Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Study of the in Vitro Cytotoxicity Testing of Nanocapsules Prepared from Deer Antler Extract
*Corresponding author: Sevil Mehraliyeva Jabrail, PhD, Associate Professor, Pharmaceutical technology and management, Azerbaijan Medical University, Baku, Azerbaijan.
Received: March 13, 2024; Published: May 27, 2024
DOI: 10.34297/AJBSR.2024.22.002997
Abstract
An extract rich in amino acids was obtained from deer antlers grown in Azerbaijan, and nanocapsules were prepared on its basis. For this purpose, 2 versions of nanocapsules were prepared. In the first version, the surface of the extract is covered with the biopolymer kappa-carrageenan (K+P), and in the other, with tragacanth (T+P) [1-4]. Subsequently, in vitro cytotoxic analysis of the samples was carried out at the Central Research Testing and Analytical Laboratory of the Ege University of the Republic of Turkey Application and Research Center (Ege MATAL). As a result of the MTT test carried out at all concentrations (0.01-0.1-1 mg/ml) of samples on HaCat cells, it was found that cell viability exceeds 70% and that the samples do not exhibit a cytotoxic effect.
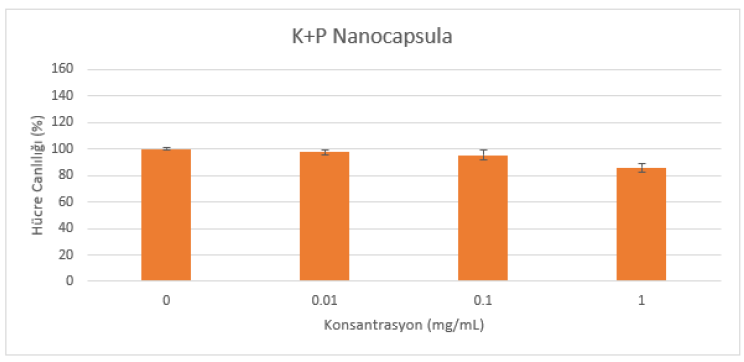
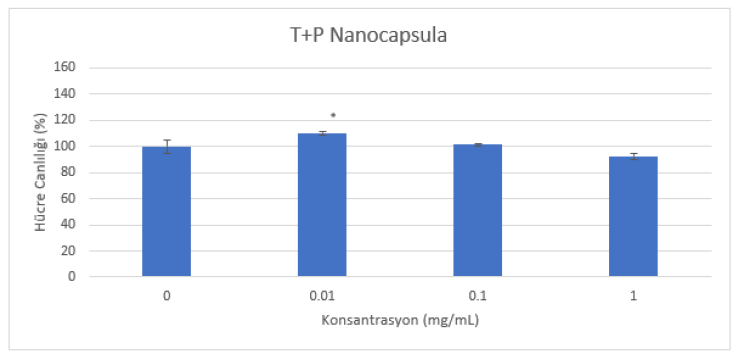
All experiments were repeated three times and the results were analyzed statistically using Graphpad Prism 8 as the statistical program. The results were analyzed using One-Way ANOVA test and Dunnett’s method was selected as the post hoc method. When all the results were statistically processed, it was found that there was a significant difference compared to the control at a concentration of 1 mg/ml of groups of cells to which the K+P Nanocapsula sample was applied (** p < 0.01). In the groups of cells to which the T+P Nanocapsula sample was applied, it was found that there was a significant difference compared to the control only at a concentration of 0.01 mg/ml (*p<0.05).
Keywords: Cytotoxic analysis, Deer antler extract, Carrageenan, Tracacanthus, Nanocapsules
Introduction
One of the priority issues of pharmaceutical technologies in the modern era is the development of new non-toxic drug delivery systems for the treatment and prevention of Alzheimer's disease based on local natural raw materials. The purpose is to test the toxicity of nanocapsules prepared on the basis of deer antler extract in an experiment in vitro with sufficient supplies of raw materials. To achieve the goal, the cytotoxic reaction of nanocapsules prepared in 2 variants was studied. It is known that over the past 15 years, the cytotoxicity test has been widely used as one of the tests for biological assessment and screening using tissue cells in vitro to monitor cell growth, proliferation, and morphological effects with the help of medical devices. Cytotoxicity is preferred in scientific research. This method is also important because it is simple, fast, highly sensitive and excludes animals from toxicity tests. It is known that the International Organization for Standardization 109993-5 defines three types of cytotoxicity tests: extraction, direct contact tests and indirect contact tests [5-9]. In our experiment, cytotoxicity was performed by applying samples on HaCat cells by MMT method.
