Mini Review 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Transfusion Medicine in Lower- and Middle-Income Countries - Impact of Universal Health Coverage and the WHO Model List of Essential Medicines
*Corresponding author: Cees Th. Smit Sibinga, Professor of International Development of Transfusion Medicine, University of Groningen and IQM Consulting, Zuidhorn, Netherland.
Received: June 06, 2024; Published: June 13, 2024
DOI: 10.34297/AJBSR.2024.22.003021
Introductory Thoughts
Despite the numerous blood safety initiatives and projects since the first World Health Assembly Resolution WHA28.72 in 1975 [1], there are still substantial differences in blood availability and safety among countries in the four different Human Development Index (HDI) [2] groups. There is definitely progress, more on the primary technical operational side than on the organization, regulatory oversight, governance, leadership, stewardship and financing of the blood supply and clinical use. The latest WHO Global Status Report on blood safety and availability 2021 [3], based on data collected in 2018 through a survey linked with Global Database on Blood Safety (GDBS), shows a clear picture in four major areas of attention-availability; quality and testing; clinical use; policy, legislation, regulatory oversight and governance mechanisms.
Universal Health Coverage and Essential Medicines
In many resource-poor countries, inadequacy of blood supply often coexists with inadequate funding and less vigorous government legislation and regulation (oversight). Safe blood chains (vein-to-vein) depend on healthy non-remunerated and regular blood donors, strong quality system management and an effective TTIA screening system, and effective clinical practice (patient blood management) and adverse event monitoring mechanisms or national hemovigilance systems.
In 2010 during the World Health Assembly, Resolution WHA63.12 was adopted [4] which expresses serious concerns on the fact that large groups of patients in developing countries still have no access to safe blood and blood products/components, including plasma derived medicinal products (PDMP) despite these being listed as Essential Medicines (EMs) since 1979 [5]. The concern expressed regarded the observation that many patients e.g., obstetric, paediatric, trauma, thalassemia were still left without needed treatment, and of those with severe congenital and acquired disorders e.g., Hemophilia, without adequate and safe plasma-derivative treatment. In this Resolution Member States were urged “… to take all the necessary steps to update their national regulations on donor assessment and deferral, the collection, testing, processing, storage, transportation and use of blood and blood products, and operation of regulatory authorities in order to ensure that regulatory control in the area of quality and safety of blood products across the entire transfusion chain meets internationally recognized standards.” Two years later, in the year 2012, WHO provided countries a useful instrument to assess the existence of blood regulatory systems [6]. However, progress has been very limited and many lower- and middle-income countries (LMICs) still have a long way to go in providing universal access to safe and quality assured blood and blood products. Additionally, a growing number of people being pushed into extreme poverty because of catastrophic out-of-pocket payments for health care costs. [7] By 2012 this number has grown to over 100 million, where over half of the global population still does not have full coverage of essential health services, medicines and blood products.
In 2012 the UN General Assembly adopted Resolution 67/81 on Global Health and Foreign Policy where all Member States agreed to work towards achieving Universal Health Coverage (UHC) by 2030, which was later included as one of the UN Sustainable Development Goals (SDG) 2016-2030. [8,9] UHC means that all people and communities can use the promotive, preventive, curative, rehabilitative and palliative health services they need, of sufficient quality to be clinically effective, while also ensuring that the use of these services does not expose the user to financial hardship. UHC embodies three important and related objectives:
1. Equity in access to health services - everyone who needs services should get them, not only those who can afford and pay for them.
2. The quality of health services should be good enough to improve the health of those receiving services.
3. People should be protected against financial risk, ensuring that the cost of using services does not put people at risk of financial harm and/or poverty.
UHC, as related to transfusion medicine, means that all individuals and communities have access to affordable. adequate and timely supplies of safe and quality-assured blood and blood products [10]. These products are included in the WHO Model List of Essential Medicines since 1977 with biannual updates. More recently, the list included blood and labile blood products (components) in 2013 [5], and the WHO Model List of Essential in vitro Diagnostics established and added in 2018. [11] To support countries in the management of blood and blood products as essential medicines, the WHO Expert Committee on Biological Standardization in 2017 developed clear supportive ‘guidelines on management of blood and blood components as essential medicines’. [12] Member States have been and are continuously urged to take appropriate actions to recognize the cruciality of an effective regulation for the establishment of blood and blood products as EMs. The guidelines cover the key aspects needed-
1. Blood and blood components as biological therapeutic products.
2. Preparation of blood and blood components.
3. Comparison of blood components with PDMPs.
4. The blood regulatory system.
5. The blood supply system.
6. The blood transfusion system.
7. Stepwise implementation of a nationally regulated blood system.
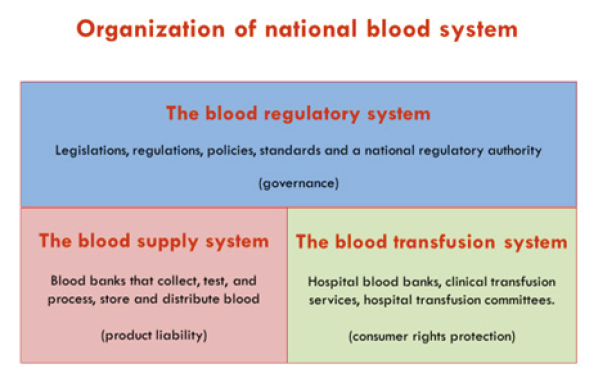
From the design and approach of these guidelines a simple and illustrative work scheme can be extracted, recognizing the interrelated triad of governing and operational systems each with its specific responsibilities in the health care-the blood regulatory system, the blood supply system and the blood transfusion system (Figure 1).
