Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Trichomonas vaginalis Interactions with Fibronectin and Fibronectin Domains
*Corresponding author: JF Alderete, School of Molecular Biosciences, MC7520, College of Veterinary Medicine, Washington State University, Pullman, WA 99164, USA.
Received: May 09, 2024; Published: May 14, 2024
DOI: 10.34297/AJBSR.2024.22.002976
Abstract
Fibronectin plays an important role in cell adhesion, growth, migration, and differentiation of eukaryotic cells. Earlier studies showed that live Trichomonas vaginalis binds to glass surfaces coated with plasma fibronectin (FN) and extracellular matrix (ECM) with cellular FN. Acquisition of FN by organisms was found to occur via specific ligand-receptor type interactions. In this work it was important to extend these earlier observations and examine the binding to trichomonads of the cell-binding domain (CBD), gelatin-binding domain (GBD), and N-terminal domain (NTD) of FN. It was first determined that FN was proteolytically degraded and released from the parasite surface, a process abrogated by the cysteine proteinase inhibitor N-α-p-Tosyl-L-Lysine Chloromethyl Ketone (TLCK). Therefore, experiments with TLCK showed that as with 125I-FN, each of the domains bound to the surface of organisms in a concentration-and time-dependent manner, affirming the specific nature of interactions. Further, trichomonads bound the distinct CBD, GBD, and NTD of FN but with different affinities. FN had a binding affinity higher than that of the individual domains. Not unexpectedly, the unlabeled domains inhibited the binding of the homologous 125I-radioiolabeled domain, and transferrin as a control glycoprotein was ineffective in competing with binding of FN and domains. Interestingly, FN and NTD, but not CBD and GBD, bound higher amounts to trichomonads grown in iron-restricted medium. Importantly, only pretreatment of FN with monoclonal antibodies (MAbs) to NTD and CBD inhibited associations of FN with T. vaginalis organisms. Data indicate that the high-affinity binding of FN with the surface of trichomonads results from the cumulative interactions of the individual domains.
Keywords: Cell-Binding Domain (CBD), Extracellular Matrix (ECM), Fibronectin (FN), Gelatin-Binding Domain (GBD), Monoclonal Antibody (MAb), N-Terminal Domain (NTD), Sexually Transmitted Infections (STIs), N-α-p-Tosyl-L-Lysine Chloromethyl Ketone (TLCK), Vaginal Epithelial Cells (VECs).
Introduction
Trichomonas vaginalis causes trichomoniasis, the number one, non-viral and curable sexually transmitted infection (STI) worldwide. Considered one of the most neglected, persistent and asymptomatic STIs, the detrimental outcomes in health to patients has been well established. Therefore, it is important to continue to define and understand the mechanisms that mediate successful host infection. It is appreciated that infection and persistence are complex and multifactorial, especially given the constantly-changing urogenital environment of women. For example, it is conceivable that the interactions of trichomonads with mucin [1] and vaginal epithelial cells (VECs) [2] fluctuate. We hypothesize that parasite proteinases [3-5] may erode the vaginal and cervical epithelium to permit access of organisms to the Extra Cellular Matrix (ECM)-basement membrane components, such as Fibronectin (FN), and that this enhances host colonization and possibly the non-self-limiting nature of infection.
Associations of T. vaginalis with host macromolecules has been reported and may be important to survival of parasites in patient environments [6-10]. Step-wise approaches examined the acquisition and identification of human plasma proteins with the surface T. vaginalis [6,7], and the biological properties of these proteins on the parasite following such associations were examined [8,9]. For example, this early work showed the mechanism of uptake of human lipoproteins and low-density lipoproteins as a source of lipids for in vitro trichomonal growth and multiplication [8]. Equally noteworthy was the finding of receptor-binding of lactoferrin, but not transferrin, as prerequisite for iron acquisition [10]. Other plasma proteins found to associate with trichomonads include the protease inhibitor α1-antitrypsin, plasminogen, fibronectin (FN), fibrinogen, immunoglobulins, and albumin [7-10], each of which may have unique functions that permit successful infection. Additionally immune evasion from host surveillance may be an outcome of masking of the parasite surface with host proteins.
Fibronectin is a large glycoprotein characterized as an important cell adhesion-promoting ECM glycoprotein. FN possesses functional domains that also have important roles in cell growth and migration as well as the differentiation of eukaryotic cells [11-13]. The interaction of FN with T. vaginalis organisms has been examined and found to be regulated by both iron and calcium [14-16]. This earlier work showed that parasites grown in iron-restricted medium bound higher numbers of FN molecules with lower affinity compared to iron-replete trichomonads, and addition of calcium at physiological levels immediately restored levels and affinities of FN associated with both low-and high-iron organisms [15]. While it was established that T. vaginalis parasites readily adhered to glass coverslips coated with FN, cell-binding domain (CBD), gelatin-binding domain (GBD) and N-terminal domain (NTD) [16], the nature of the interaction by parasites with the domains and the role of iron in these associations was undefined. The purpose of this work, therefore, was to examine the specific binding of each of the FN domains to T. vaginalis surfaces. The data presented in this report show the existence of specific sites on trichomonal membranes that bind the FN domains.
