Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Remote Continuous Patient Monitoring Using Early Warning System (Iews) for the Detection of Clinical Deterioration in Patients Receiving Active Cancer Treatment: A Phase II Randomized Controlled Trial
*Corresponding author: Sachin Suresh Jadhav, Group Head of Department, Haematology, Bone Marrow Transplantation unit, HCG Group of Hospitals.
Received: May 14, 2024; Published: May 17, 2024
DOI: 10.34297/AJBSR.2024.22.002982
Abstract
Background: Cancer patients admitted to non-intensive care unit settings and at home, who are undergoing active cancer treatment, often undergo clinical deterioration. Early identification of deterioration provides an opportunity to intervene early and improve outcomes. The trial was intended to investigate whether remote continuous patient monitoring using Dozee® Early Warning System (iEWS, Dozee®) can generate early actionable alerts leading to timely interventions which could potentially improve outcomes.
Method: In this phase II, open-labelled, randomized controlled trial, conducted in a tertiary care centre in Bangalore (India). In the ‘Group I: Hospital Cohort’, we randomly assigned 60 patients who were admitted for surgery, chemotherapy, stem cell transplant or those who were admitted in the hospital Day-Care Unit or the Inpatient Unit, for the treatment of complications due to surgery, chemotherapy, or stem cell transplant to receive either remote continuous patient monitoring using the iEWS (Dozee® Arm) or spot check monitoring which is the Standard of care (Control Arm). On discharge from the Day-Care or the Inpatient Unit of the hospital these patients then continued in their previously randomized arms as ‘Group II: Home Cohort’. The patients in Dozee® arm were monitored with Dozee® (iEWS) in hospital & also at home after discharge. The patients in Control arm were monitored with spot checks done by healthcare workers in hospital and by caregivers at home after discharge. The primary outcome measures were time to actionable alert by Dozee® or healthcare worker, time to intervention and outcomes. The secondary outcome measure was mortality.
Result: Patients in the Hospital Cohort were monitored from randomization until they were discharged from the hospital, median day 1, range (0-39). Those in the Home Cohort were monitored from their date of randomization until their next hospital admission or until the closure of data collection on 11 Sep 2023, whichever was earlier mean ± 2SD, 54 ± (2*14). In the Hospital Cohort, 7 out of 30 (23.3%) patients in the Dozee® Arm had actionable alerts compared to none in the control cohort, p=0.006. These actionable alerts led to 32 interventions [enhanced monitoring (8), diagnostic intervention (8) & therapeutic intervention (16)]. 6/30 (20%) patients in Dozee® hospital cohort needed therapeutic interventions. One patient in each arm required Intensive Care Unit (ICU) transfer. The patient from the Dozee cohort required ICU care for 3 days while the one from the Control Cohort was in the ICU for 17 days. In the Home Cohort, 2/18 (11.11%) patients in Dozee® Arm had 2 clinically significant alerts. These actionable alerts led to changes in their medication and a visit to hospital in both the patients. There were no alerts in the control cohort, p= 0.071.
Conclusion: Remote continuous patient monitoring using the Dozee® iEWS led to higher number of alerts in both the Hospital and Home Arms, which led to closer monitoring, diagnostic tests, and therapeutic interventions. Larger studies need to be performed to assess the impact of this on clinical outcomes.
Keywords: Cancer patients, Non-intensive care unit settings, Clinical deterioration, Early identification, Remote continuous patient monitoring, Dozee® Early Warning System (iEWS, Dozee®), Early actionable alerts
Introduction
Delayed identification of clinical deterioration is an important cause of preventable morbidity & mortality in non-ICU settings in hospitals, as vital cardiorespiratory parameters may change several hours prior to the occurrence of an adverse event [1-6]. Therefore, early detection of clinical deterioration and timely intervention could potentially reduce or prevent ICU transfer, cardiopulmonary arrest, or death [7-12]. Given these challenges, the need for an effective system that can bridge the monitoring gap between ICU and non-ICU settings is evident. In this study we assess the utility of a centralized, remote, ‘Intelligent Early Warning System’ (iEWS) via the Dozee® Pro contactless vital sign monitoring system. The "Dozee® Pro'' device is certified for usage in India and has been validated for its capacity to monitor Heart Rate (HR), Respiration Rate (RR), and Blood Pressure (BP) without direct contact [13-16].
