Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Benefits and Pitfalls of Plasmin Derivatives Application for Thrombolysis: A Narrative Review
*Corresponding author: Robert Mikulik, International Centre for Clinical Research, St. Ann’s Hospital, Brno, Czech Republic, C2P NEXARS, The Campus Science Park, Palachovo namesti 2, 62500 Brno, Czech Republic, Department of Neurology, St. Anne’s Hospital and Faculty of Medicine, Masaryk University, Brno, Czech Republic and Jaroslav Turanek, International Centre for Clinical Research, St. Ann’s Hospital, Brno, Czech Republic, C2P NEXARS, The Campus Science Park, Palachovo namesti 2, 62500 Brno, Czech Republic, Department of Immunology, Faculty of Medicine and Dentistry, Palacky University Olomouc, Hněvotínská 3, 775 15 Olomouc, Czech Republic.
Received: July 27, 2024; Published: August 08, 2024
DOI: 10.34297/AJBSR.2024.23.003101
Abstract
Cardio- and cerebrovascular thrombosis is a serious pathology with the highest lethality and disability rates in the world. The current thrombolytic therapy is mainly based on plasminogen activators, such as streptokinase, urokinase and tissue plasminogen activator. As soon as their target is ad hoc plasminogen activation, an addressed plasmin(ogen) delivery to a clot could have at least the same efficiency. Here we present in a form of narrative review thrombolytic potential of plasmin(ogen) and its derivatives, which in some experiments outperformed approved plasminogen activators. We believe that these factors will find their niches in clinical thrombolytic therapy in the near future.
Keywords: Myocardial infarction, Ischemic stroke, Thrombosis, Thrombolysis, Thrombolytics, Plasmin(ogen), Alteplase, Liposome
Introduction
Cardio- and cerebrovascular thrombosis causes the highest rates of deaths and disabilities in the world, being the most lethal pathology for many years. Taking into consideration ageing of the world population, its frequency will only grow in the future perspective. The gold standard of the thrombolysis treatment now is plasminogen activators infusion, with the most popular current thrombolytic agent being alteplase [1]. Its administration at 0.9 mg/kg rate for stroke treatments within 4.5 hrs time window promotes up to 43% reperfusion within 24 hours and causes intracerebral hemorrhages in ~3-6% of patients [2,3]. It should be noted that current rates of deleterious side effects are lower in comparison with previous generations of fibrin nonspecific thrombolytics, such as, for example, streptokinase and urokinase [4]. Despite serious progress in recanalization strategies, including, for example, mechanical thrombectomy, around half of patients with severe intracerebral thrombosis remains disabled or dies [5]. This stimulates development of new approaches for effective thrombolysis with minimal side effects, which were summarized in a number of recent reviews [4,6-9]. From the other hand, in this narrative review we focus on a thrombolytic potential of plasmin(ogen) and its derivatives, which could be a new generation approach for effective and safe thrombolysis.
Materials and Methods
For this narrative review an electronic search was performed of Pubmed and Scopus databases in English with time limits from 2000 year to present. A key word used was “plasmin(ogen) derivatives”. The electronic searches revealed 3,375 entries, which were then manually curated with special emphasis on role in the body, evaluation in vivo, and targeting by liposomes.
Results and Discussion
Selected articles were categorized by derivative’s types, which are presented below. Each chapter includes detailed structural information to differentiate them; in vitro and in vivo evaluation summary; and practical applications, if they exist. For plasmin(ogen), its pleiotropic roles in the body are presented to better realize its therapeutic and side effects. A comparison of thrombolytic potency of plasmin versus plasminogen activators is presented in a separate chapter.