Methods
Culture and Growth of T. vaginalis
The fresh clinical T. vaginalis isolate T016 was grown to mid-logarithmic phase in trypticase-yeast extract-maltose (TYM) medium with 10% heat-inactivated horse serum, as before [6,9,14,17]. After centrifugation, organisms were suspended to the same final density in medium for an additional 2-h incubation at 37°C. Parasites were then harvested by centrifugation and washed three times in cold minimal binding (MB) buffer (120mM NaCl, 1.3mM KCl, 0.9mM Na2HPO4, 5.5mM glucose, and 26mM NaHCO3, pH 5.0) [14] prior to use [14,15].
Purification of FN
FN was purified from human plasma by gelatin-Sepharose (Sigma Chemical Co., St. Louis, MO USA) affinity chromatography, as detailed previously [18,19]. Briefly, the protease inhibitors 5mM EDTA, 1mM phenylmethylsulphonyl fluoride (PMSF; Sigma) and 50mM 𝜀-amino-n-caproic acid were added to plasma prewarmed at 37°C. Plasma was then centrifuged for 15-min at 10,000 xg followed by rapid passage over the Sepharose CL-4B precolumn prior to gelatin-Sepharose chromatography. The recovered FN was then added to the gelatin-Sepharose, which was washed extensively in buffer containing 1M NaCl, 50mM Tris-HCl, 50mM 6-aminohexanoic acid, and 20mM sodium citrate, pH 7.6. This was followed by immediate washing of the column in buffer composed of 50mM Tris-HCl, 50mM 𝜀-amino-n-caproic acid, and 20mM sodium citrate, pH 7.6. Finally, FN was eluted with 100mM NaCl with 50mM sodium citrate, pH 5.5 and the column further cleaned with 4M urea in Tris-buffered saline (150mM CaCl and 10mM Tris-HCl, pH 7.0). The pooled fractions with FN were precipitated with 50% NH4SO2(w/v) at 4°C for 2-h. Precipitated FN was centrifuged for 15-min at 10,000xg before suspending FN in PBS for dialysis at 4°C in 3.5 liters of PBS with two changes of PBS per day for 3 days to remove residual ammonium sulfate.
Generation of FN Domains
Purified FN (330mg) was dialyzed and concentrated at 4°C to 3 mg/ml in a buffer of 30mM NaCl, 50mM Tris-HCl and 1mM CaCl2, pH 7.0. FN was then digested with 0.66mg TPCK-trypsin (Sigma) for 30-min at 30°C. The reaction was stopped by adding 1mM PMSF, pH 7.0, followed by dialysis in buffer composed of 10mM Tris-HCl and 1mM PMSF, pH 7.0. Digested protein was subjected to affinity chromatography, and after washing of the column, the domains were eluted as before [17,18, 20-22]. The cell-binding domain (CBD), gelatin-binding domain (GBD), and N-terminal domain (NTD) were precipitated with 70% NH4SO2 (w/v) and dialyzed at 4°C with 4 changes of PBS [17,18, 20-22]. The protein concentration was determined using Pierce™ BCA Protein assay (Thermo Scientific, Rockford, IL).
Iodination of Proteins
Iodination of FN and domains was by chloramine T, as before [8,10,23]. Proteins at 1mg/ml were incubated at RT for 5-min with 0.1ml of 2mg/ml chloramine T in PBS and 1mCi 125Iodine (Amersham Corp., Arlington Heights, IL). The iodination was terminated by addition of 0.1ml of 4mg/ml sodium metabisulfite in PBS followed by G25-Sephadex column chromatography. Radioactivity of 0.5ml fractions was determined as Counts Per Minute (cpm) by scintillation spectroscopy. The protein concentration was determined as above. Specific activity (cpm/pmol) was calculated for each protein.
Assays for Binding and Competition of FN and Domains with T. vaginalis
Unless otherwise stated, the binding assay had 5x105 trichomonads suspended in MB buffer [14-16] containing 400μM N-α- p-Tosyl-L-Lysine Chloromethyl Ketone (TLCK) and incubated with iodinated proteins at a final volume of 0.1 ml per sample. Samples were incubated at 37°C for 20-min followed by three washes in MB buffer. Bound radioiodinated protein was determined by scintillation spectroscopy, and where described, cpm was converted to fmol of protein. TLCK had no effect on the viability and motility of T. vaginalis organisms throughout the experimental protocols. All assays were performed on at least three separate occasions using triplicate samples. Competition experiments were performed using different concentrations of unlabeled, purified FN with radioiodinated FN, as described above [18,19]. Likewise, purified FN domains CBD, GBD and NTD and commercially-available human FN alpha-chymotryptic fragment of the CBD (120-kDa) that contains the cell attachment region (Chemicon Int’l, Inc., Temecula, CA) were used in experiments. Finally, purified NTD, GBD and the RGD-containing CBD domains and Monoclonal Antibody (MAb) 333 to CBD and MAb 304 to NTD were also kindly provided by Dr. Steve Akiyama of the National Institutes of Environmental Health Sciences of the Research Triangle Park, NC. Low-iron-grown T. vaginalis organisms were obtained as previously detailed [14,24]. Briefly, trichomonads at 105 organisms/ml were cultured overnight in medium with 50μM of 2,2-dipyridil (2,2-DP; Sigma) for 24-h at 37°C [14,24]. Parasites were then suspended in medium with 75μM 2,2-DP for an additional 24-h. High-iron organisms were grown in medium containing 250μM ferrous ammonium sulfate (Sigma) and incubated for 20-h at 37°C. Both low-and high-iron cultures were used for binding assays as above. Importantly, the iron status of trichomonads was confirmed on the basis of synthesis of adhesins and cytoadherence of organisms to host epithelial cells [24].
