Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Reformulated Post-Weaning Diets Combined with A Β-Mannanase Enzyme Supplementation Resulted in Equal Performance and An Economic Benefit
*Corresponding author: Frédéric Vangroenweghe, BU Food Animals, Elanco Benelux, Generaal Lemanstraat 55/3 (Building D, 1st floor), 2018 Antwerpen, Belgium.
Received: May 14, 2024; Published: May 21, 2024
DOI: 10.34297/AJBSR.2024.22.002986
Abstract
Many vegetable feed ingredients contain β-mannans, known as strongly antinutritive polysaccharide fibers. In swine diets, the content of soluble β-mannans commonly ranges between 0.15 to 0.40%. As little as 0.05% soluble β-mannan content in feed can elicit a strong innate immune response. Hemicell HT (Elanco) is a β-mannanase enzyme used to supplement animal feed, breaking down β-mannans and preventing economic losses due to the wasteful immune response elicited by these β-mannans. This field study compared pig performances on a control diet to a reformulated diet. The reformulated diet included a β-mannanase enzyme with either a lower energy content - a reduction of 45kcal/kg NE (Trial 1) - or a substitution of expensive protein sources with cheap alternatives (Trial 2). A seven-week feeding trial was conducted on a commercial post-weaning facility with TN70 x Tempo piglets starting at 28 days of age. Standard three-phase (0-10d, 11-28d, and 28-48d post-weaning) control diets were compared to reformulated diets with a 45kcal/kg NE reduction or a substitution of expensive protein sources along with the inclusion of a β-mannanase enzyme (Hemicell HT; Elanco) at 300g/tonne. Standard production data were collected, and the data were analyzed using JMP 15.0 statistical program. Overall, performance data did not differ significantly between treatment groups in all three phases and overall, during the entire post-weaning period for both Trial 1 and Trial 2. Mortality was only numerically, but not significantly higher in the Control as compared to the Enzyme-treated group in Trial 1. In Trial 1, Hemicell HT had an overall benefit of € 2.02 per piglet and € 8.05 per ton of feed due to the 45kcal/kg NE reduction. In Trial 2, Hemicell HT had an overall benefit of € 0.15 per piglet and € 8.80 per ton of feed due to substitution of expensive protein sources. The current trial demonstrated that the inclusion of Hemicell HT in reformulated diets with a lower energy content (45kcal/kg NE) or substitution of protein sources was able to retain production performance in post-weaned piglets with an additional economic benefit.
Keywords:β-mannanase, Post-weaning, Net energy reduction, Equal performance, Economic benefit
Abbreviations:ADFI: Average Daily Feed Intake; ADWG: Average Daily Weight Gain; FCR: Feed Conversion Rate; NSP: Non-Starch Polysaccharide; PCV-2: Porcine Circo Virus-Type 2; PRRSV: Porcine Reproductive and Respiratory Syndrome Virus; PWD: Post- Weaning Diarrhea; SBM: Soybean Meal
Introduction
Polysaccharides, which are polymers of monosaccharides linked by glycosidic bonds, are major components of all vegetable feed ingredients commonly used in swine diets. Starch, a polymer of glucose units linked by α-(1-4) with a few α-(1-6) bonds, is digested in the small intestine of pigs through endogenous enzyme activity. Non-Starch Polysaccharides (NSPs) are fibrous materials found in the plant cell wall, including celluloses, hemicelluloses, pectins, and oligosaccharides. Monogastric animals like pigs lack the endogenous enzymes required to digest β-linked NSPs like β-mannans [14]. β-Mannans-an antinutritive factor present in many common feed ingredients [4] - have gained increasing attention in recent years. β-Mannans are linear polysaccharides composed of repeating units of β-1,4-mannose and α-1,6-galactose and/or glucose units attached to the β-mannan backbone [7,11]. High concentrations of these β-mannans are considered unsuitable in monogastric diets due to their antinutritive properties, mainly due to stimulation of the innate immune response. The innate immune cells recognize pathogens through distinct molecules, called Pathogen-Associated Molecular Patterns (PAMPs), which are expressed on the pathogen surface [5]. The binding of PAMPs to Pathogen Recognition Receptors (PRR) present on innate immune cells, results in the release of innate defense molecules such as reactive oxygen and nitrogen species, bacteriolytic enzymes, antimicrobial peptides and complement proteins [19]. These PAMPs include complex polysaccharides that resemble β-mannans [5]. Consequently, β-mannans present in the feed may be mistaken by the immune system in the gastrointestinal tract for invading pathogens causing an unwarranted immune activation [1,15], also known as a feed-induced immune response (FIIR); [2]. This misrecognition of β-mannans as invading pathogens results to a futile immune response that wastes energy and nutrients [7]. The hydrolysis of β-mannans through the inclusion of exogenous β-mannanase enzyme can reduce and potentially eliminate their ability to induce a FIIR. β-Mannans in swine diets have been suggested to hinder the nutrient utilization [18]. Favorable effects on nutrient digestibility and growth performance have been observed following β-mannanase supplementation to maize-Soybean Meal (SBM)-based diets [17]. In poultry, the inclusion of dietary β-mannanase has been shown to improve daily weight gain and feed efficiency, while decreasing digesta viscosity [3], and upregulating a broad range of metabolic functions related to digestion, metabolism, and immunity [2]. Moreover, beneficial effects of β-mannanase addition in chickens, challenged with Eimeria sp. and Clostridium perfringens, were observed with improved performance and reduced lesion scores in these disease-challenged birds [10]. Supplementation of β-mannanase to low- and high-mannan diets has the potential to improve the performance of growing pigs [13]. Others have concluded that β-mannanase improved growth performance in both weanling and grow-finishing pigs on corn-SBM diets [16,17,12] with minimal effects on nutrient digestibility [17]. Additionally, β-mannanase supplementation to corn-SBM diets has reduced the population of fecal coliforms and tended to reduce the NH3 concentration of fecal slurry after 24 hours of fermentation [20]. The reduction of fecal coliforms might impact the environmental infection pressure from coliforms related to clinical problems of Post-Weaning Diarrhea (PWD) [21]. Another study demonstrated the in vivo anti-inflammatory activity of mannanase-hydrolyzed copra meal in a porcine colitis model with decreased expression of mRNA for ileal IL-1β, IL-6, IL-17 and TNF-α [9]. Innate immune activation is accompanied by downregulation of anabolic functions [8], resulting in a reduced performance capacity. Therefore, supplementation of a β-mannanase enzyme to post-weaning diets could reduce or eliminate the occurrence of FIIR and increase available energy and proteins for growth.
