Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Role of Formyl Peptide Receptor 1 (FPR1) in Prostate Cancer Progression: Insights from Expression Analysis and Therapeutic Intervention
*Corresponding author: Fatima Khurshid, Medical Officer, Department of Radiation Oncology, Shifa International Hospital ltd. Islamabad, Pakistan.
Received: April 23, 2024; Published: April 30, 2024
DOI: 10.34297/AJBSR.2024.22.002950
Abstract
Background: Given that prostate cancer causes a significant global health burden, it is imperative to comprehend the molecular mechanisms underlying the disease. While its precise role remains unclear, formyl peptide receptor 1 (FPR1) is identified as a putative regulator in the advancement of prostate cancer.
Objective: The study employed flow cytometry to assess FPR1 expression in mouse tumor xenografts, examine the antagonistic effect of ICT12035, analyze FPR1 expression in prostate cancer cell lines PC-3 and DU-145, and assess FPR1 receptor functionality.
Methodology: The study employed the FPR1 agonist fMLF and the FPR1 antagonist ICT12035, as well as the human prostate cancer cell lines PC-3 and DU145. Standard operating procedures were followed to maintain cell cultures, and FPR1 expression was measured by flow cytometry. FPR1 levels were measured in xenograft tissues using immunohistochemistry. Calcium flux assays were performed to see how cells responded to fMLF and ICT12035. Scratch experiments revealed cell migration following fMLF therapy. Furthermore, cell viability was assessed following exposure to fMLF and ICT12035 using the MTT assay. The statistical evaluation was carried out in Microsoft Excel, with a significance level of P < 0.05.
Results: The study revealed that castration-resistant prostate cancer cell lines PC-3 and DU-145 express high levels of FPR1, with DU-145 displaying slightly greater levels. Immunohistochemistry analysis demonstrated that cancerous tissues expressed higher levels of FPR1 than normal prostate cells. ICT12035, a medication, inhibited prostate cancer cell proliferation and had an antagonistic effect on fMLF-induced calcium mobilization in DU145 cells, indicating its potential utility as an FPR1 antagonist in prostate cancer therapy.
Conclusion: FPR1, a key regulator of prostate cancer development, has been identified as a potential therapeutic target. ICT12035, a small molecule FPR1 antagonist, has shown potent anti-proliferative activity without toxicity, suggesting potential for prostate cancer treatment. Further investigation is needed to fully understand the therapeutic benefits of FPR1 inhibition in prostate cancer treatment.
Keywords: Metastasis, Prostate cancer, Calcium flux, Therapeutic target
Introduction
Prostate cancer is one of the most frequent malignancies in men worldwide, with significant rates of morbidity and mortality [1,2]. Despite advances in diagnostic and treatment techniques, our understanding of the underlying biological pathways of this illness is constantly evolving [3,4]. The role of formyl peptide receptor 1 (FPR1) in the metastasis and development of prostate cancer has received more attention in recent years [5]. The G protein-coupled receptor FPR1, which is mostly found in immune cells, has been connected to a variety of physiological and pathological processes, including inflammation, the immune system, and cancer [6].
An accumulating body of data suggests that FPR1 may be critical to the pathogenesis of prostate cancer [7]. According to research, prostate cancer cells express FPR1 differently than normal prostate tissues, implying that this protein may be involved in the disease's pathogenesis [8]. FPR1 expression levels have been associated to tumor aggressiveness, metastatic potential, and overall patient prognosis, emphasizing its significance in prostate cancer biology [9]. Despite these exciting discoveries, the particular pathways underlying FPR1's participation in prostate cancer remain unclear [10]. Clarifying FPR1's functional involvement in prostate cancer development and its possible therapeutic target is thus critical [11]. In order to fill this knowledge gap, we comprehensively investigated the function of FPR1 in prostate cancer in our research. We investigated the in vivo importance of FPR1 expression using mouse tumor xenografts and flow cytometry on the PC-3 and DU-145 cell lines. In addition, we employed calcium flux and scratch studies to evaluate FPR1's functional activity and interaction with the N-Formylmethionyl-Leucyl-Phenylalanine (fMLF) ligand. Finally, we conducted the MTT test to determine the maximum tolerated dose of ICT12035, a potential FPR1 antagonist, which set the ground for future therapeutic approaches.