Plasmin(ogen)
Plasminogen (Plg) is a zymogen form of serine protease plasmin (Plm), responsible for fibrin clot degradation and many other processes [10,11]. It consists of 7 domains: Pan-apple (PAp), 5 kringle domains (KR 1-5) and serine protease domain (Figure 1). The kringle domains interact with fibrin molecules and cellular receptors. These interactions are crucial for open form plasminogen conversion, which can be easily recognized by plasminogen activators. These factors stimulate production of catalytically active plasmin (Plm) by disruption of Arg561/Val562 covalent bond. The most critical step for Plg transformation from closed to open state is dissociation of PAp-KR5 interactions [11]. Generated Plm cuts off the first 71 aa from its own molecule, thus producing Lys-Plm isoform (Figure 1). Lys-Plm has higher fibrin affinity in comparison with the native Glu-Plm molecules [12]. Plasmin generation from inactive zymogen Plg is tightly regulated in the body to avoid systemic fibrinolysis or tumour progression.
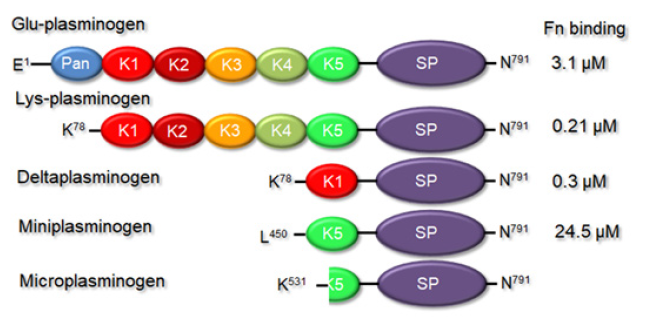
Figure 1: Domain organization of different plasminogen derivatives.
Note*: Pan= Pan-apple; K1-5=kringle domains; SP=serine protease domain. Right column represents fibrin affinities of the corresponding factors, taken from Kim, et al., 2012 and Hunt, et al., 2008 [12,13].
As it was mentioned above, besides the fibrin the plasmin has many other targets in the body, thus being involved in a number of physiological processes, including wound healing, cell signalling, extracellular matrix degradation and inflammatory response (Figure 2). Plm was shown to be a complement inhibitor. The complement system includes ~20 proteases circulating in the body fluids. They are activated in a cascade manner to increase antibody efficiency to attack pathogens, thus being an important part of the inflammatory response. Plasminogen can bind to C3, C3b, C3d, C4b and C5 complement proteins via Lysine residues reducing their activating capacities [14,15]. Some pathogenic bacteria exploit this Plg feature to survive from the host response [16].
Extracellular matrix (ECM) degradation is involved into cell migration and infection development. Plm can directly or indirectly degrade many ECM proteins, thus playing a critical role in such physiological processes as development and tissue remodelling. ECM degradation by Plm also promotes bacteria and parasites as well as immune cells to penetrate in different tissues [17]. Indirect ECM degradation is promoted by Plm via matrix metalloproteases activation, including the key ones MMP-2 and MMP-9 [18]. In the brains laminin degradation by Plm can disrupt ECM interactions with neurons, thus leading to hippocampal neuronal death [19] (Figure 1).
Plasmin binding proteins, Plm receptors, include a wide variety of proteins, such as membrane-bound, intracellular, nuclear proteins and integrins, with C-terminal Lysines [20]. Cell surface plasmin(ogen) binding is crucial for many its functions.
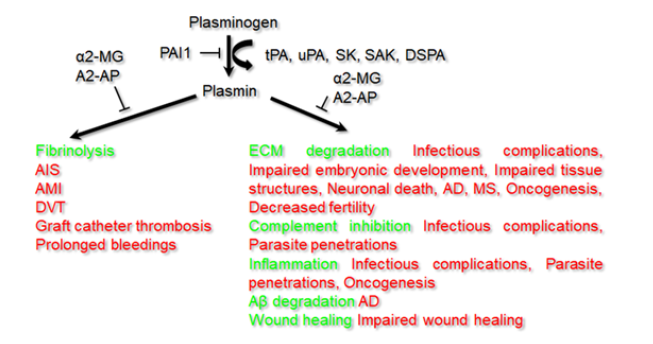
Figure 2: Pleiotropic roles of plasmin(ogen) in the body.