The objective of the current study was to evaluate the effects of β-mannanase supplementation of post-weaning diets with a reduced net energy content of 45kcal/kg of feed or an alternative protein source containing an increased level of β-mannans on piglet performance and economic parameters during the post-weaning phase.
Materials and Methods
Description of Experimental Farm
The field trial was conducted at a conventional post-weaning unit in the Netherlands comprising 7 compartments, each containing 4-6 pens. A total of 24 pens were included in the study, of which 12 pens were assigned to the Control group and 12 pens to the Enzyme-treated group. Each pen housed 11 post-weaned piglets. Compartments were ventilated through mechanical ventilation with an air inlet through the door. All pens were equipped with partially slatted plastic floors, dry feeders, and water was distributed through nipples in the feeders. Meal feed consumption was registered at group level. Both study groups were randomly allocated within the post-weaning compartments.
Experimental Design
Treatment Groups
At weaning, the piglets were assigned to one of the two treatment groups: Control and Enzyme-treated, respectively. A three-phase diet was distributed. The groups were blinded to the farm personnel and only distinguished by color codes (red and blue). Piglets from each individual pen were considered one experimental unit and were weighed together.
Experimental Diets
The pigs were fed a three-phase mash diet consisting of Phase 1 (0-10 days), Phase 2 (11-28 days), and Phase 3 (29-48 days) in each of the treatment groups. In Trial 1, the main difference between the diets for the Control and the Enzyme-treated groups was the reduction in net energy content of 45kcal/kg of feed in Phases 1, 2, and 3, respectively (Table 1). In Trial 2, the main difference between the diets for the Control and the Enzyme-treated group was the replacement of expensive protein sources with cheaper alternative protein sources in Phases 1, 2, and 3, respectively (Table 2). In both trials, the Enzyme-treated group was supplemented with a β-mannanase enzyme (Hemicell HT; Elanco, Indianapolis; IN) at an inclusion rate of 300 g per tonne of feed, according to the manufacturer’s instructions for use. Tonne enzymes (xylanase and phytase) in the diets remained at the same levels in both study groups (Table 1,2).

Table 1: Feed formulation (expressed as % of total feed) of the different phases (Phase 1-2-3) for Control and Enzyme-treated diets in Trial 1 with a 45kcal/kg NE reduction in the Enzyme-treated diets. Only feed ingredients that differ between both treatment groups are mentioned. Premix composition is identical in both treatment groups. Β-Mannan content (expressed as %) is given for each of the feed formulations.
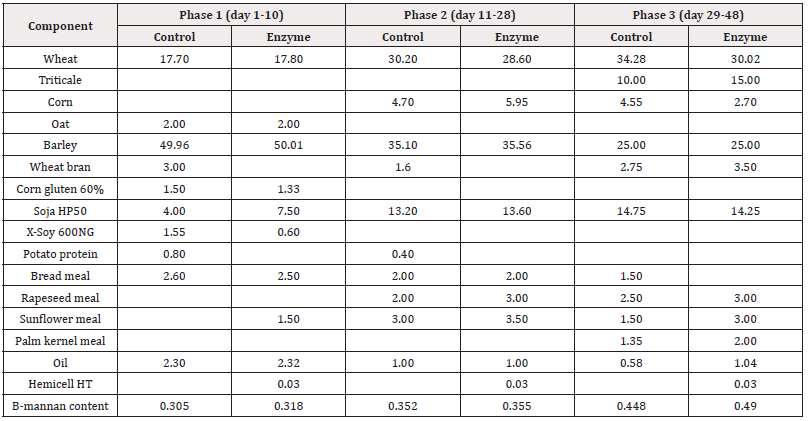
Table 2: Feed formulation (expressed as % of total feed) of the different phases (Phase 1-2-3) for Control and Enzyme-treated diets in Trial 2 with alternative protein substitution in the Enzyme-treated diets. Only feed ingredients that differ between both treatment groups are mentioned. Premix composition is identical in both treatment groups. Β-Mannan content (expressed as %) is given for each of the feed formulations.