The purpose of this study was to determine the highest permissible dose of ICT12035 that is safe to use using MTT, investigate the antagonistic impact of ICT12035 in prostate cancer cell lines using the calcium flux assay, evaluate the functioning of the FPR1 receptor and its reaction when exposed to fMLF ligand using the calcium flux and scratch assays, and use flow cytometry to assess FPR1 expression in prostate cancer cell lines PC-3 and DU-145.
Material and Methods
Cell Lines, Chemicals, and Assay Kits
The research used the medication ICT12035, which was produced by Dr. Victoria Vinader at the University of Bradford's Institute of Cancer Therapeutics, and the FPR1 agonist fMLF, which was acquired from Sigma-Aldrich. The American Type Culture Collection (ATCC) human prostate cancer cell lines PC-3 and DU145 were used. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide reagent was employed in the MTT experiment. Chemicals of analytical grade were used. A BD Biosciences FACS-Calibur flow cytometer was used for the flow cytometry analysis. Thermo Fisher Scientific's Invitrogen Molecular Probes TM Fluo-4 NW calcium assay kit was employed for calcium flow analysis.
Cell Culture
The PC3 and DU145 cell lines were maintained in RPMI 1640 that was enhanced with 2 mM glutamine, 12.5 units/ml penicillin, 6.5 μg/ml streptomycin, 10% fetal bovine serum, and 10 nM human insulin. The cells were grown at 3 × 10^5 cells/well in 6-well plates at 37°C in a humid environment with 95% air and 5% CO2.
Flow Cytometry
After harvesting the cells, they were treated with a primary polyclonal antibody against FPR-1 and a monoclonal antibody that was an isotype control. FPR1 expression was examined in fixed, permeabilized, and blocked cells using the FACS-Calibur flow cytometer.
IHC and staining of FPR1 in Xenografts
Sections of paraffin-embedded tissues were prepared for immunostaining with FPR1 primary antibody. The steps involved were antigen retrieval, blocking, primary and secondary antibody incubation, DAB solution detection, and counterstaining. After dehydrating, sections were mounted for examination under a microscope.
Calcium Flux Assay: A 96-well plate was seeded with DU145 cells, filled with Fluo-4 NW dye, and subjected to ICT12035 and fMLF treatments. A microplate reader was used to measure the fluorescence throughout a 120-second period.
Scratch Assay
PC3 and DU145 cells underwent fMLF treatment, starvation, scratching, and seeding. After 14 hours, gap closing was seen using microscopy.
MTT Assay
After being planted in 96-well plates, PC3 and DU145 cells were treated with fMLF and ICT12035, and they were then incubated for four days. To determine the vitality of the cells, formazan crystals were dissolved, MTT solution was added, and absorbance was measured. For information on the plate design utilized in this experiment, see Figure 1. Control measurements were made using DMSO and a medication at 0nM.
Statistical Analysis
Microsoft Excel was employed for all statistical analysis. At P < 0.05, a statistically significant difference was established. One-tailed and two-tailed t-tests evaluating two variables were used to compare the groups statistically (where necessary). Excel bar and line graphs were used to construct the plots, and the mean of a series of tests was used to get the data.
Results
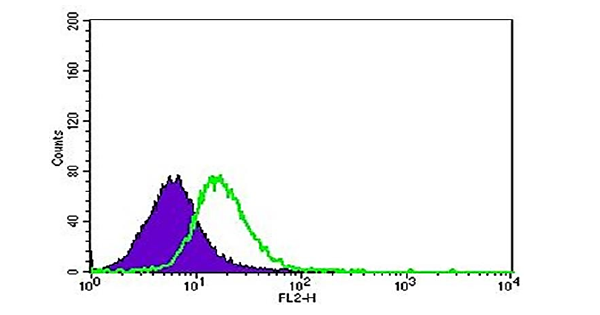
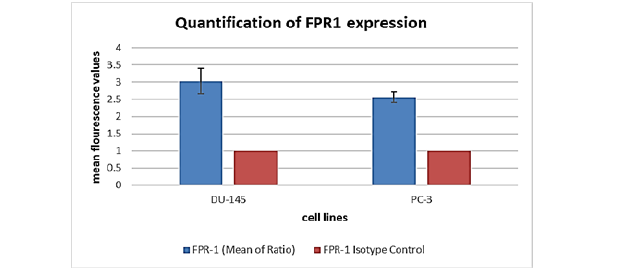
FPR1 expression was assessed by flow cytometry in the androgen receptor (AR)-negative "classical" prostate cancer cell lines DU-145 and PC-3, which are resistant to castration. Because these cell lines have a higher propensity for metastasis than androgen-dependent LNCaP cells, they were selected to investigate metastatic castration-resistant prostate cancer (mCRPC). DU-145 and PC-3 were the main subjects of our inquiry because of their tendency to form distant metastases quickly. Predominant FPR1 expression was found in both cell lines after analysis (see Figures 2 and 3). Interestingly, one-tailed t-test analysis revealed that FPR1 expression was somewhat greater in DU-145 than in PC-3, with statistical significance (p=0.05) supported (Figure 4). This discrepancy may be explained by the increased proliferative ability of DU-145 or by protein-protein interactions that cause downregulation in PC-3 cells, which might lead to a lower antibody signal. To determine the functional state of the expressed receptors, further investigation is necessary. Visualization of FPR-1 surface expression was made possible by flow cytometry analysis, where FPR-1 signals are shown in green and isotype control antibody is shown in purple.