In order to monitor the patients remotely & continuously in non-ICU settings, while improving patient comfort, Dozee® uses a combination of Ballistocardiography and next-generation AI algorithms to monitor the vital health parameters (Heart Rate, Respiratory Rate, Blood Pressure, Oxygen Saturation & Temperature) of the patient on near real-time basis and shares them with the health care providers on a web-based patient monitoring system and mobile apps. Dozee® comes with intelligent Early Warning System (iEWS) algorithms which track the trends of various health parameters for early detection of clinical deterioration of patients and to enable timely medical intervention. In an earlier prospective, observational clinical study, Dozee® had 91% sensitivity for identifying patient deterioration as compared to the 67% sensitivity of spot checks at a tertiary multispecialty hospital in Lucknow, India (currently under review for publication).
Method
Study Design
The study was designed as a phase II open-labelled randomized controlled trial in which patients were randomized to either the Dozee® Arm or Control Arm. The patient population can be classified into 2 groups. Group I (Hospital Cohort) was those who were admitted for surgery, chemotherapy, stem cell transplant or complication arising due to surgery, chemotherapy, stem cell transplant in the hospital Inpatient Unit or the Day-Care Unit. Group II (Home Cohort) were those patients who were being discharged after surgery, chemotherapy, or stem cell transplant. Patients in both the Groups were randomly assigned to Dozee® Arm or Control Arm using a simple randomization table and study CONSORT diagram represented in Fig;1. The trial was approved by the Institutional Ethics Committee of study centre and initiated on June 30th, 2023. All the patients provided written informed consent (Figure 1).
Study Population
The study was conducted in a referral Cancer Centre at Bangalore, India. The inclusion criteria for the Hospital Cohort included all consecutive, unselected patients who were admitted for surgery, chemotherapy, stem cell transplant or complication due to surgery, chemotherapy, stem cell transplant in the hospital Inpatient Unit or the Day-Care Unit and were willing to give informed consent. On discharge from the hospital, these same patients were then followed up as the home cohort. Exclusion criteria included patients <18 years of age, Patients <40 Kg & >120Kg, patients experiencing symptoms of deterioration at the time of randomization, patients in ICU and refusal to consent.
Study Procedure
Baseline vitals (Heart Rate, Respiratory Rate, Blood Pressure, Mean Arterial Blood Pressure, Oxygen Saturation, Temperature, Glasgow Coma Scale (GCS)) were recorded at the time of randomization for all the patients.
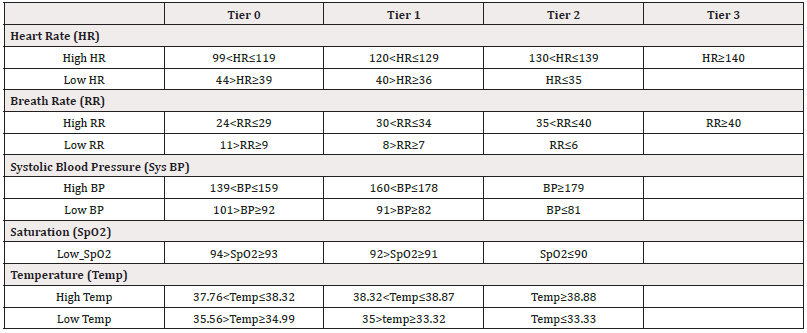
Vitals monitoring in the Dozee® Arm: In Dozee® Arm, the patients were monitored remotely & continuously using Dozee® A dynamic tier based alerting system was developed that utilized median vitals for every 10 minutes for Heart Rate (HR) and Respiratory Rate (RR), 30 minutes for Blood Pressure (BP) moving with an increment of two minutes to assess an alert positive condition. Furthermore, alerts were categorized in to 4 tiers (0,1,2,3), depending on the degree of worsening, refer Table 1. Once an alert was triggered, “Snooze time” or periods of no alerts were set to three hours unless the next tier was breached. In the final tier, i.e. Tier 3, there was no snooze condition and alerts would occur every 10-minute period. If no alert occurred in the snooze period, the threshold would be set to the lowest tier (Table 1).