Experimental Animals
TN70 (Topigs Norsvin)* Tempo piglets were obtained from a conventional commercial sow farm linked to the post-weaning facility. The piglets were vaccinated to protect against Mycoplasma hyopneumoniae, Porcine Reproductive and Respiratory Syndrome Virus (PRRSV), and Porcine Circovirus type 2 (PCV-2). A total of 264 piglets were enrolled in each feed trial.
Performance Data Collection
Pig body weight per pen was measured at 0-, 10-, 28- and 49-days post-weaning. Feed provision (ad libitum) was recorded at treatment group level. Average Daily Weight Gain (ADWG; expressed as g/d), Average Daily Feed Intake (ADFI; expressed as g/d) and Feed Conversion Rate (FCR; expressed as kg feed per kg of weight gain) were calculated for Phase 1, Phase 2, and Phase 3, respectively. Mortality was recorded along with the date of death and the number of dead animals.
Veterinary Treatments
Individual antibiotic treatments were administered as needed due to the critical state of the piglets and in case of a broader health issue in the barn, group treatment could be administered. The same veterinary products and dosages (ml/kg) were used throughout the entire study period. Individual antibiotics treatments or group treatments were recorded daily including the date, product, dose, ID number of treated piglets, presumed cause of treatment, and the number of times the treatment was repeated.
Economic Benefit Per Piglet and Per Ton of Feed
The economic benefit of β-mannanase supplementation combined with a reduction in net energy of approximately 45kcal/kg feed (Trial 1) or alternative protein substitution (Trial 2) was calculated both at the piglet level and at the feed cost level. For the calculation of economic benefit at the piglet level, the following parameters were considered: feed cost reduction, piglet price correction (standard price for 25 kg piglet), and opportunity costs of mortality. For the calculation of economic benefit at the feed cost level, the following parameters were considered: total feed cost and the total amount of feed consumed.
Data Management and Statistical Analysis
Data were hand-recorded by the farm personnel and stored in MS Excel on OneDrive at the end of each day. Following the end of the feed trial, the data were extracted from Excel into JMP 15.0 and the blinded color-coded treatments were unblinded to reveal the respective treatment groups. Calculations, exploratory data analysis and quality review, and subsequent statistical analysis were all performed in JMP 15.0. All data were presented as means with their respective pooled Standard Error of The Mean (SEM). All means were tested for significant differences (P<0.05) using a T-test.
Results
Piglet Weight
For Trial 1, involving a reduction of 45kcal/kg NE, data on pig weight is given in Table 3. The piglets arrived at the post-weaning facility at an average weight of 6.9kg. There were no significant differences (P>0.05) observed in the start weight (d0) between both treatment groups. At day 10, piglets in the Enzyme-treated group were slightly, but non-significantly (P>0.05) lighter as compared to the Control group (8.3kg±0.14kg vs. 8.4±0.06kg, respectively). Similarly, by day 28 and day 48, the piglets in the Enzyme-treated group remained slightly lighter than the Control group, with no significant differences observed (P>0.05; d28: 15.9±0.29kg vs. 16.4±0.20kg, respectively; d48: 26.2±0.64 kg vs. 26.5±0.46 kg respectively). For Trial 2, involving alternative protein substitution, pig weight data are presented in Table 4. The piglets arrived at the post-weaning facility at an average weight of 7.1kg. No significant differences (P>0.05) were observed in the start weight (d0) between both treatment groups. At day 10, piglets in the Enzyme-treated group were slightly, but non-significantly (P>0.05) lighter as compared to the Control group (8.5±0.14kg vs. 8.6±0.00kg, respectively). Similarly, by day 28 and day 48, the piglets in the Enzyme-treated group remained slightly lighter than the Control group with no significant differences observed (P>0.05; d28: 18.0±0.38kg vs. 18.2±0.38kg, respectively; d48: 28.7±0.51 kg vs. 28.9±0.61 kg, respectively).