Evaluating Immunohistochemistry-Based Functional Expression of FPR1
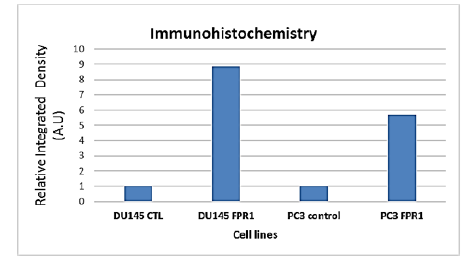
A study investigated the expression of FPR1 proteins in animal tumor xenografts grown subcutaneously. Results showed that normal prostate cells stained negatively for FPR1, but prostate tissue samples from PC-3 and DU-145 cells showed significantly higher expression levels than normal tissues. This was observed in Figures 5 and 6 (A: Prostate tissue in normal condition; B: Malignant tissue that expresses FPR1 as dark stains) and 6 (A: Prostate tissue in normal condition; B: Brown stains indicating FPR1 transcription in malignant tissue), where FPR1 transcription is noticeably elevated close to the tumor's necrotic region. Densitometric analysis of FPR1 expression in tumor xenograft samples compared to normal prostate tissue showed a much greater integrated density of FPR1. Immunohistochemistry analysis also showed that FPR1 is expressed more in DU145 than in PC3.
Analyzing FPR1's Functional Activity and Response to fMLF Using the Scratch Assay
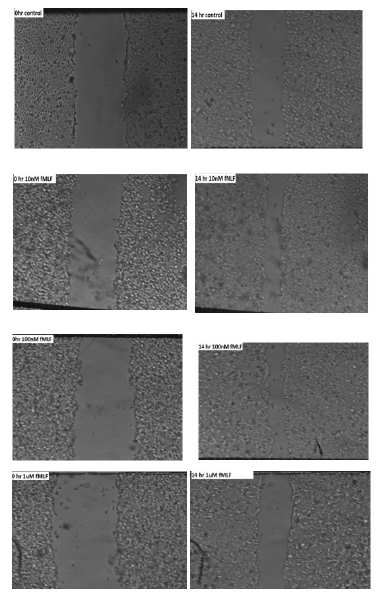
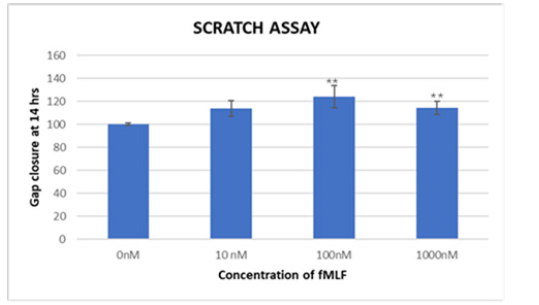
Cell migration plays a critical role in tumor metastasis. One method that is frequently used to calculate cell migration is the scratch test. Our findings indicate that fMLF increases the propensity for migration, indicating that FPR1 activation promotes tumor cell dispersion in prostate cancer. When fMLF is present, gap closure is shown in Figure 8, suggesting increased invasiveness.