Release and Degradation of FN by Live Organisms
After performing a binding assay as above with 125I-FN, T. vaginalis organisms were incubated in MB buffer for up to 60-min in the absence of any TLCK inhibitor of cysteine proteinases. At different times, parasites were removed by centrifugation, and supernatants were examined for released radioactivity by scintillation spectroscopy. Throughout this work the viability and motility of parasites was monitored by trypan blue exclusion [2,25]. In separate experiments, we examined the radiolabeled proteins released into supernatants by sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) [23,25]. Organisms in the binding assay were incubated with iodinated FN in the absence or presence of TLCK. Supernatants were then collected as above at different time points up to 60-min. Released radiolabeled proteins in supernatants were precipitated by addition of 10% Tri Chloroacetic Acid (TCA) at 4°C for 16-h [25]. Pellets of precipitated protein were processed by washing three times with cold PBS [23,25], and pelleted proteins were dissolved by boiling in electrophoresis dissolving buffer (6.25mM Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 2% β-mercaptoethanol, and 2% bromophenol blue) [26]. SDS-PAGE was performed using 4% stacking and 7% separating acrylamide gels [25,26]. After staining with Coomassie Brilliant blue R, gels were dried for autoradiography, as before [23,25].
Results
Release and Degradation of FN by Live Organisms
For accurate quantitation of both FN and FN domains bound to T. vaginalis surfaces it was important to determine the integrity of the associated FN, especially given the secretion of and presence on the trichomonad surface of cysteine proteinases [3-5]. Therefore, after performing a binding assay (Materials and Methods), washed organisms were monitored for bound radioiodinated FN at different times. Figure 1 for two representative experiments showed 70% (part A) and 90% (part B) reduced amounts of 125I-FN associated with live trichomonads at 45-min and 60-min, respectively. Further, Figure 2 (part A) presents SDS-PAGE-autoradiography of protein released into the supernatant during incubation of organisms with bound radioiodinated FN in the absence of TLCK. The time-dependent degradation of 125I-FN released into the supernatant was evident. Part B shows the abrogation of digestion of FN by TLCK and indicates that cysteine proteinases mediate the release and proteolysis of FN on the parasite surface.
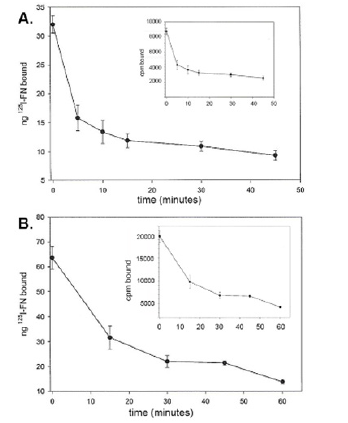
Figure 1: Representative experiments showing release of 125I-radiolabeled FN (125I-FN) bound to the surface of T. vaginalis organisms after performing the binding assay (Materials and Methods). No TLCK cysteine proteinase inhibitors were added to assess the role of proteases [3-5] in removal and/or degrading of FN. These data show removal of 40% (part A) and 60% (part B) of 125 I-FN from the parasite surface by 45-min and 60-min, respectively. Inserts show the radioactive FN removed from the surface as measured by scintillation spectroscopy in counts per minute (cpm). In data not shown, experiments with TLCK during the incubation of trichomonads with radioiodinated FN resulted in less than 5% of iodinated protein released from the parasitesurface, which is consistent with the autoradiogram results presented in Figure 2(part B).
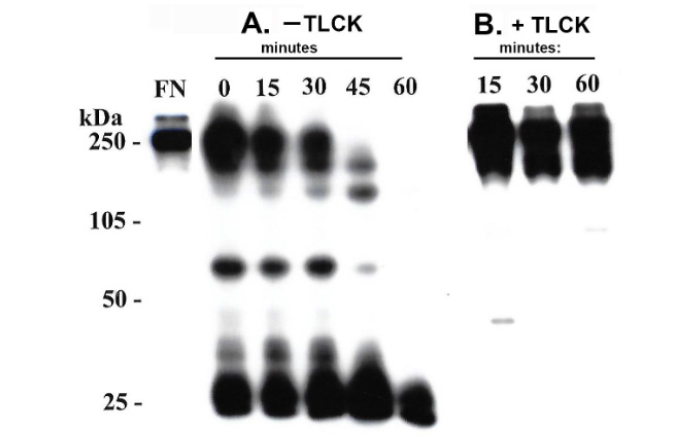
Figure 2: Autoradiogram after SDS-PAGE of the released radioiodinated protein at different time points as described in Materials and Methods. The binding assay was performed in the absence (part A) and presence (part B) of the cysteine proteinase inhibitor N-α-p-Tosyl-L-Lysine Chloromethyl Ketone (TLCK). Kilodaltons (kDa) of molecular weight protein markers used in the SDS-PAGE are included to show the Mr of the degradation products of FN (part A). The purified, radioiodinated FN used in the experiment is presented in the lane labeled FN. Part B shows that there was no proteinase digestion of FN evident in the presence of TLCK throughout the incubation period.