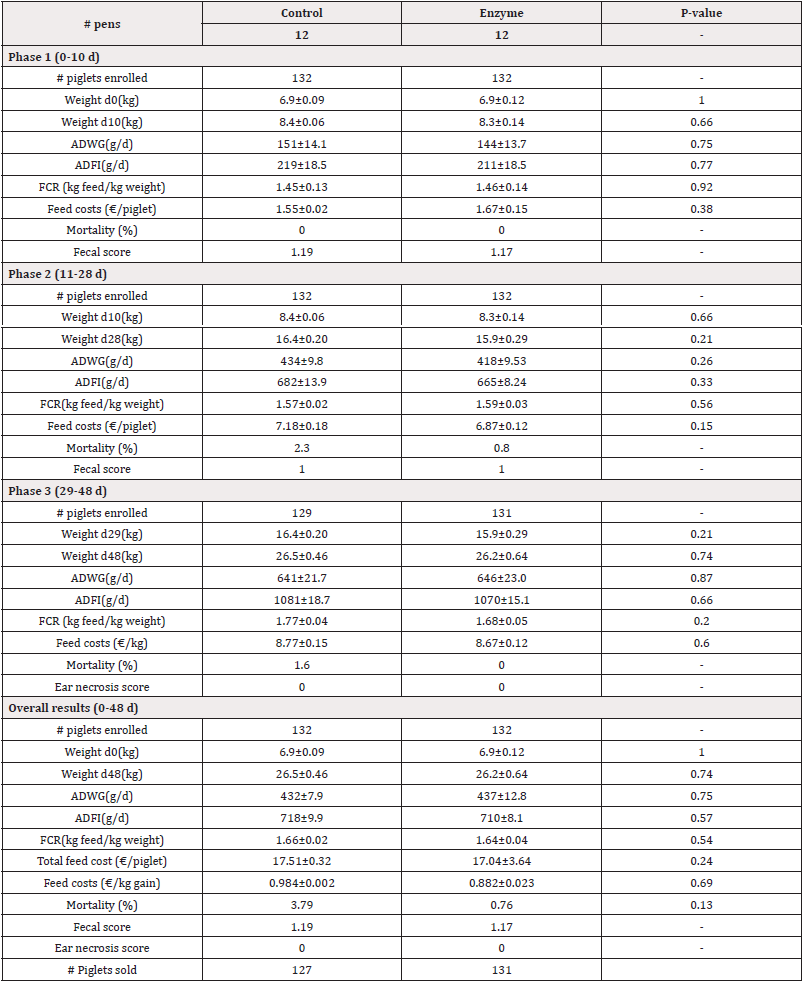
Table 3: Performance parameters for both Control and Enzyme-treated groups with a 45 kcal/lkg NE reduction in Enzyme-treated diets in Phases 1, 2, 3 and overall. Weight, Average Daily Weight Gain (ADWG), Average Daily Feed Intake (ADFI), Feed Conversion Rate (FCR), and feed costs are given as mean±SEM. Mortality is given as mean. P-values<0.05 represent statistically significant differences.
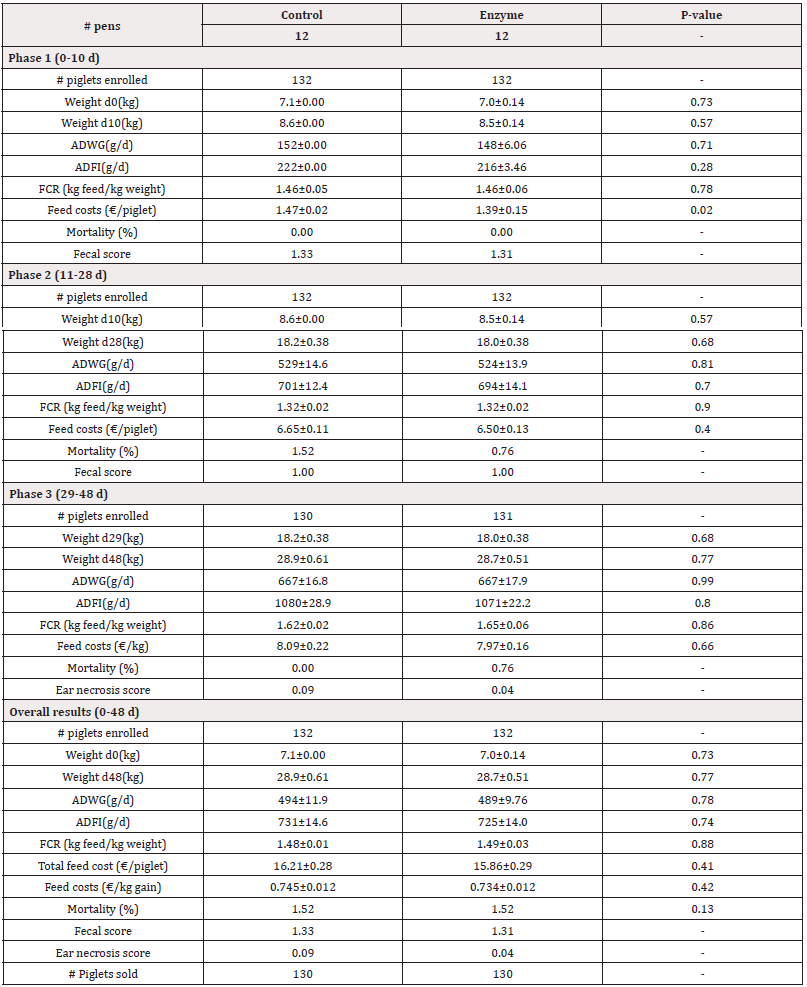
Table 4: Performance parameters for both Control and Enzyme-treated groups with an alternative protein substitution in Enzyme- treated diets in Phases 1, 2, 3 and overall. Weight, Average Daily Weight Gain (ADWG), Average Daily Feed Intake (ADFI), Feed Conversion Rate (FCR), and feed costs are given as mean±SEM. Mortality is given as mean. P-values<0.05 represent statistically significant differences.