The fMLF-treated cells traveled faster toward the center of the cell monolayer gaps than did the vehicle-treated cells. As seen in Figure 6, the cells treated with 100nM of fMLF for 14 hours had the largest gap closing, around 24% greater than the control. Figure 9 displays the average of the outcomes from the three runs of this experiment. Three trials' worth of data were averaged, and the results showed significant p-values of 0.01** and 0.02*. The graph indicates a peak response at 100nM, most likely due to receptor desensitization as ligand concentration increases, since it exhibits greater cell motility at 100nM fMLF-induced FPR1 activation in contrast to 10nM and 1000nM concentrations. Following 30 seconds of FPR1 binding, fMLF internalizes and starts downstream signaling pathways, which are mediated by phosphorylation of the carboxy-terminal serine and threonine residues of the receptor, an arrestin-induced desensitization mechanism. G-protein dissociation and the onset of intracellular signaling follow from this. It appears that homologous desensitization occurs between 10nM and 100nM, indicating the need for additional study. Statistical analysis reveals a significant change in cell migration at different doses (p-value <0.05).
Determining the ICT12035 Maximum Tolerated Dose By applying the MTT Assay
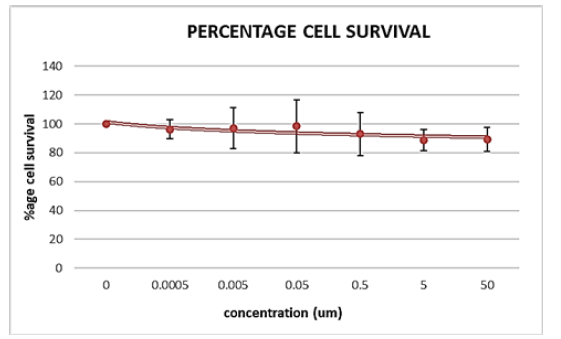
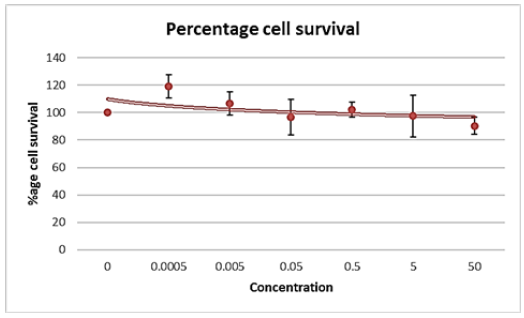
Our objective was to determine the Maximum Tolerated Dosage (MTD) of the small molecule drug ICT12035 in order to assess its effectiveness in prostate cancer cell lines. The MTT assay was employed for this purpose because to its widespread use as a quantitative cytotoxicity test due to its sensitivity, accuracy, speed, and ease of use. The test was conducted in three independent trials and evaluated cell viability at various drug dosages relative to untreated cells. Figures 10A and B display the average percentage of viability that was determined from these investigations.
The maximum tolerated dosage (MTD) for ICT12035 was determined to be around 0.05uM for PC-3 cell lines and 0.0005uM for DU145 cell lines. The highest concentration of ICT12035 that was employed was 1uM. ICT12035 exhibits anti-proliferative rather than cytotoxic effects on prostate cancer cell lines via reducing fMLF function. Conversely, higher observed toxicity could be the result of uneven cell concentration or bacterial contamination during the course of the experiment. Despite a 10% drop in viability over the course of the 4-day therapy (Figure 10A), cells withstood treatment with ICT12035, suggesting that higher drug dosages may be used in future research. However, adhering to the proper methods for incubation length and cell concentration is crucial for more accurate MTD findings. Since the MTT assay employed cannot distinguish between cytotoxic and cytostatic effects, more tests are necessary for a comprehensive assessment.
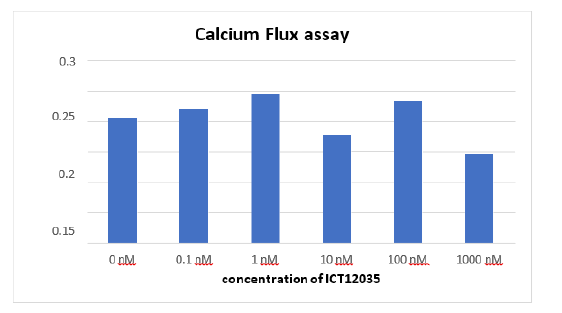
Analysis of Antagonistic Activity of IC12035 Using Calcium Flux Assay
The aim of the study was to investigate if IC12035 inhibits the intracellular Ca2+ mobilization of DU145 prostate cancer cells in response to fMLF. fMLF activates FPR1, which leads to the release of PIP3 and PLC, which in turn increases calcium mobilization and chemotaxis. We used a functional, cell-based method called the calcium flux assay to measure absorbance changes as a proxy for drug efficacy. The medicine's antagonistic effect was demonstrated through the plotting of normalized data from every well that received varying doses of ICT12035. With an IC50 of around 0.5 uM, the maximum degree of inhibition was observed at 1 uM ICT12035 (Figure 10). The decrease in calcium flow at this dosage validates the antagonistic activity of the drug. Further investigation is necessary, nevertheless, as Figure 11's unusual behavior at 10nM and 100nM concentrations suggests that the experimental design may be the source of variability. More consistent and dependable results could be obtained by conducting the experiment again.