The alerts (Tier 0,1,2,3) based on individual vital or based on composite score of vitals were raised on centralized monitoring station which was placed in the Inpatient Unit or the Day-Care Unit for the Hospital Cohort or was an App-based alert for the Home Cohort. The alerts by Dozee® were informed to the treating Physician or their designated team member by the nursing staff.
Vitals monitoring in the Control Arm: In the Hospital Cohort, patients in the Control Arm were monitored by spot checks conducted by nurses as per the hospital protocol and as per the instructions of the treating physician. These alerts were informed to treating Physicians or their designated team by the nursing staff. In the Home Cohort, those patients who were randomized to the control arm were monitored by the patient’s caregivers based on the discharge instructions given to them by their treating team of doctors and nurses. The alerts, events, interventions & outcomes were recorded for patients monitored at home in similar manner as it was done in hospital. The emergency revisit to hospital based on Dozee® alert, caregiver alert was recorded for patients after discharge from hospital.
Alerts: The alerts on which any intervention (whether increased monitoring, diagnostic intervention, or therapeutic intervention) was done were classified as ‘actionable alerts.’ In the Dozee® arm, tier-based alerts were raised by the Dozee® While in the Control Arm they were raised by either the caregivers (in both the Hospital and Home Cohorts) or the healthcare workers such as the doctors or nurses in the Hospital Cohort. The details of all actionable alerts were recorded for both Dozee® and control arms. Values of all the vitals (Heart Rate, Respiratory Rate, Blood Pressure, Mean Arterial Blood Pressure, Oxygen Saturation, Temperature, GCS) were recorded at the time of actionable alert.
Events, Interventions, and Outcomes: Details of ‘events’ such as bleeding, seizure, cardiac arrest, deterioration in level of consciousness were recorded with date and time. ‘Interventions’ which were conducted done based on actionable alerts and/or events were recorded with date and time. The interventions were categorized as increased monitoring, diagnostic interventions, and therapeutic interventions. Diagnostic interventions included blood test, urine test, ECG, Radiologic tests (CT- Scan, MRI, PET Scan etc.). The therapeutic interventions included a change in medication, administration of Intravenous (IV) fluids, administration of oxygen, transfer to ICU, Cardio Pulmonary Resuscitation (CPR) or an emergency revisit to hospital in the Home Cohort. The details of ‘outcomes’, such as the necessity of admission to the ICU were recorded. In the patients who required ICU care, the details of, the duration of ICU treatment, the requirement of assisted ventilation, inotropes and dialysis were documented.
Statistical Analysis: The descriptive data were reported as means with standard deviation or medians with range and frequencies with percentages as appropriate. Continuous data were compared with t‑test or Mann–Whitney U‑test as appropriate. Proportions were compared using the Pearson’s Chi‑square test or Fisher’s exact test. All statistical tests were two‑tailed, with a P = 0.05 or less considered statistically significant. Data were analysed using the IBM SPSS Statistics for Windows, Version 23.0 /Stata 16.0.1.