Trichomonas vaginalis Binding to 125I-FN is Time and Concentration Dependent and Competition with Unlabeled FN
Next, prior to experiments with the FN domains, it was important to reaffirm the concentration and time- dependent nature of binding of FN by live T. vaginalis organisms, as before [14,15]. (Figure 3) indeed shows the concentration-and time-dependent kinetics of FN associations with live organisms (part A and insert). Unlabeled FN readily competed with the binding of 125I-FN (part B). Unlabeled transferrin as a negative control had no effect on FN acquisition (not shown), as before [14]. These data affirms that specific receptor-ligand type interactions mediate FN associations with the parasite surface.
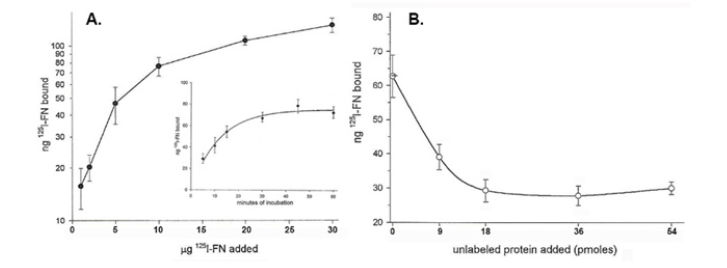
Figure 3: Kinetics of 125I-FN binding (part A) and competition by unlabeled FN with the association of 125I-FN (part B) binding to live T. vaginalis organisms. Part A. Saturation-binding kinetics is shown in a binding assay performed with increasing amounts of radioiodinated FN added to 5x105 trichomonads suspended in 100μl binding buffer containing TLCK and incubated for 20-min at 37°C (Materials and Methods). As expected, the insert shows the time-dependent kinetics of 125I-FN associations in minutes of incubation with parasites. Part B. Competition of iodinated FN acquisition by organisms incubated with increasing amounts of unlabeled FN in the binding assay as in part A. In this experiment and as shown previously [14,15], unlabeled transferrin as a negative control did not inhibit the binding of radiolabeled FN to T. vaginalis organisms.
T.vaginalis Organisms Bind the NTD, GBD and CBD Domains of FN
As with FN (Figure 3) each of the domains showed saturation-binding kinetics to live parasites as shown in Figure 4. Not unexpectedly, the acquisition of domains was also in a time-dependent manner. Interestingly, saturation binding of the CBD was ̴10- fold less than the GBD and NTD. These data suggest that, like FN binding, each domain is associated via specific sites on the surface of live organisms. Scatchard analysis [10,27] of the data for FN and FN domain binding are presented in (Table 1). The number of molecules of GBD was higher than FN and the domains CBD and NTD. FN and CBD bound with higher affinity than NTD and GBD, suggesting that the CBD may be the primary domain interacting with the parasite surface. This analysis provided evidence of a single class of receptors for the domains with apparent KD ranging from 0.50 to 2.00. Low affinity (nonspecific) interactions were also evident at very high concentrations of FN and domains added to organisms. Importantly, only unlabeled FN domains competed for binding with homologous domains. As with FN, transferrin as a control also did not inhibit the association of domains with live organisms (data not shown).
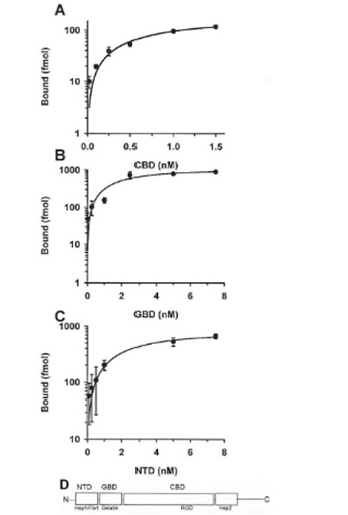
Figure 4: Saturation-binding kinetics of the FN Cell Binding Domain (CBD, part A), Gelatin Binding Domain (GBD, part B), and N Terminal-binding Domain (NTD, part C) to live T. vaginalis organisms. The binding assays was as described in Materials and Methods and incubation was for 20-min at 37°C. Part D illustrates the structure of FN with each of the domains indicated, as previously reported [29]. The amino acid sequence RGD tripeptide that associates with integrin receptors [12,13,30] is within the CBD. As shown in Figure 3, each of the domains bound in a concentration-dependent manner to trichomonads. In data not shown and not unexpectedly, the association with each domain was time-dependent. Interestingly, the saturation binding of CBD (part A) was ten-fold less than for GBD (part B) and for NTD (part C).
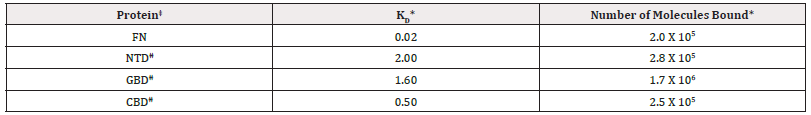
Table 1: Summary of Scatchard analysis* and KD for FN and FN-domains‡ bound to Trichomonas vaginalis.