Discussion
Our analysis, which is consistent with prior findings [12], revealed a high level of FPR1 expression in the prostate cancer cell lines DU-145 and PC-3. Interestingly, we discovered that DU-145 cells expressed FPR1 slightly higher than PC-3 cells, implying that distinct prostate cancer morphologies may have varied degrees of FPR1 abundance. This finding is consistent with previous research that has found differential FPR1 expression between prostate cancer and normal prostate tissues [13]. These findings highlight the heterogeneity of FPR1 expression levels in prostate cancer, as well as the need for additional study to better understand the functional repercussions in various disease scenarios.
We used the scratch test to study the functional relevance of FPR1 in prostate cancer cell migration. Our findings, which are consistent with earlier studies [14,15], show that activating FPR1 with the fMLF ligand dramatically increases cell migration, implying that FPR1 may play a role in tumor dissemination. Furthermore, our dose-response research demonstrated that 100nM fMLF induced peak cell motility, shedding fresh light on the dynamic nature of FPR1 signaling in prostate cancer metastasis. These findings are consistent with previous research that has highlighted the importance of FPR1 signaling in regulating cancer cell motility and invasion [16]. The dose-dependent response found in our work emphasizes the complicated signaling pathways involving FPR1 and its potential as a therapeutic target to prevent prostate cancer metastasis.
In our investigation of prospective FPR1-targeting therapeutic options, we assessed the efficacy of the FPR1 antagonist ICT12035 in suppressing prostate cancer cell proliferation and calcium flow. Our findings showed that ICT12035 has high anti-proliferative capabilities without producing cytotoxicity, indicating that it could be a promising therapeutic agent for prostate cancer treatment [17,18]. Furthermore, our calcium flux experiment results supported ICT12035's activity as an FPR1 antagonist by reducing fMLF-induced intracellular calcium mobilization in a dose-dependent manner. These findings are consistent with prior research demonstrating the therapeutic potential of targeting FPR1 signaling pathways in several forms of cancer [19]. ICT12035 has a great potential as a targeted treatment to slow the growth of prostate cancer since it effectively modulates FPR1-mediated signaling.
Our study builds on prior studies into the role of FPR1 in prostate cancer progression. The elevated expression of FPR1 in prostate cancer cell lines and xenografts lends credence to its role in tumor formation and metastasis [20,21]. Furthermore, our findings shed new light on how FPR1 regulates prostate cancer cell migration and invasion, as well as the potential therapeutic implications of ICT12035 as an FPR1 antagonist. These discoveries open up new opportunities for the development of targeted therapeutics and increase our understanding of the molecular processes that underpin prostate cancer etiology. Our findings are consistent with prior research, highlighting FPR1's potential as a druggable target for therapeutic intervention and its importance in prostate cancer biology [20,21].
Conclusion
This study has shed light on FPR1’s critical involvement in the aggressiveness, invasion, and metastasis of many cancers, including prostate cancer. Notably, FPR1 has been identified as a crucial oncogene in the progression of metastatic castration-resistant prostate cancer. Our findings confirmed the elevated expression of FPR1 in PC3 and DU145 prostate cancer cell lines, emphasising its functional significance in tumour growth. Furthermore, FPR1 over-expression has been linked to increased prostate tumour growth, spread, and neovascularization. ICT12035, a small molecule FPR1 antagonist, showed substantial anti-proliferative action without toxicity, indicating that it could be used as a therapeutic agent for prostate cancer. Although the MTT assay produced conflicting findings, the calcium mobilisation test demonstrated the compound’s effectiveness, suggesting its potential for further study.
Acknowledgments
None.
Declaration of Interest
None.
References
- Rawla P (2019) Epidemiology of prostate cancer. World J Oncol 10(2): 63-89.