Results
Baseline Characteristics
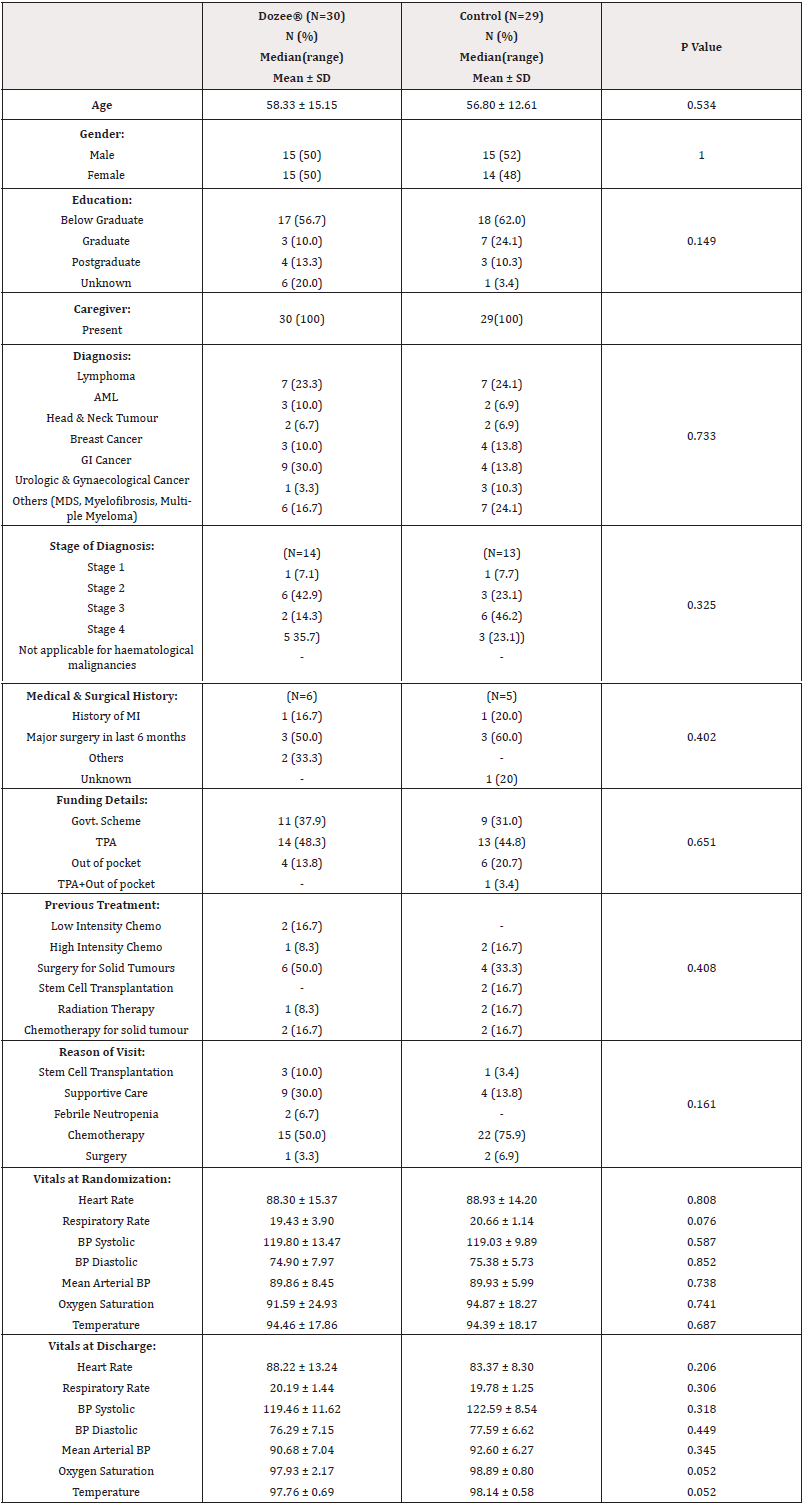
The patient demographics, disease and treatment details and the baseline vital parameters given in Table 2. All the baseline characteristics were comparable between the Dozee® and control arms. There was no statistically significant difference in the underlying diagnosis, past medical and surgical history, previous treatment and reason for admission between two groups at randomization.
The study involved a comparison between two arms: the Dozee®-monitored arm (30 patients) and the control arm (29 patients).