*Note: *Scatchard analyses [27] of data as presented in Figures 3 and 4 that demonstrated saturation-binding kinetics of radioiodinated FN and domains binding to T. vaginalis organisms. Trichomonads were incubated with 125I-FN and radioiodinated domains as per Figure 1 for 20-min followed by washing once and suspending in MB buffer (Materials and Methods). Viability of organisms was present throughout the experiment as determined by trypan blue-exclusion. The relative KD value was defined as the amount (nM) required to reach half saturation binding, and the number of molecules bound per cell were determined.
‡FN and domains were generated and interacted with live parasites grown in normal medium as in Figures 3 and 4 and as described in Materials and Methods.
ǂǂAbbreviations: FN- Fibronectin; NTD- N-Terminal Domain; GBD- Gelatin-Binding Domain; CBD-Cell-Binding Domain.
T.vaginalis Binding of the NTB of FN is Iron Regulated
An earlier report showed that low-iron parasites bound higher amounts of FN compared to normal-and high-iron-grown parasites [15]. Therefore, it was important to determine the role of iron in the association with the distinct domains. As presented in Figure 5 (part A) low-iron trichomonads bound higher amounts of FN in contrast to high-iron organisms. Likewise, higher amounts of associated NTD were seen for low- versus high-iron cells. The CBD and GBD did not show any regulation by iron on levels of these FN domains bound to trichomonads (not shown). These data suggest that NTD may be responsible for the increased numbers of FN molecules bound by organisms grown in low-iron medium.
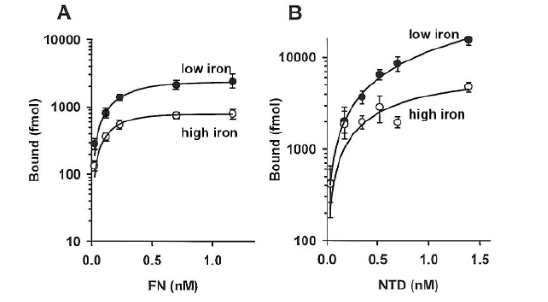
Figure 5: Saturation-binding kinetics of 125I-labeled FN (part A) and 125I-labeled N Terminal Domain (NTD) (part B) to live T. vaginalis organisms grown in low-iron (solid circles) versus high-iron (open circles) medium (Materials and Methods). Part A shows the higher amounts of radioiodinated FN bound by low iron-versus high-iron-grown trichomonads, as before [15]. Part B presents similar results for the NTD of FN with increased NTD bound to low-iron parasites compared to high-iron organisms.
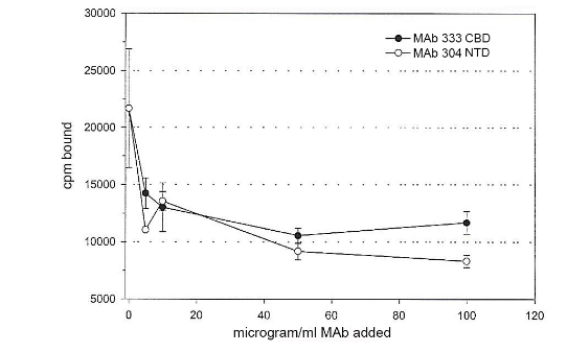
Figure 6: Decreased binding to live T. vaginalis organisms of 125I-FN in a binding assay with increasing amounts of monoclonal antibody (MAb 333) to CBD and MAb 304 to NTD. The binding assay was as described in Materials and Methods, and the different amounts of MAbs were mixed with radiolabeled FN prior to addition to 5x105 trichomonads followed by incubation for 20-min at 37°C. MAb 333 to CBD inhibited binding by ~50%, and MAb 304 inhibited NTD binding by ~60%. For both CBD and NTD, ~50μg/ml of MAbs gave maximal inhibition of FN acquisition to trichomonads in the binding assay. No decreased association with radioiodinated FN was evident with irrelevant MAb of the same IgG1 isotype.
Monoclonal Antibodies to the CBD and NTD Inhibit T. vaginalis Binding of FN
Finally, the effect of monoclonal antibodies (MAbs) to the individual FN domains was examined. Surprisingly, (Figure 6) presents data that pretreatment of FN in MB buffer with MAb 333 reactive to CBD and MAb 304 to NTD prior to addition to live organisms inhibited associations. This is evidenced by 50% and 65% decreased binding of FN by T. vaginalis with the MAb 333 to CBD and MAb 304 to NTD, respectively. Fifty micrograms/ml of MAbs gave maximal inhibition of binding for both CBD and NTD. No MAb was available to the GBD to examine for inhibition of FN binding.