National Blood System
The guidelines recommend that a national blood system should consist of three interrelated subsystems, i.e., the blood regulatory system, the blood supply system and the blood transfusion system, with clearly defined roles and responsibilities that are defined by a framework law. Because blood and blood products are biomedicines, the production and use of these products should be effectively managed and regulated by a competent regulatory authority (RA) based on a quality system, standards and structured documentation.
Blood and blood products are produced or manufactured by the blood supply system (blood banks/establishments)-similar to manufacturers of medicines. The product liability lies with the manufacturer. The blood transfusion system (hospitals, diagnostic and treatment institutions) is the user/customer-the patient and treating clinicians who prescribe and administer blood components and medicines in hospitals (supported by therapeutic and transfusion committees). These have the legal obligation to protect rights of the final consumer-the patient in need, from faulty products.
Challenges-Developing Bridging Efforts
The 2021 Global Status Report [3] presents as most important challenges the continued existence of incompetent regulatory oversight due to inappropriate governance; weak leadership and management; poor stewardship; weak to absent public and clinical awareness; inconsistent quality, economies of scale and documentation (traceability); and financial budget constraints with protracting dependence on external donors. Most of the donor financed development projects so far have suffered from lack of vision and adaptation to the local country circumstances, capacities and resilience to change. Additionally, they were timewise too short to generate and root any sustainable effect, were largely focused on the day-to-day primary technical operations, and were limited in their monitoring and evaluation over time of the outcomes (hemovigilance) within the perspective of a country’s health care system and the related UHC development.
However, there is noticeable a growing political awareness in lower- and middle-income countries, focused on the need for an effective regulatory framework consisting of appropriate legislative instruments-
1. Legislation, framework law or act providing the scope and anchoring the international and ethical principles.
2. Regulations supplemented by policies and implementable strategies, standards, guidelines, and guidance documents, spelling out implementation details of the daily management and operation of the blood supply and consumption.
3. National Regulatory Authority (NRA) to oversee and control the systems of procurement and consumption.
In general, it is recommended to allow a risk-based development of the composite triad-system as described (Figure 1). For a stepwise implementation of a nationally well-regulated blood system, fundamental to assuring the quality, safety, availability and affordability of blood and blood products as essential medicines, a risk-based strategy is recommended and preferred, when considering the development of an effective regulatory structure for the blood system and a national roadmap for its efficacious and sustainable implementation. Despite the observation that there are different starting situations from fragmented, small scale and non-coordinated to national and consolidated (regional), a prime prerequisite is the existence of a documented political commitment of the ministry of (public) health and social affairs to establish a road map for implementation and maintenance of a nationally regulated blood system. Such road map needs sustained consensus of all stakeholders and related parties and a strong and competent leadership.
Impact on Blood Transfusion
Key are an initial review and improvement of an existing structure, including the financing mechanisms (e.g., cost recoery, health insurance, mix); development or strengthening of the legal framework and NRA; development and/or adoption of standards, preferably in line with accepted international standards-quality and technical; continued interaction among key stakeholders, including the community and the health facilities (hospitals); development of a comprehensive quality management system; capacity building (competency and stewardship) and leadership development (Result Oriented/Based Management); network of collaboration and cooperation; development of sufficient economies of scale to allow efficient processing and QC/testing to achieve equal standards of practice and reduce costs; strong clinical interface and rational use of blood; development of sustainable awareness campaigns-public, clinical and political.
Expected Outcomes
When these measures are implemented effectively at national level, lives of hundreds of millions of people will be influenced in a positive way, leading to important improvement of quality and efficacy of the health care system in many developing countries (LMICs). Ensuring appropriate access to affordable and quality assured blood and blood products as EMs based on a sound regulatory system plays an important role in achieving UHC and SDGs targets by 2030, reduce the burden of disease (QALY and costs), and be given due consideration by all stakeholders working toward achieving these targets. To support and guide these LMICs WHO launched in 2020 the Action Framework to Advance Universal Access to Safe, Effective and Quality-assured Blood Products 2020-2023 [13] and a series of extremely useful Guidance recommendations [14].
Conflict of Interest
The author has no conflicting interest.
References
- World Health Organization (1975) WHA28.72. Utilization and supply of human blood and blood products. Geneva: World Health Organization.
- UNDP Human Development Index.
- WHO Global Status Report on Blood Safety and Availability 2021. Geneva: World Health Organization 2022.
- World Health Organization (2010) WHA63.12. Availability, safety and quality of blood products. Geneva: World Health Organization.
- World Health Organization (2013) WHO Model Lists of Essential Medicines. Geneva: World Health Organization.
- WHO Assessment Criteria for National Blood Regulatory Systems.
- World Health Organization Regional Office for the Eastern Mediterranean (EMRO) (2016) . Framework for action on advancing universal health coverage in the Eastern Mediterranean Region. Cairo: EMRO.
- UN Resolution 67/81 Global Health Policy, 2012. New York.
- United Nations. Sustainable Development Goals. New York: United Nations; 2015.
- World Health Organization. Draft thirteenth general programme of work 2019- 2023 Geneva.
- WHO Model List of Essential in-vitro Diagnostics, 1st ed., Geneva, Switzerland; 2018.
- WHO technical report. Series no. 1004:67, 2017-Annex 3. Guidelines on management of blood and blood components as essential medicines.
- (2020) Action framework to advance universal access to safe, effective and quality-assured blood and blood products 2020-2023. Geneva World Health Organization.
- Abdella YE, Mariuningsih Soedarmo YS, Smit Sibinga CTh (2023) Historical background and current global efforts. In: Eichbaum QG. Global Perspectives and Practices in Transfusion Medicine. AABB Press, Bethesda MD. Chapter 1: 1-26



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.