Hospital Cohort
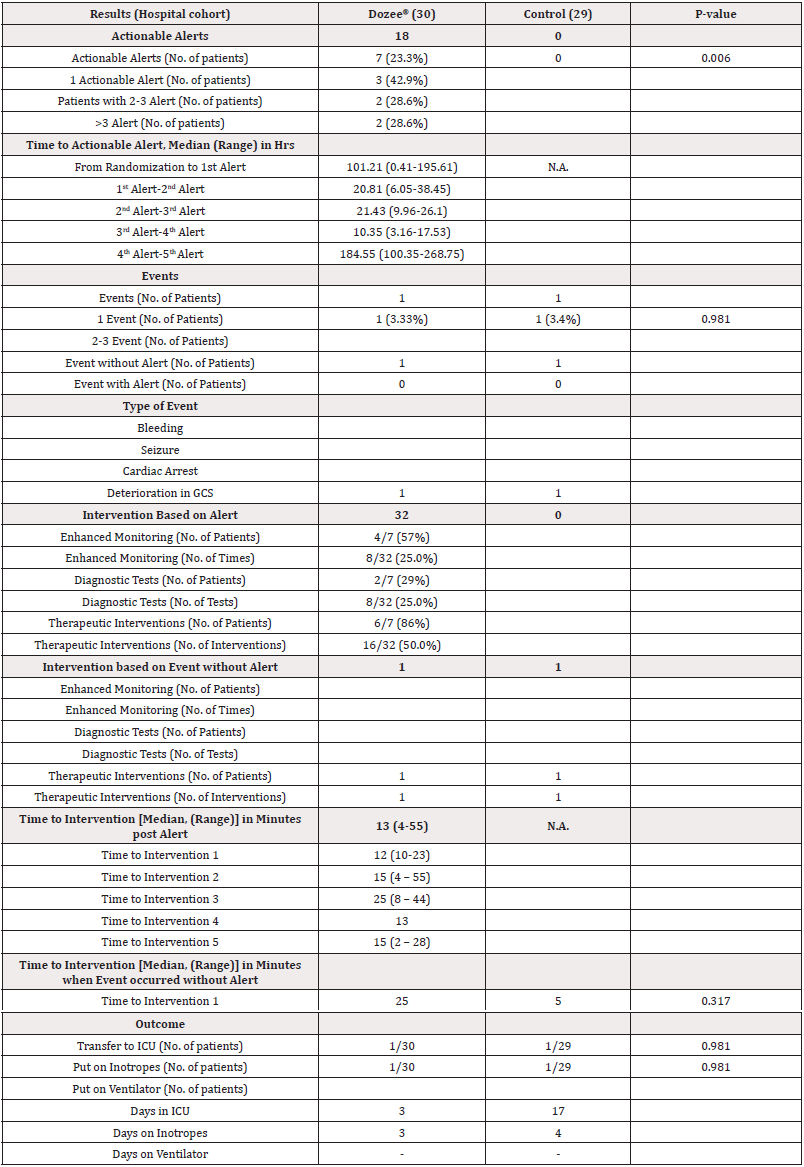
In the Dozee® monitored arm, as indicated in Table 3, 7 patients (23.3%) received a total of 18 actionable alerts, whereas none were observed in the control arm (p=). These alerts were triggered after a median duration of 101.21 hours (range: 0.41 - 195.61) following randomization. As a result of these alerts in 7 patients in the Dozee® arm, 32 interventions were carried out. 4/7 (57%) patients underwent enhanced monitoring, 2/7 (29%) patients underwent additional diagnostic tests and 6/7 (86%) patients received additional therapeutic interventions. Out of these, 50% of the interventions were therapeutic interventions, 25% were diagnostic, and 25% were increased monitoring. Time to interventions post-alert in Dozee® was median 13 minutes (range 4-55 minutes). One patient in each arm required ICU care for ionotropic support. The patient in the Dozee® arm, who required ICU care, did so or 3 days while the one from the control arm was in the ICU for 17 days. In both arms, 1 patient experienced reduction in GCS scale without an alert, which required oxygen administration (Table 3).
Home Monitoring Cohort
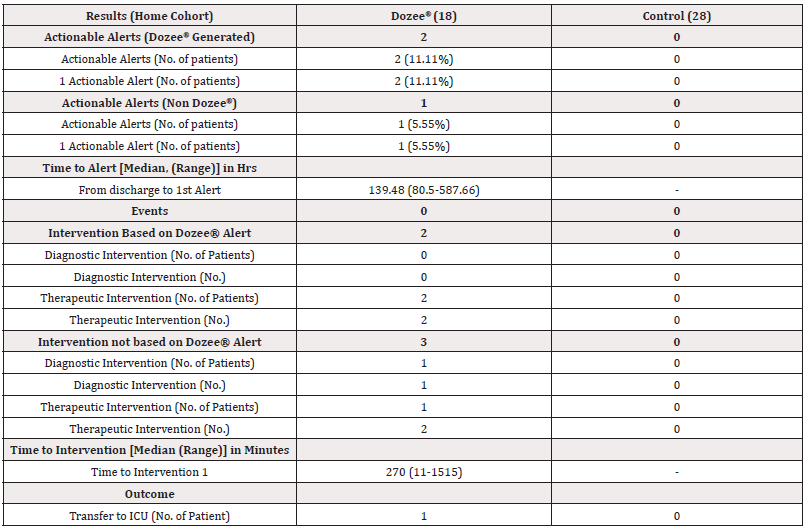
Although 30 patients were randomized to the Dozee®-monitored arm, the Dozee® device could be fitted in the home of only 18 patients. Failure of installing the Dozee® device in the homes of 8 patients was due to multiple reasons like unavailability of proper bed to install device, patient relocated to some other place, one patient reported discomfort with technology. The control arm in the Home Cohort had 28 patients, there was no dropout. 2 (11.11%) patients in the Dozee® monitored arm received 2 actionable alerts. There were no actionable alerts in control arm. The time from discharge to actionable alert in Dozee® arm was a median of 139.48 hours (range 80.5 -587.66 hours). 2/2 (100%) patients had therapeutic intervention in Dozee® arm. One patient visited emergency department based on the Dozee® alert and had to be transferred to the ICU due to sepsis. The other patient required an increase in the dose of antihypertensive medication based on the Dozee® alert. Details of this are given in (Table 4).