Discussion
Fibronectin (FN) is a large dimeric glycoprotein with functional domains organized on two polypeptide chain subunit arms of 220,000 linked by disulfide bonds at the COOH-terminal end of the molecule [11- 13,18,20,22]. This glycoprotein is one of the best characterized cell adhesion-promoting ECM proteins and is found in blood (plasma FN) and immobilized on surfaces of cells and tissues (cellular FN) [28]. FN is implicated in numerous cellular functions, including adhesion, cell migration, differentiation, phagocytosis and arrangement of cytoskeleton. FN forms a network of fibers that connect cells to the extracellular matrix (ECM) [28-30]. Importantly, FN is comprised of functional domains with unique binding activities and are characterized as the cell-binding domain (CBD) that interacts with integrins via the RGD amino acid sequence and is in the central region of the protein [31] as shown in part D of (Figure 4). There is also the gelatin-binding domain (GBD) and the N-terminal domain (NTD) that binds heparin and fibrin 1 [28,30].
Trichomonas vaginalis associations with FN and the FN domains via specific interactions (Figures 3-5 and Table 1) are consistent with what has been reported for mammalian cells [31,32]. Each organism possesses ~2x105 specific sites for human plasma FN with an apparent KD estimated to be 0.02nM, indicating high affinity interactions between FN and the parasite surface. Interestingly, the number of sites on trichomonads is consistent with what has been reported before for fibroblast cells [32]. Each of the FN domains, on the other hand, has a higher apparent KD of lower affinity than that of FN. This may indicate the existence of distinct sites on the parasite membrane for recognizing a unique FN domain. Thus, it can be envisioned that the high-affinity binding of FN may be the cumulative, synergistic result of the domains interacting with the cell surface concurrently, which is also consistent with the proposed models of FN interactions with fibroblastic cells [32]. Finally, it is noteworthy that the MAb 333 to CBD inhibited FN binding to organisms by 50% at 50μg/ml concentration, and the epitope recognized by this MAb is within the small Mr protein that contains the RGD sequence known to bind to cells [32]. Nonetheless, competition experiments performed here with synthetic peptides containing the RGD sequence did not indicate any role for this region in the binding of FN to parasites (data not shown).
Other bacterial pathogens, yeast and parasites are known to bind the FN domains [33-46]. For example, Staphylococcus aureus recognizes both the NTD as well as the heparin-binding domain of the C-terminal end of FN [36,37,43]. The F1 protein of Streptococcus pyogenes associates with the NTD and a second 70-kDa region overlapping the NTD and GBD [39]. Interestingly, Trypanosoma cruzi [46] and Treponema pallidum [33,34] bind the CBD via the RGD sequence. Candida albicans interacts with both FN and laminin [44,45] and has also been shown to degrade both of these ECM proteins [45]. That low-iron T. vaginalis organisms exist during an infection was established by showing that patients with trichomoniasis have serum antibody to immunogenic proteins synthesized only by trichomonads grown under low-iron conditions [24,47]. Further, it has been shown that iron-depleted parasites have longer generation times and do not synthesize and secrete the cysteine proteinases that remove and degrade FN after binding to live organisms (Figure 2) [3,47-49]. Data presented here show that low-iron-grown organisms bound higher amounts of NTD (Figure 5 and Table 1) and also of FN, as before [15]. Further, it is known that patients with trichomoniasis make serum antibody to the cysteine proteinases [4,5]. These facts suggest strongly that a) the existence of low-iron parasites during infection, b) the production of antibody by patients to cysteine proteinases, and c) the absence of these proteinases synthesized by low-iron organisms may indicate the availability of FN on parasite surfaces during infections may promote associations with ECMs that enhance host colonization.
Trichomonas vaginalis undergoes dramatic morphologic changes from the batch culture ellipsoid forms to amoeboid forms upon contact and cytoadherence to vaginal epithelial cells [50]. This change in morphology was accompanied with pseudopodia and membrane extensions, and it is noteworthy that similar amoeboid trichomonads are observed upon parasite binding to glass surfaces coated with FN, the domains, and commercially-available ECM [15,16]. Thus, it is conceivable that this morphologic form of T. vaginalis also exists during infection upon organisms residing within the ECM of the vaginal epithelium. Finally, the surface acquisition by T. vaginalis organisms of the human plasminogen [51,52], a host pathogenicity factor, and the activation of plasminogen to plasmin during infection may result in the ability of this parasite to penetrate the vaginal epithelium. This may permit establishment of T. vaginalis residence at the ECM. A hypothesis, therefore, is that this scenario may contribute to the continued existence of the parasite within infected patients and, thus, persistence, which results in the non-self-limiting nature of infection for this STI.
Conclusion
FN is a dimeric glycoprotein with functional domains [11- 13,32] and is one of the best studied cell-adhesin molecules with numerous roles in cell biology. This report shows for the first time the interaction of the CBD, GBD, and NTD FN domains with specific sites on the surface of T. vaginalis. That cysteine proteinases and iron influence the nature and stability of FN on the parasite surface illustrates the complex FN associations that, in turn, affects the role of FN and ECM during host infection. The number of sites and affinity for FN and the involvement of multiple binding regions on FN by T. vaginalis organisms are consistent with prior reports on FN [32] and permits understanding the higher affinity of FN than that of the individual domains. Overall, this paper contributes to the understanding of this important cell adhesion protein to the host-parasite interrelationship that may help in our understanding of the mechanism for persistence and the long-term nature of infection.
