades, the recognition of collecting duct carcinoma and chromophobe RCC as distinct entities marked the initial steps in defining RCC subtypes [10]. Through extensive pathological and genetic investigations, various types of tumors with presumed diverse cellular origins, phenotypes, and clinical characteristics [11] have been identified. The discovery of specific genetic alterations underlying pathogenesis has led to the acknowledgment of distinct tumor types. This classification, based on specific genetic features, was initially proposed by Kovacs [12], with a consensus on a new classification system being reached in 1996 at the Heidelberg conference [13]. Subsequently, in 1997, this classification system was reaffirmed through a consensus workshop between the Union International Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC).
The interpretation of survival data regarding different RCC types remains controversial. However, numerous studies suggest that chromophobe RCC exhibits significantly better survival outcomes compared to conventional and papillary RCC have an intermediate prognosis [14, 15]. Our study indicated that clear cell renal cell carcinoma has a better prognosis than other solid RCC subtypes. Other histological factors including tumor grade and lymph vascular invasion have also been studied in several studies.
Tumor grade is considered one of the most important histological prognostic factors. The Fuhrman nuclear grade [16] has now been replaced by the World Health Organization/International Society of Urological Pathology (WHO/ISUP) classification [17]. This relies solely on nuclear prominence for grades 1 to 3, allowing for less inter-observer variability [18]. It has been demonstrated that the WHO/ISUP classification provides superior prognostic information compared to the Fuhrman grading, especially for grade 2 and 3 tumors. In all types of RCC, prognosis worsens with stage and histopathological grade. The 5-year OS for all types of RCC is 49%, which has improved since 2006, likely due to an increase in incidentally detected RCC and new systemic treatments. [19,20] Clinical factors include performance status (PS), local symptoms, cachexia, anemia, platelet count, neutrophil count, lymphocyte count, C-reactive protein (CRP) [21], albumin and various indices derived from these factors such as neutrophil count.
Other factors such as age, sex, and race do not influence the clinical course of the disease. Although younger patients may lose more years of expected survival, there is no difference in actual survival duration compared to older patients [22]. General presentation symptoms such as weight loss, fatigue, persistent fever, and pain are concerning prognostic variables indicating a negative impact on survival. Numerous molecular markers such as carbonic anhydrase IX (CaIX), VEGF, HIF, Ki67 (proliferation), p53, p21 [23], PTEN (phosphatase and tensin homolog) cell cycle [24], E-cadherin, osteopontin [25], CD44 (cell adhesion) [230, 231], CXCR4, PD-L1 miRNAs, SNPs, genetic mutations, and gene methylations have been studied (LE: 3). While most of these markers are associated with prognosis and many of them enhance the discrimination of current prognostic models, there has been little emphasis on external validation studies. Moreover, there is no conclusive evidence regarding the value of molecular markers for treatment selection in mRCC. Therefore, their systematic use in clinical practice is not recommended.
Conclusion
Histological subtype (p<0.003), Fuhrman grade (p=0.005), vascular invasion (p=0.002), type of surgery (p<0.002), and performance status index were significantly associated with death and/or disease progression in univariate analysis. However, only histological subtype, vascular invasion, type of surgery, and performance status index are prognostic factors for survival in multivariate analysis. In the future, a deeper genetic and molecular understanding of tumor growth will contribute to the development of even more precise integrated predictive systems for RCC.
References
- Qarro, A Ammani, K Bazine, M Asseban, M Najoui, et al. (2013) Alami: Chirurgie conservatrice dans le cancer du rein Départemen t d’urologie, Hôpital Militaire Moulay Ismail, Meknès, Maroc, African journal 0f Urology 19: 205-210.
- Alma Demirovic, Davor Tomas, Karla Tomić, Borislav Spajić, Amir Ibukić, et al. (2014) Correlation of vascular endothelial growth factor and hypoxia-inducible factor- 1α expression with pathological renal artery changes in patients with renal cell carcinoma Scand J Urol 48(1): 34- 40.
- M Peycelon, R Renard Penna, M Rouprêt, Tumeurs du rein( 2011) Elsevier Masson SAS, 5 -0620 (EMC).
- Ferlay J (2002) International Agency for research on cancer (IARC) GLOBOCAN: cancer incidence, mortality and Prevalence Worldwide. Lyon, France: IARC Press.
- (1986) B Ljungberg et coll. Signification pronostique de la teneur en ADN dans le carcinome renal. Jurol.