Discussion
The results of our study demonstrate the potential clinical utility of Dozee® monitoring in both hospital and home settings. In the hospital monitoring phase, Dozee® was associated with increase in significant actionable alerts, leading to therapeutic and diagnostic interventions as well as enhanced monitoring in a subset of patients. These findings are consistent with previous research indicating the effectiveness of continuous remote monitoring systems in identifying early signs of deterioration and facilitating timely interventions [17-20].
While the overall outcomes, such as transfer to the Intensive Care Unit (ICU) and use of inotropes, did not show statistically significant differences between the Dozee® and control arms, it is important to note the numerical trend towards fewer days spent in the ICU in the Dozee® arm. The current findings, which align with earlier studies, indicate that the use of Continuous Monitoring System Technology in hospital settings to optimize clinical practices may yield favourable outcomes, including reductions in ICU utilization, Length of Stay, and associated costs [21]. This suggests a potential benefit of Dozee® monitoring in preventing or mitigating the need for intensive care, although further studies with larger sample sizes are warranted to confirm these observations. In the home monitoring phase, Dozee® continued to demonstrate its ability to generate clinically significant actionable alerts, resulting in therapeutic interventions and timely hospital admissions. The variability in the time to the first alert after discharge underscores the importance of continuous monitoring in detecting subtle changes in patients' health status, particularly in the post-discharge period when they may be at risk of deterioration [22-24].
The interventions triggered by Dozee® alerts, including adjustments to medication dosages and timely hospital admissions, highlight its role as a valuable tool in remote patient management. These findings align with previous studies indicating the potential of remote monitoring technologies to improve patient outcomes and reduce healthcare costs by preventing unnecessary hospital readmissions and complications [25-27]. The observed increase in actionable alerts presents a promising avenue for potentially enhancing clinical outcomes, including reductions in mortality rates and decreased incidence of ICU transfers. These assertions find support in our study's findings, as well as corroborating evidence from previous research. [28,29]. Collectively, the evidence suggests that increasing the frequency and sensitivity of actionable alerts within remote monitoring systems has the potential to yield tangible improvements in patient outcomes, including reductions in mortality rates and mitigated risks of ICU transfers.
However, it is important to acknowledge the limitations of our study, including the relatively small sample size and the need for further analysis to fully understand the clinical implications of Dozee® alerts. Future research should explore the long-term impact of Dozee® monitoring on patient outcomes, as well as its integration into existing healthcare systems to optimize its effectiveness in routine clinical practice.
Conclusion
Our phase II randomized controlled trial evaluating remote continuous patient monitoring using the Dozee® Early Warning System (iEWS) in cancer patients undergoing active treatment demonstrates promising results for improving clinical outcomes. The use of Dozee® led to a higher number of actionable alerts, facilitating timely interventions and closer monitoring both in hospital and home settings. These interventions ranged from enhanced monitoring to diagnostic and therapeutic interventions, potentially averting adverse events and reducing the need for ICU transfers. While our study suggests the potential clinical utility of Dozee® monitoring, larger-scale investigations are needed to confirm these findings and assess its long-term impact on patient outcomes. Nevertheless, the observed increase in actionable alerts highlights the importance of continuous remote monitoring in detecting early signs of deterioration and enabling timely interventions. Incorporating technologies like Dozee® into routine clinical practice may offer a valuable tool for improving patient care and reducing healthcare costs by preventing complications and optimizing resource utilization.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of HealthCare Global (HCG) Hospital, Bengaluru, India. Written informed consent to participate in this study was provided by the participants.