Acknowledgements
This study was supported by Public Health Service grants AI- 43940 and AI-39803 from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health. As per instructions by the International Committee of Medical Journal Editors on “Who Is an Author?,” (http://www.icmje.org/recommendations/browse/ roles-and-responsibilities/defining-the-role-of-authors-and-contributors.html), I want to acknowledge Marie Crouch and Andrew P. Alderete, both of whom generated the data under my supervision in my laboratory while at The University of Texas Health Science Center at San Antonio, Texas. Special acknowledgement to Dr. Steve Akiyama of the National Institutes of Environmental Health Sciences of the Research Triangle Park, NC for his gifts of FN domain proteins and monoclonal antibodies to FN and for advising Marie Crouch during her experiments.
Conflict of Interest
I declare that there are no conflicts of interest. I alone designed the study and was responsible in the collection, analyses, and interpretation of data, in the writing of the manuscript, and in the decision to publish the results.
References
- Lehker MW, Sweeney D (1999) Trichomonad invasion of the mucous layer requires adhesins, mucinases, and motility. Sex Transm Infect 75(4): 231-238.
- Arroyo R, Engbring J, Alderete JF (1992) Molecular basis of host epithelial cell recognition by Trichomonas vaginalis. Mol Microbiol 6(7): 853-862.
- Neale KA, Alderete JF (1990) Analysis of the proteinases of representative Trichomonas vaginalis isolates. Infect Immun 58(1): 157-162.
- Alderete JF, Newton E, Dennis C, Neale KA (1991) Antibody in sera of patients infected with Trichomonas vaginalis is to trichomonad proteinases. Genitourin Med 67(4): 331-334.
- Alderete JF, Newton E, Dennis C, Neale KA (1992) The vagina of women infected with Trichomonas vaginalis has numerous proteinases and antibody to trichomonad proteinases. Genitourin Med 67(6): 469-474.
- Peterson KM, Alderete JF (1982) Host plasma proteins on the surface of pathogenic Trichomonas vaginalis. Infect Immun 37(2): 755-762.
- Peterson KM, Alderete JF (1983) Acquisition of alpha 1-antitrypsin by pathogenic Trichomonas vaginalis. Infect Immun 40(2): 640-646.
- Peterson KM, Alderete JF (1984) Selective acquisition of plasma proteins by Trichomonas vaginalis and human lipoproteins as a growth requirement by his species. Mol Biochem Parasitol 12(1): 37-48.
- Peterson KM, Alderete JF (1984) Selective acquisition of plasma proteins by Trichomonas vaginalis and human lipoproteins as a growth requirement by his species. Mol Biochem Parasitol. 12(1): 37-48.
- Peterson KM, Alderete JF (1984) Iron uptake and increased intracellular enzyme activity follow lactoferrin binding by Trichomonas vaginalis receptors. J Exp Med 160(2): 398-410.
- Proctor RA (1987) Fibronectin: a brief overview of its structure, function and physiology. Rev Infect Dis 4: S317-321.
- Akiyama SK, Yamada YM, Hayashi M (1981) The structure of fibronectin and its role in cellular adhesion. J Supramolec Struct Cell Biochem 16(4): 345-358.
- Sekiguchi K, Hakomori S (1980) Functional domain structure of fibronectin. Proc Natl Acad Sci USA 77(5): 2661-2665.
- Crouch ML, Alderete JF (1999) Trichomonas vaginalis interactions with fibronectin and laminin. Microbiology 145(pt10): 2835-2843.
- Crouch ML, Benchimol M, Alderete JF (2001) Binding of fibronectin by Trichomonas vaginalis is influenced by iron and calcium. Microbiol Pathogen 31(3): 131-144.
- Alderete JF, Benchimol M, Lehker MW, Crouch ML (2002) The complex fibronectin-Trichomonas vaginalis interactions and trichomonosis. Parasitol Intl 51(3): 285-292.
- Alderete JF (2021) An mRNA-binding protein of the ancient protist Trichomonas vaginalis. American J Biomed Sci Res 13(3): 302-312.
- Akiyama SK, Yamada KM (1995) Fibronectin and fibronectin fragments. In Extracellular Matrix: a Practical Approach ed. Haralson MA and Hassell JR: 175-185.
- Akiyama SK (1999) Purification of fibronectin. Curr Protocols Cell Biol 60: 10.5.1-10.5.13.
- Hayashi M, Yamada KM (1983) Domain structure of the carboxyl-terminal half of human plasma fibronectin. J Biol Chem 258(5): 3332-3340.
- Zardi L, Carnemolla B, Balza E, Borsi L, Castellani P, Rocco M, Siri A (1985) Elution of fibronectin proteolytic fragments from a hydroxyapatite chromatography column. A simple procedure for the purification of fibronectin domains Eur J Biochem 146(3): 571-579.
- Sekiguchi K, Hakomori S (1980) Functional domain structure of fibronectin. Proc Natl Acad Sci USA 77(5): 2661-2665.
- Alderete JF (1983) Identification of immunogenic and antibody-binding proteins on the membrane of pathogenic Trichomonas vaginalis. Infect Immun 40(1): 284-291.
- Lehker MW, Arroyo R, Alderete JF (1991) The regulation by iron of the synthesis of adhesins and cytoadherence levels in the protozoan Trichomonas vaginalis. J Exp Med 174(2): 311-318.