- (1990) GF Murphy et coll. Analyse de la forme nucléaire pour l'évaluation du pronostic du carcinome renal. J Urol
- (2007) Belldegrun AS: Carcinome rénal: Facteurs pronostiques et sélection des patients. Eur Urol Suppl 6: 477-483.
- Suarez C, Campayo M, Bastus R, Castillo S, Etxanitz O, Guix M, Sala N, et al. (2018) Gallardo E : Facteurs pronostiques et prédictifs du carcinome rénal. Cible Oncol. 13: 309-331.
- Jonas D, Thoma B, Beckert H, Weber W (1985) The value of morphological prognostic criteria in the assessment of renal cell carcinoma. Urol Int 40: 148-154
- Thönes W, Störkel S, Rumpelt HJ (1986) Histopathology and classification of renal cell tumors (adenomas, oncocytomas and carcinomas): the basic cytological and histopathological elements and their use for diagnostics. Pathol Res Pract 181(2): 125-143.
- Kovacs G, Wilkens L, Papp T de Riese W (1989) Differentiation between papillary and nonpapillary renal cell carcinomas by DNA analysis. J Nat Cancer Inst 81(7): 527-530.
- Kovacs G (1993) Molecular cytogenetics of renal cell tumors. Adv Cancer Res 62: 89-124.
- Kovacs G, Akhtar M, Beckwith BJ, Bugert P, Cooper CS, et al. (1997) The Heidelberg classification of renal cell tumours. J Pathol 183(2): 131-133.
- Beck SD, Patel MI, Snyder ME, Kattan MW, Motzer RJ, et al. (2004) Effect of papillary and chromophobe cell type on disease-free survival after nephrectomy for renal cell carcinoma. Ann Surg Oncol 11(1): 71-77.
- Amin MB, Amin MB, Tamboli P, Javidan J, Stricker H, et al. (2002) Prognostic impact of histologic subtyping of adult renal epithelial neoplasms: an experience of 405 cases. Am J Surg Pathol 26(3): 281-291.
- Fuhrman SA, L C Lasky, C Limas. (1982) Prognostic significance of morphological parameters in renal cell carcinoma. Am J Surg Pathol 6(7): 655-663.
- Delahunt B, John C Cheville, Guido Martignoni, Peter A Humphrey, Cristina Magi Galluzzi, et al. (2013) International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am J Surg Pathol 37(10): 1490-1504.
- Paner GP, Walter M Stadler, Donna E Hansel, Rodolfo Montironi , Daniel W Lin, et al. (2018) Updates to the eighth edition of the tumor-node-metastasis staging classification for urologic cancers. Eur Urol 73(4): 560-569.
- Wahlgren T, U Harmenberg, P Sandström, S Lundstam, J Kowalski, et al. (2013) Treatment and overall survival in renal cell carcinoma: A population-based Swedish study (2000-2008). Br J Cancer 108(7): 1541-1549.
- Li P, Yu Ning Wong, Katrina Armstrong, Naomi Haas, Prasun Subedi, et al. (2016) Survival in patients with advanced renal cell carcinoma in the era of targeted and precision therapies. Cancer Med 5(2): 169-181.
- Fukuda S, Kazutaka Saito, Yosuke Yasuda, Toshiki Kijima, Soichiro YoshidaKazutaka Saito, et al. (2021) Impact of enhanced C-reactive protein response on on oncological outcomes in patients with metastatic renal cell carcinoma treated with nivolumab. J Immunother Cancer 9(2): e001564.
- Abou El Fettouh HI, Cherullo EE, El Jack M, Al Maslamani Y, Novick AC: Sporadic renal cell carcinoma in young adults: press
- Hammers, HJ et al. CheckMate 214: Phase III, randomized, open-label study of nivolumab plus ipilimumab versus sunitinib monotherapy in previously untreated patients with metastatic renal cell carcinoma. Registered under ClinicalTrials.gov identifier NCT02231749 in 2015.
- Fan D, Qiang Liu, Fei Wu, Na Liu, Hongyi Qu, et al. (2020) Prognostic significance of members of the PI3K/AKT/mTOR signaling pathway in clear cell renal cell carcinoma. Peer J 1:8: e9261.
- Sim SH, et al. Prognostic utility of preoperative circulating osteopontin, carbonic anhydrase IX, and CRP in renal cell carcinoma. Published in the British Journal of Cancer in 2012, this study examines the prognostic significance of these biomarkers in renal cell carcinoma.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.