Author Contributions
SJ involved in the Conception, Design of the work, Analysis, Interpretation of data, and Revision of drafted work. AA involved in the Acquisition, Analysis, Interpretation of data, and Revision of drafted work. KC involved in the Interpretation of data, and Revision of drafted work. GP involved in the Conception, Design of the work, Analysis, Interpretation of data, and Revision of drafted work. PK involved in the Acquisition, Analysis, Interpretation of data, and drafted the work and Revision of drafted work. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Funding
In this study, no external funding was received.
Acknowledgements
We are grateful to all of the patients for their assistance and cooperation in this study.
Guarantor
No specific guarantor was designated for this study.
Conflicting Interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Summary
Our study underscores the importance of leveraging innovative technologies for remote patient monitoring in non-ICU settings, particularly in high-risk populations such as cancer patients undergoing active treatment. Further research is warranted to elucidate the full potential of Dozee® monitoring and its integration into comprehensive care strategies aimed at enhancing patient outcomes and reducing healthcare burden.
References
- Thompson R, Luettel D, Healey F, Scobie S, Beaumunt K, et al. (2007) Safer care for the acutely ill patient: learning from serious incidents. The fifth report from the Patient Safety Observatory. Report, The National Patient Safety Agency, UK.
- Luettel D, Beaumont K and Healey F (2007) Recognising and responding appropriately to early signs of deterioration in hospitalised patients. Report, The National Patient Safety Agency, UK.
- Hillman KM, Bristow PJ, Chey T, Daffurn K, Jacques T, et al. (2002) Duration of life-threatening antecedents prior to intensive care admission. Intensive Care Med 28(11): 1629-1634.
- Schein R, Hazday N, Pena M, Ruben BH, Sprong CL, et al. (1990) Clinical antecedents to in-hospital cardiopulmonary arrest. Chest 98(6): 1388-1392.
- Buist MD, Jarmolowski E, Burton PR, Bernard SA, Waxman BP, et al. (1999) Recognising clinical instability in hospital patients before cardiac arrest or unplanned admission to intensive care: a pilot study in a tertiary-care hospital. Med J Aust 171(1): 22-25.
- Goldhill DR, McNarry AF, Hadjianastassiou VG, Tekkis PP (2004) The longer patients are in hospital before intensive care admission the higher their mortality. Intensive Care Med 30(10): 1908-1913.
- Mokart D, Lambert J, Schnell D, Louis Fouché, Antoine Rabbat, et al. (2013) Delayed intensive care unit admission is associated with increased mortality in patients with cancer with acute respiratory failure. Leuk Lymphoma 54(8): 1724-1729.
- Song JU, Suh GY, Park HY, So Yeon Lim, Seo Goo Han, et al. (2012) Early intervention on the outcomes in critically ill cancer patients admitted to intensive care units. Intensive Care Med 38(9): 1505-1513.
- Lee DS, Suh GY, Ryu JA, Chi Ryang Chung, Jeong Hoon Yang, et al. (2015) Effect of early intervention on long-term outcomes of critically ill cancer patients admitted to ICUs. Crit Care Med 43(7): 1439-1448.
- Kipnis P, Turk BJ, Wulf DA, Juan Carlos LaGuardia, Vincent Liu, et al. (2016) Development and validation of an electronic medical record-based alert score for detection of inpatient deterioration outside the ICU. J Biomed Inform 64: 10-19.
- Churpek MM, Yuen TC, Winslow C, David O Meltzer, Michael W Kattan, et al. (2016) Multicenter comparison of machine learning methods and conventional regression for predicting clinical deterioration on the wards. Crit Care Med 44(2): 368-374.
- Bailey TC, Chen Y, Mao Y, Chenyang Lu, Gregory Hackmann, et al. (2013) A trial of a real-time alert for clinical deterioration in patients hospitalized on general medical wards. J Hosp Med 8: 236-242.