- Alderete JF (1983) Antigenic analysis of several pathogenic strains of Trichomonas vaginalis. Infect Immun 39(3): 1041-1047.
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259): 680-685.
- Feldman HA (1972) Mathematical theory of complex ligand-binding systems at equilibrium: some methdos for parameter fitting. Anal Biochem 48(2): 317-338.
- Gao M, Craig D, Lequin O, Campbell ID, Vogel V, et al. (2003) Structure and functional significance of mechanically unfolded fibronectin type III1 intermediates. Proc Natl Acad Sci USA 100(25): 14784-14789.
- Fruh SM, Schoen I, Ries J, Vogel V (2015) Molecular architecture of native fibronectin fibrils. Nat Comm 6: 7275.
- Schor SL, Ellis I, Banyard J, Schor AM (1999) Motogenic activity of IGD-containing synthetic peptides. J Cell Sci 112(pt22): 3879-3888.
- Akiyama SK, Olden K, Yamada KM (1995) Fibronectin and integrins in invasion and metastasis. Cancer Metastasis Rev 14(3): 173-189.
- Akiyama SK, Hasegawa E, Hasegawa T, Yamada KM (1985) The interaction of fibronectin fragments with fibroblastic cells. J Biol Chem 260(24): 13256-13260.
- Thomas DD, Baseman JB, Alderete JF (1985) Putative Treponema pallidum cytadhesins share a common functional domain. Infect Immun 49(3): 833-835.
- Thomas DD, Baseman JB, Alderete JF (1985) Fibronectin tetrapeptide is the target for syphilis spirochete cytadherence. J Exp Med 162(5): 1715-1719.
- Kemper L, Hensel A (2023) Campylobacter jejuni: targeting host cells, adhesion, invasion, and survival. Appl Microbiol Biotech 107(9): 2725-2754.
- Hammerschmidt S, Rohde M, Preissner KT (2019) Extracellular matrix interactions with gram-positive pathogens. Microbiol Spectr 7(2).
- Henderson B, Nair S, Pallas J, Williams MA (2011) Fibronectin: a multidomain host adhesin targeted by bacterial fibronectin-binding proteins. FEMS Microbiol Rev 35(1): 147-200.
- Grab DJ, Givens C, Kennedy R (1998) Fibronectin-binding activity in Borrelia burgdorferi. Biochim Biophys Acta 1407(2): 135-145.
- Ozeri V, Tovi A, Burstein I, Natanson Yaron S, et al. (1996) A two-domain mechanism for group A streptococcal adherence through protein F to the extracellular matrix. EMBO J 15(5): 989-998.
- Wyler DJ (1987) Fibronectin and parasitic diseases. Rev Infect Dis 4: S391-399.
- Brittingham A, Chen G, McGwire BS, Chang KP, Mosser DM (1999) Interaction of Leishmania gp63 with Cellular Receptors for Fibronectin. Infect Immun 67(9): 4477-4484.
- Maeda FY, Cortez C, Izidoro MA, Juliano L, Yoshida N (2014) Fibronectin-degradaing activity of Trypanosoma cruci cysteine proteinase plays a role in host cell invasion. Infect Immun 82(12): 5166-5174.
- Bozzini S, Visai L, Pignatti P, Petersen TE, Speziale P (1992) Multiple binding sites in fibronectin and the staphylococcal fibronectin receptor. Eur J Biochem 207(1): 327-333.
- Skerl JG, Calderone RA, Segal E, Sreevalsan T, Scheld WM (1984) In vitro binding of Candida albicans yeast cells to human fibronectin. Can J Microbiol 30(2): 221-227.
- Parnanen P, Meurman JH, Virtanen I (2009) Laminin-511 and fibronectin degradation with Candida yeast. J Oral Pathol Med. 38(10): 768-772.
- Ouaissi MA, Cornette J, Afchain D, Capron A, Gras-Masse H, et al. (1986) Trypanosoma cruzi infection inhibited by peptides modeled from a fibronectin cell attachment domain. Science 234(4776): 603-607.
- Lehker MW, Alderete JF (1992) Iron regulates growth of Trichomonas vaginalis and the expression of immunogenic proteins. Mol Microbiol 6(1): 123-132.
- Provenzano D, Alderete JF (1995) Analysis of human immunoglobulin-degrading Trichomonas vaginalis cysteine proteinases. Infect Immun 63(9): 3388-3395.
- Provenzano D, Alderete JF (1997) The vagina has reducing level sufficient for activation of Trichomonas vaginalis cysteine proteinases. Genitourin Med 73: 291-296.
- Arroyo R, González Robles A, Martínez Palomo A, Alderete JF (1993) Signaling of Trichomonas vaginalis for ameboid transformation and adhesin synthesis follows cytoadherence. Mol Microbiol 7(2): 299-309.
- Mundodi V, Kucknoor AS, Alderete JF (2008) Immunogenic and plasminogen-binding surface-associated alpha-enolase of Trichomonas vaginalis. Infect Immun 76(2): 523-531.
- Lama A, Kucknoor AS, Mundodi V, Alderete JF (2009) Glyceraldehyde-3-phosphate dehydrogenase is a surface- associated fibronectin-binding protein of Trichomonas vaginalis. Infect Immun 77(7): 2703-2711.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.