Average Daily Weight Gain
In Trial 1, involving a reduction of 45kcal/kg NE, data on ADWG is given in Table 3. In Phase 1 (0-10 d), piglets in the Enzyme-treated group had slightly, but not significantly lower (P<0.05) ADWG as compared to the Control group (211±18g/d vs. 219±18g/d, respectively). Similarly in Phase 2 (11-28d), piglets in the Enzyme-treated group had slightly, but not significantly lower (P>0.05) ADWG as compared to the Control group (418±10 g/d vs. 434±10g/d, respectively). In Phase 3 (29-48d), piglets in the Enzyme-treated group had slightly, but not significantly higher (P>0.05) ADWG as compared to the Control group ((646±23g/d vs. 641±22g/d, respectively). Overall, ADWG was not significantly different (P>0.05) between both study groups (437±13g/d vs. 432±8g/d in Enzyme-treated and Control group, respectively). In Trial 2, involving alternative protein substitution, data on ADWG is given in Table 4. In Phase 1 (0-10d), piglets in the Enzyme-treated group had slightly, but not significantly lower (P>0.05) ADWG as compared to the Control group (148±6g/d vs. 152±0g/d, respectively). In Phase 2 (11-28d), piglets in the Enzyme-treated group had slightly, but not significantly lower (P>0.05) ADWG as compared to the Control group (524±14g/d vs. 529±15g/d, respectively). In Phase 3 (29- 48d), piglets in the Enzyme-treated group had equal (667±17g/d; P>0.05) ADWG compared to the Control group. Overall, ADWG did not significantly (P>0.05) differ between the Enzyme-treated and the Control group (489±10g/d vs. 494±12g/d, respectively).
Average Daily Feed Intake and Feed Conversion Rate
In Trial 1, involving a reduction of 45kcal/kg NE, data on ADFI and FCR are given in Table 3. In Phase 1 (0-10d), the ADFI was slightly, but not significantly lower (P>0.05) in the Enzyme-treated group as compared to the Control group (211±18g/d vs. 219±18g/d, respectively). In Phase 2 (11-28d), the ADFI was lower (P>0.05) in the Enzyme-treated group as compared to the Control group (665±8g/d vs. 682±14g/d, respectively). In Phase 3 (29-48d), the ADFI was lower (P>0.05) in the Enzyme-treated group as compared to the Control group (1070±15g/d vs. 1081±19g/d, respectively). Overall, ADFI was 8 g/d lower (P>0.05) in the Enzyme-treated group as compared to the Control group (710±8g/d vs. 718±10g/d, respectively). The FCR was 0.01 higher (P>0.05) in the Enzyme-treated group as compared to the Control group ((1.46±0.14kg feed/kg gain vs. 1.45 kg±0.13 feed/kg gain, respectively) in Phase 1 (0-10 d). In Phase 2 (11-28 d), the FCR was slightly, but not significantly higher (P>0.05) in the Enzyme-treated group as compared to the Control group (1.59±0.03kg feed/kg gain vs. 1.57±0.02kg feed/ kg gain, respectively). However, in Phase 3, the FCR was slightly, but not significantly higher (P>0.05) in the Enzyme-treated group as compared to the Control group (1.68±0.05kg feed/kg gain vs. 1.77±0.04kg feed/kg gain, respectively). Overall, FCR was 0.02 lower (P>0.05) in the Enzyme-treated group as compared to the Control group (1.64±0.04kg feed/kg gain vs. 1.66±0.02kg feed/kg gain, respectively).
In Trial 2, with an alternative protein substitution, data on ADFI and FCR are given in Table 4. In Phase 1 (0-10d), the ADFI was slightly, but not significantly lower (P>0.05) in the Enzyme-treated group as compared to the Control group (216±3g/d vs. 222±0g/d, respectively). In Phase 2 (11-28d), the ADFI was lower (P>0.05) in the Enzyme-treated group as compared to the Control group (694±14g/d vs. 701±12g/d, respectively). In Phase 3 (29-48 d), the ADFI was lower (P>0.05) in the Enzyme-treated group as compared to the Control group (1071 ±22 g/d vs. 1080±29g/d, respectively). Overall, ADFI was 8 g/d lower (P>0.05) in the Enzyme-treated group as compared to the Control group (725±14g/d vs. 731±15g/d, respectively). In Phase 1 (0-10 d) and Phase 2 (11-28d), the FCR was equal (P>0.05) in the Control and Enzyme-treated group (1.46±0.06kg feed/kg gain and 1.32±0.02 kg feed/kg gain, respectively in both phases). However, in Phase 3 (29-48d), the FCR was slightly, but not significantly higher (P>0.05) in the Enzyme-treated group as compared to the Control group (1.65±0.06kg feed/kg gain vs. 1.62±0.02kg feed/kg gain, respectively). Overall, FCR was 0.01 higher (P>0.05) in the Enzyme-treated group as compared to the Control group (1.49±0.03kg feed/kg gain vs. 1.48±0.01kg feed/kg gain, respectively).
Antimicrobial Treatment
No significant differences were observed at neither the level of individual treatment or at the level of group treatment between both treatment groups during both feed trials.