Materials and Methods
a) Test Applied
Cytotoxicity test* (with MTT method)
b) Test Material
Powder sample (T+P Nanocapsula, K+P Nanocapsula)
c) Solvent*
Dimethyl sulfoxide (DMSO)
d) Type and source of cells used*
HaCat, Human keratinocyte cell line (Monolayer)
e) Passage Number of Cells Used
26.
f) Culture Medium
Dulbecco's MEM High Glucose, 10% Fetal Bovine Serum (FBS, S181G, Biowest, France), 1% Sodium Pyruvate (S8636, Sigma, Germany), 0.1% Penicillin-Streptomycin (A2213, Biochrom, Germany).
g) Negative Control
Material known to have no cytotoxic effect on the cell, Polyethylene (PE) (0.2g/mL extract in medium).
h) Positive Control
Material known to have a cytotoxic effect on the cell, Dimethyl Sulfoxide (DMSO) (20% (v/v) in the medium).
i) Reagent Control (Blank)
Culture prepared with cell culture medium that does not contain the test substance. *Regulated according to IS0 10993-12:1998 Sample Preparation and Reference Material Selection standard.
j) Sample Preparation Method
Powder samples were dissolved in DMSO to 1-10-100mg/mL, then 1% of these solutions were taken and diluted in Dulbecco's MEM High Glucose medium to prepare solutions with 0.01-0.1 and 1mg/mL concentrations. Since DMSO has a toxic effect on cells, it was added to the medium at a maximum rate of 1% (Figure 1 and Figure 2).
Discussion and Results
a) Cytotoxicity Analysis Method
A cytotoxicity test was performed with HaCat cells. The cytotoxic effect of the samples applied at different concentrations for 24 hours (in an environment of 5% CO2 and 95% humidity at 37°C) on the cells planted at a concentration of 1x105 cells/mL on 96 well microplates was evaluated by reading their absorbance on a spectrophotometer (570 nm) with the MTT test (Figure 1 and Figure 2).
b) Comment
The results of the 4-hour MTT test performed as a result of incubation of the cells with the test materials for 24 hours are given below.
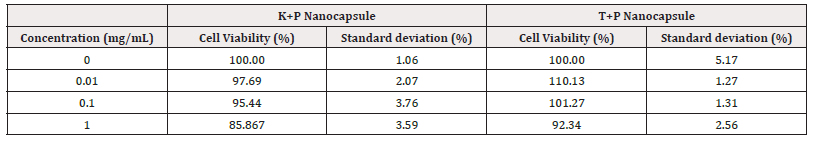
Negative Control cell viability rate: 96.8% ±1.13
Positive Control cell viability rate: 2.7% ±0.62
c) Test Materials Cell Viability Rates
(Table 1) When the MTT results performed with reference to the control of test materials are interpreted according to the ISO 10993-5:2009 Tests for In Vitro Cytotoxicity standard. As a result of the MTT test performed on HaCat cells at all concentrations (0.01-0.1-1mg/mL), it was determined that the samples did not show a cytotoxic effect since the cell viability was over 70%.
Conclusions
Nanocapsules as a new drug delivery system prepared by covering the surface of the extract obtained from deer antlers adapted to the climatic conditions of Azerbaijan have been proven to be non-toxic as a result of cytotoxic analysis. This also necessitates the use of nanocapsules prepared as a biologically active food additive from natural raw materials in a dose of 0.01-1mg/ml for the prevention of Alzheimer's disease.
Acknowledgments
None.
Conflict of Interest
None.
References
- Sevil Mehralıyeva (2023) The method of obtaining nanocapsules. Patent AZİ :0075.
- Sevil Mehraliyeva (2023) Preparation of Nano Medicines from Deer Antones in Azerbaijan and Study of Quality Criteria. Biom J Sci Tech Res: 40379-40385.
- Mehralıyeva SC (2022) Nanotechnologies in the field of development of medicines on the basis of natural raw materials Karabakh III İnternational congress of applied sciences Year of Shusha-2022. Karabakh /Azerbaijan. Proceeding book 2: 158-164.
- Mehralıyeva SC (2022) Analysis of nanocapsules prepared from deer antlers. Actual problems of medicine, "Shusha 270", Baku.
- IS0 10993-12:1998 Sample Preparation and Reference Material Selection standard.
- International Standard ISO 10993-5. Biological evaluation of medical devices- Part 5: Tests for in vitro cytotoxicity
- ISO 10993-12:2021(E) Biological evaluation of medical devices - Part 12: Sample preparation and reference materials.
- ISO 10993-5:2009(en) Biological evaluation of medical devices - Part 5: Tests for in vitro
- Weijia Li, Jing Zhou, Yuyin Xu (2015) Study of the in vitro cytotoxicity testing of medical devices. Biomed Rep 3(5): 617-620.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.