- Gandaglia G, Leni R, Bray F, Fleshner N, Freedland SJ, et al. (2021) Epidemiology and prevention of prostate cancer. Eur Urol Oncol 4(6): 877-892.
- Gandhi J, Afridi A, Vatsia S, Joshi G, Joshi G, et al. (2018) The molecular biology of prostate cancer: current understanding and clinical implications. Prostate Cancer Prostatic Dis 21(1): 22-36.
- Nakazawa M, Paller C, Kyprianou (2017) Mechanisms of therapeutic resistance in prostate cancer. Curr Oncol Rep 19(2): 1-13.
- Tian C, Chen K, Gong W, Yoshimura T, Huang J, et al. (2020) The G-protein coupled formyl peptide receptors and their role in the progression of digestive tract cancer. Technol Cancer Res Treat 19:1533033820973280.
- Vacchelli E, Le Naour J, Kroemer G (2020) The ambiguous role of FPR1 in immunity and inflammation. Oncoimmunology 9(1): 1760061.
- Gastardelo TS, Cunha BR, Raposo LS, Maniglia JV, Cury PM, et al. (2014) Inflammation and cancer: role of annexin A1 and FPR2/ALX in proliferation and metastasis in human laryngeal squamous cell carcinoma. PLoS One 9(12): e111317.
- Reed AB, Parekh DJ (2010) Biomarkers for prostate cancer detection. Expert Rev Anticancer Ther 10(1): 103-114.
- Park SJ, Greer PL, Lee N (2024) From odor to oncology: non-canonical odorant receptors in cancer. Oncogene 43(5): 304-318.
- Cheng B, Li L, Wu Y, Luo T, Tang C et al. (2023) The key cellular senescence related molecule RRM2 regulates prostate cancer progression and resistance to docetaxel treatment. Cell Biosci 13(1): 211.
- Kori M, Yalcin Arga K (2018) Potential biomarkers and therapeutic targets in cervical cancer: Insights from the meta-analysis of transcriptomics data within network biomedicine perspective. PLoS One 13(7): e0200717.
- Wang X, Wang X, Jin S, Muhammad N, Guo Z (2018) Stimuli-responsive therapeutic metallodrugs. Chem Rev 119(2):1138-1192.
- Mota ST, Vecchi L, Alves DA, Cordeiro AO, Guimarães GS et al. (2020) Annexin A1 promotes the nuclear localization of the epidermal growth factor receptor in castration-resistant prostate cancer. Int J Cell Biol 127: 105838.
- Minopoli M, Polo A, Ragone C, Ingangi V, Ciliberto G, et al. (2019) Structure-function relationship of an Urokinase Receptor-derived peptide which inhibits the Formyl Peptide Receptor type 1 activity. Sci Rep 9(1): 12169.
- Kretschmer D, Rautenberg M, Linke D, Peschel A (2015) Peptide length and folding state govern the capacity of staphylococcal β-type phenol-soluble modulins to activate human formyl-peptide receptors 1 or 2. J Leucoc Biol 97(4): 689-697.
- Cattaneo F, Russo R, Castaldo M, Chambery A, Zollo C et al. (2019) Phosphoproteomic analysis sheds light on intracellular signaling cascades triggered by Formyl-Peptide Receptor 2. Sci Rep 9(1): 17894.
- Ahmet DS, Basheer HA, Salem A, Lu D, Aghamohammadi A et al. (2020) Application of small molecule FPR1 antagonists in the treatment of cancers. Sci Rep 10(1): 17249.
- Ahmet DS. Targeting the formyl peptide receptor 1 for treatment of glioblastoma (Doctoral dissertation, University of Bradford).
- Ahmet DS, Basheer HA, Salem A, Lu D, Aghamohammadi A et al. (2020) Application of small molecule FPR1 antagonists in the treatment of cancers. Sci Rep 10(1): 17249.
- Cheng B, Li L, Wu Y, Luo T, Tang C et al. (2023) The key cellular senescence related molecule RRM2 regulates prostate cancer progression and resistance to docetaxel treatment. Cell Biosci 13(1): 211.
- Bizzarro V, Belvedere R, Milone MR, Pucci B, Lombardi R et al. (2015) Annexin A1 is involved in the acquisition and maintenance of a stem cell-like/aggressive phenotype in prostate cancer cells with acquired resistance to zoledronic acid. Oncotarget 6(28): 25076-92.







 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.