Mortality
For Trial 1, involving a reduction of 45kcal/kg NE, data on mortality is given in Table 3. Overall, mortality was slightly, but not significantly (P>0.05) lower in the Enzyme-treated group as compared to the Control group (0.76% vs. 3.79%, respectively).
For Trial 2, involving alternative protein substitution, data on mortality is given in Table 4. Overall mortality was equal (P>0.05) in the Control and Enzyme-treated group (1.52%).
Economic Benefit Per Piglet and Per Ton of Feed
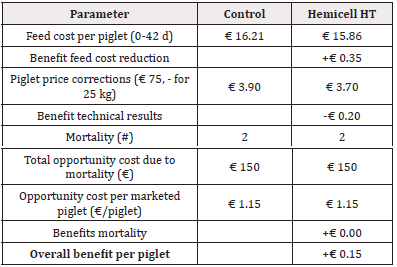
For Trial 1, involving a reduction of 45kcal/kg NE, the detailed calculation of economic benefit per piglet is given in Table 5. Overall, supplementation of a β-mannanase enzyme combined with a reduction of net energy by 45 kcal/kg feed over the three phases resulted in an economic benefit per piglet of € 2.02. The detailed calculation of economic benefit per ton of feed is given in Table 6. Overall, supplementation of a β-mannanase enzyme resulted in a feed cost reduction of € 8.05 per ton of feed.
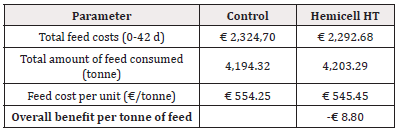
For Trial 2, involving alternative protein substitution, the detailed calculation of economic benefit per piglet is given in Table 7. Overall, supplementation of a β-mannanase enzyme combined with an alternative protein substitution over the three phases resulted in an economic benefit per piglet of € 0.15. The detailed calculation of economic benefit per ton of feed is given in Table 8. Overall, supplementation of a β-mannanase enzyme resulted in a feed cost reduction of € 8.80 per ton of feed (Tables 5-8).
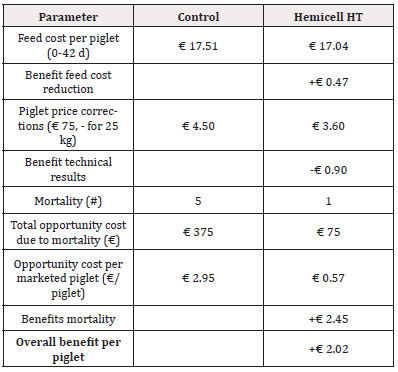
Table 5: Detailed calculation of economic benefit per piglet considering a reduction in feed cost, piglet price corrections (standard price at 25 kg) and the opportunity cost of mortality for Trial 1 - reduction of 45 kcal/kg NE in Enzyme-treated diets.
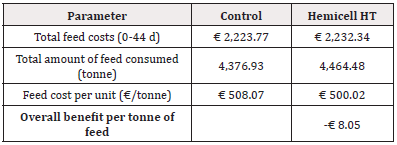
Table 6: Detailed calculation of economic benefit of feed cost per tonne of feed considering total feed costs and total amount of feed consumed for Trial 1 - reduction of 45 kcal/kg NE in Enzyme- treated diets.
Discussion
In Trial 1, involving the 45kcal/kg NE reduction, the β-mannan content in all three phases ranged from 0.304 to 0.344%, which was sufficiently high to maintain the standard feed composition without the need for additional protein substitutions as previously reported [21]. The relatively high level of β-mannans, a known antinutritive factor [4], which may stimulate an innate immune response through their resemblance with PAMPs [5], may induce FIIR (Feed Induced Immune Response) [2] and lead to an unnecessary immune activation, causing energy and nutrients to be wasted [6]. Therefore, 300 g/ton of an exogenous β-mannanase enzyme (Hemicell HT; Elanco, Greenfield, IA) was added to hydrolyze these antinutritive β-mannans in the trial feed. The results in all phases demonstrated no significant differences in the measured (piglet weight, ADFI) or calculated (ADWG, FCR) performance parameters between both treatments. Although minor numerical differences were observed, the overall result confirmed that the addition of an exogenous β-mannanase to adapted formulations with a reduction in net energy content of 45kcal/kg of feed, in the presence of a sufficient level of β-mannans, allowed them to perform equally to the standard post-weaning Control diets. These results are consistent with other recent studies in low- and high-mannan diets [13,21-23].