- Kedia S, Ur Rahman I, Kaushal A, Mahizhvannan E, Jain RS, et al. (2023) Clinical Validation of an Indigenous Micro-Vibration Vital Parameter Monitor Dozee® 2023 15th International Conference on COMmunication Systems & NETworkS (COMSNETS) :141-146.
- Saran V, Kumar G, Parchani G (2019) Unsupervised Extraction of Respiration Cycles Through Ballistocardiography. Advanced Informatics for Computing Research, Springer Singapore: 136-147.
- Saran V, Kumar R, Kumar G, Chokalingam K, et al. (2022) Validation of Dozee®, a Ballistocardiography-based Device, for Contactless and Continuous Heart Rate and Respiratory Rate Measurement. Conf Proc IEEE Eng Med Biol Soc 2022:1939-1943.
- Saran V, Kumar G, Dhawan U, Parchani G (2018) Unsupervised Identification of Cardiac Contraction through Ballistocardiography. 2018 IEEE-EMBS Conference on Biomedical Engineering and Sciences (IECBES): 42-47.
- Areia C, Biggs C, Santos M, Neal Thurley, Stephen Gerry, et al. (2021) The impact of wearable continuous vital sign monitoring on deterioration detection and clinical outcomes in hospitalised patients: a systematic review and meta-analysis. Crit Care 25(1): 351.
- Vandenberk B, Raj SR (2023) Remote Patient Monitoring: What Have We Learned and Where Are We Going? Curr Cardiovasc Risk Rep 17(6): 103-115.
- Subbe CP, Duller B, Bellomo R (2017) Effect of an automated notification system for deteriorating ward patients on clinical outcomes. Crit Care 21(1): 52.
- Smith AC, Thomas E, Snoswell CL, Helen Haydon, Ateev Mehrotra, et al. (2020) Telehealth for global emergencies: Implications for coronavirus disease 2019 (COVID-19). J Telemed Telecare 26(5): 309-313.
- Dykes P, Lowenthal G, Lipsitz S, Suzanne M Salvucci, Catherine Yoon, et al. (2022) Reducing ICU Utilization, Length of Stay, and Cost by Optimizing the Clinical Use of Continuous Monitoring System Technology in the Hospital. The American Journal of Medicine135(3): 337-341.
- Kessler AJ, Besculides M, Kisswany C (2022) Promoting digital health equity through remote patient monitoring: A feasibility study. Journal of Clinical Oncology [Internet].
- Londral A, Azevedo S, Dias P, C Ramos, J Santos, et al. Developing and validating high-value patient digital follow-up services: a pilot study in cardiac surgery. BMC Health Serv Res 22(1): 680.
- Leijdekkers P, Gay V (2015) Mobile health for community-based organizations: a case study. Health Informatics J 21(2):101-112.
- Dawson NL, Hull BP, Vijapura P, Adrian G Dumitrascu, Colleen T Ball, et al. (2021) Home Telemonitoring to Reduce Readmission of High-Risk Patients: a Modified Intention-to-Treat Randomized Clinical Trial. J Gen Intern Med 36(11): 3395-3401.
- Ong MK, Romano PS, Edgington S, Harriet U Aronow, Andrew D Auerbach, et al. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the Better Effectiveness After Transition -- Heart Failure (BEAT-HF) randomized clinical trial. JAMA Intern Med. 2016;176(3):310-318.
- Wakefield BJ, Ward MM, Holman JE, Annette Ray, Melody Scherubel, et al. (2008) Evaluation of home telehealth following hospitalization for heart failure: a randomized trial. Telemed J E Health 14(8): 753-761.
- Iqbal FM, Lam K, Joshi M, Khan S, Ashrafian H, et al. (2021) Clinical outcomes of digital sensor alerting systems in remote monitoring: a systematic review and meta-analysis. NPJ Digit Med 4(1):7.
- Acharya M, Ali MM, Bogulski CA, Ambrish A Pandit, Ruchira V Mahashabde, et al. Association of Remote Patient Monitoring with Mortality and Healthcare Utilization in Hypertensive Patients: a Medicare Claims–Based Study. J GEN INTERN MED 39(5): 762-773.




 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.