In addition to similar results in production performance, a substantial economic benefit of supplementation of a β-mannanase enzyme could be calculated. Based on the actual feed prices and measured feed intake, we obtained a 2.7% reduction in the feed cost (€ 17.04 vs. € 17.51 in the Enzyme-treated vs. the Control group, respectively) per piglet produced and a 1.6% reduction in feed cost per ton of feed (€ 500.02 vs. € 508.07, in Enzyme-treated vs. Control group, respectively). Considering all costs (feed cost, basic piglet market price at 25kg, and opportunity costs for mortality) the income per produced piglet was € 2.02 higher for the Enzyme-treated group. Others concluded that β-mannanase improved growth performance in both weanling and grow-finishing pigs on corn-SBM diets [15,17,12]. A diet with a 150kcal/kg reduction in digestible energy supplemented with β-mannanase outperformed in weight gain and feed efficiency [15]. Others have also observed the energy-sparing effect from the supplementation of β-mannanase. For example, the supplementation to a common nursery diet resulted in similar effects on the performance of a comparable diet supplemented with 2% soya oil [17]. In poultry, beneficial effects of β-mannanase supplementation on the performance of chickens challenged with Eimeria sp. and Clostridium perfringens were observed together with reduced lesion scores in disease-challenged birds [10]. This observation was confirmed by a recent study in post-weaned piglets, where antimicrobial use for the treatment of PWD due to Escherichia coli was significantly reduced in the Enzyme-treated group compared to the Control group [21]. However, in the current study, disease challenges during the post-weaning period were relatively low, and therefore no differences in antimicrobial treatment could be observed between both treatment groups.
In Trial 2, the β-mannan content in the Enzyme-treated group was generally higher in all three phases as compared to the Control group (0.318 to 0.490% vs. 0.355 to 0.448%, respectively), mainly due to the alternative protein substitution, such as palm kernel meal, rapeseed meal and sunflower meal that contain higher levels of β-mannans. Although the higher β-mannan levels in the Enzyme-treated group, the results in all phases demonstrated no significant differences in the measured (piglet weight, ADFI) or calculated (ADWG, FCR) performance parameters between both treatments. Although minor numerical differences were observed, the overall result confirmed that the addition of an exogenous β-mannanase to adapted formulations with alternative protein substitution allowed them to perform equally to the standard post-weaning Control diets. In addition to similar results in production performance, a substantial economic benefit of supplementation of a β-mannanase enzyme could be calculated. Based on the actual feed prices and measured feed intake, we obtained a 2.2% reduction in the feed cost (€ 15.86 vs. € 16.21, in Enzyme-treated vs. Control group, respectively) per piglet produced and a 1.6% reduction in feed cost per ton of feed (€ 545.45 vs. € 554.25, in the Enzyme-treated vs. the Control group, respectively). Considering all costs (feed cost, basic piglet market price at 25kg, and opportunity costs for mortality) the income per produced piglet was € 0.15 higher for the Enzyme-treated group. These observations were in line with previous results [21]. where expensive protein sources, such as potato protein and extruded soy products, were replaced by soybean meal (49% crude protein) and the addition of a β-mannanase enzyme.
Overall, the results from the current trials demonstrated that in the present of a sufficient amount of β-mannans in the diet formulations, the addition of a β-mannanase enzyme (Hemicell HT; Elanco) could support piglet performances under field conditions with formulations adapted towards net energy reduction or alternative protein substitution. Both adapted diet formulations resulted in improved economic benefits at the individual piglet level and the cost per ton of feed level.
Conclusions
The current trial demonstrated that the inclusion of Hemicell HT in reformulated diets with a lower energy content (45kcal/kg NE) or alternative protein substitution was able to retain production performance in post-weaned piglets with an economic benefit. The inclusion of Hemicell HT had an overall benefit of € 2.02 / piglet and € 8.05 / ton of feed due to the reduction of net energy of 45kcal/kg, or € 0.15 / piglet and € 8.80 / ton of feed due to alternative protein substitution.
Declarations
Ethics approval and Consent to Participate
Field trial with an EFSA approved feed supplement for use in swine. No additional ethical approval was needed. Consent to participate was obtained following full information of the farmer on the protocol to be carried out.
Consent for Publication
Not applicable.
Availability of Data and Material
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare that they have no other competing interests.
Funding
The study was funded by Elanco Animal Health.
Author’s Contributions
FV and AdB were both involved in study design, data collection, data analysis and manuscript preparation. Both authors read and approved the final manuscript.
Acknowledgements
The authors greatly acknowledge the swine farmer for his participation in the study.
Author’s Information
FV is currently a Principal Technical Advisor Swine & Nutritional Health for Benelux / UK&ROI within Elanco Animal Health. He holds a DVM, a Master in Veterinary Public Health and Food Safety, a PhD in Veterinary Sciences, a PhD in Applied Biological Sciences and an EBVSTM European Specialist in Porcine Health Management. He is a resident of the American Board of Veterinary Practitioners-Swine Health Management and has a specific interest in swine intestinal health and specific approaches to improve intestinal health through non-antibiotic solutions.
Acknowledgements
The authors greatly acknowledge the technical staff for their assistance in randomization, weighing and data collection.
References
- Anderson DM, Hsiao HY, Dale NM (2008) Identification of an inflammatory compound for chicks in soybean meal. Poult Sci 88:153-157.
- Arsenault RJ, Lee JT, Latham R, Carter B, Kogut MH (2017) Changes in immune and metabolic gut response in broilers fed β-mannanase in β-mannan-containing diets. Poult Sci 96(12): 4307-3216.
- Balasubramanian B, Ingale SL, Park JH, Rathi PC, Shanmugam S (2018) Inclusion of dietary β-mannanase improves performance and ileal digestibility and reduces ileal digesta viscosity of broilers fed corn-soybean meal-based diet. Poult Sci 97(9): 3097-3101.
- Ferrel J, Anderson DM, Hsiao HY (2014) Content of soluble non-starch polysaccharides β-Mannan and xylan in legume meals, non-legume meals, and cereal grains or cereal grain by-products. J Anim Sci 92.
- Forsberg NE, Wang Y (2006) Nutrition and immunity in dairy cattle: implications to hemorrhagic bowel syndrome. Proc Mid-South Rum Nutr Conf pp: 11-20.
- Hsiao HY, Jin FL, Mathis GF (2004) Efficacy of β-mannanase (Hemicell) in broiler chickens infected with necrotic enteritis. Int Poultry Sci Forum, pp. 1790.
- Hsiao HY, Anderson DM, Dale NM (2006) Levels of β-mannan in soybean meal. Poult Sci 85(8): 1430-1432.
- Humphrey BD, Klasing KC (2005) The acute phase response alters cationic amino acid transporter expression in growing chickens (Gallus gallus domesticus). Comp Biochem Physiol A Mol Integr Physiol 142(4): 485-494.
- Ibuki M, Fukui K, Kanatani H, Mine Y (2014) Anti-inflammatory effects of mannanase-hydrolyzed copra meal in a porcine model of colitis. J Vet Med Sci 76(5): 645-651.
- Jackson ME, Anderson DM, Hsiao HY, Mathis GF, Fodge DW (2003) Beneficial effect of β-mannanase feed enzyme on performance of chicks challenged with Eimeria sp. and Clostridium perfringens. Av Dis 47(3): 759-763.
- Jackson ME, Geronian K, Knox A, McNab J, McCartney E (2004) A dose-response study with the feed enzyme β-mannanase in broilers provided with corn-soybean meal-based diets in the absence of antibiotic growth promoters. Poult Sci 83(12): 1992-1996.
- Jo JK, Ingale SL, Kim JS, Kim YW, Kim KH, et al. (2012) Effects of exogenous enzyme supplementation to corn- and soybean meal-based or complex diets on growth performance, nutrient digestibility, and blood metabolites in growing pigs. J Anim Sci 90: 3041-3048.
- Kim JS, Ingale SL, Hosseindoust AR, Lee SH, Lee JH, et al. (2017) Effects of mannan level and β-mannanase supplementation on growth performance, apparent total tract digestibility and blood metabolites of growing pigs. Animal 11(2): 202-208.
- Klein J, Williams M, Brown B, Rao S, Lee TJ (2015) Effects of dietary inclusion of a cocktail NSPase and β-mannanase separately and in combination in low energy diets on broiler performance and processing parameters. J Appl Poult Res 24: 489-501.
- Li Y, Chen X, Chen Y, Li Z, Cao Y (2010) Effects of β-mannanase expressed by Pichia pastoris in corn soybean meal diets on broiler performance, nutrient digestibility, energy utilization and immunoglobulin levels. Anim Feed Sci Technol 159: 59-67.
- Lv JN, Chen YQ, Guo XJ, Piao XS, Cao YH, Dong B (2013) Effects of supplementation of β-mannanase in corn-soybean meal diets on performance and nutrient digestibility in growing pigs. Asian- Australas J Anim Sci 26(7): 579-587.
- Pettey LA, Carter SD, Senne BW, Shriver JA (2002) Effects of beta-mannanase addition to corn-soybean meal diets on growth performance, carcass traits, and nutrient digestibility of weanling and growing-finishing pigs. J Anim Sci 80(4): 1012-1019.
- Rainbird AL, Low AG, Zebrowska T (1984) Effect of guar gum on glucose and water absorption from isolated loops of jejunum in conscious growing pigs. Br J Nutr 52(3): 489-498.
- Sukhithasri V, Nisha N, Biswas L, Kumar VA, Biswas R (2013) Innate immune recognition of microbial cell wall components and microbial strategies to evade such recognitions. Microbiol Res 168(7): 396-406.
- Upadhaya SD, Park JW, Lee JH, Kim IH (2016) Efficacy of β-mannanase supplementation to corn-soya bean meal-based diets on growth performance, nutrient digestibility, blood urea nitrogen, faecal coliform and lactic acid bacteria and feacal noxious gas emission in growing pigs. Arch. Anim Nutr 70(1): 33-43.
- Vangroenweghe F, Poulsen K, Thas O (2021) Supplementation of a β-mannanase enzyme reduces post-weaning diarrhea and antibiotic use in piglets on an alternative diet with additional soybean meal. Porcine Health Manag 7(1): 8-19.
- Vangroenweghe F, de Bruijn A, Vandenbussche G, Joye P (2023a) Supplementation of a β-mannanase enzyme to diets with a reduced net energy content supports post-weaning piglet performance during a PRRSV outbreak under field conditions. Am J Biomed Sci Res 20: 28-35.
- Vangroenweghe F, Goethals S, Van Zele D, de Bruijn A (2023b) Application of a β-mannanase enzyme in diets with a reduced net energy content in post-weaning piglets resulted in equal performance and an additional economic benefit. Med Res Arch 11: 1-12.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.