Note*: Main functions are indicated by green and corresponding pathologies - by red color. α2-MG= α2-macroglobulin; α2-AP=α2-antiplasmin; PAI1= plasminogen activator inhibitor 1; tPA=tissue plasminogen activator; uPA=urokinase, SK=streptokinase; SAK=staphylokinase; DSPA=desmoteplase; AIS=acute ischemic stroke; AMI=acute myocardial infarction; DVT=deep venous thrombosis; AD=Alzheimer’s disease; MS=multiple sclerosis.
Being involved in so many processes in the body, plasmin(ogen) plays key roles in development of many pathologies and infections (Figure 2). Its binding with subsequent proteolytic activity promotes invasion into tissues and proliferation of different bacteria, viruses, fungi and parasites. For example, the gram-negative bacteria Moraxella catarrhalis and Pseudomonas aeruginosa, and the spirocytic bacteria Borrelia crocidurae and Borrelia burgdorferi exploit Plg for the host invasion [16,21]. Staphylococcus aureus, the gram-positive pathogen of the skin and soft tissues, exploiting its own plasminogen activator, staphylokinase, decreases the host protective mechanism through fibrinolysis [22]. Due to its unique properties it can be used as a perspective thrombolytic agent [7,8,22]. Influenza A viruses, H5N1 and H1N1, depend on fibrinolysis by Plm for the lung inflammation [23]. It was recently shown that Plm cleavage of furin site in the S protein of SARS-CoV-2 increases its infectivity and virulence [24]. Moreover, significantly increased fibrin degradation product levels and reduced platelets in severe COVID-19 patients are consistent with the presence of hyperfibrinolysis or Plm hyperactivation [24]. Pathogenicity of such fungi as Cryptococcus neoformans and Aspergillus fumigatus exploits Plm activity [25,26]. Neurological problems of people infected with (Figure 2) trematode Fasciola hepatica are dependent on Plg-binding proteins, compromising blood-brain barrier [27].
Many central nervous system (CNS) cells, such as neuroendocrine cells, and cortex, hippocampus, and cerebellum neurons express Plg [28]. Microvessel endothelial cells are main source of tissue plasminogen activator (tPA), which is required for Plg activation in CNS. But tPA can also be produced by neurons, microglia, astrocytes, oligodendrocytes, perivascular mast cells, pericytes and infiltrating leukocytes, suggesting that it has many other roles in CNS [28,29]. Tissue plasminogen activator is often localized at the synapse of neurons and can activate Plg or can be inhibited by PAI1, when released into the extracellular space [30].
Microglia is formed by specialized macrophages, which provide neuroimmune response through releasing cytokines and signalling molecules, clearing cellular debris and dead neurons. Impaired activation of the glial cells can cause such heavy neurodegenerative disorders as Alzheimer’s disease (AD), multiple sclerosis and stroke. Plasmin induces microglial activation and expression of IL-1β, TNF-α, and reactive oxygen species [31,32]. On animal models it was demonstrated that alteplase infusion for acute ischemic stroke treatment seriously increases inflammation via chemokines, cytokines, and microglial recruitment [32,33]. The latter effect could be responsible for tPA neurotoxicity. In brain samples from AD patients tPA and Plm levels were found to be decreased, and thus fibrin clamps can increase vascular damage and inflammation in the course of the disease [34]. These multiple plasmin(ogen) functions can be a limitation for its therapeutic applications, because it is impossible to target one function without affecting another.
Miniplasmin
Miniplasmin (Mini-Plm) contains only KR5 and serine protease (SP) domains from the native molecule (Figure 1). It can be produced from Plm by a limited proteolysis with porcine pancreatic elastase [35]. The most crucial step of its production is Val441/Val442 covalent bond disruption. It is curious that despite much lower fibrin affinity (24.5 vs .0.21 μM, Figure 1;[12]) Mini-Plm demonstrated almost twice the fibrinolytic capacity of Plm [36]. In a canine model of arterial thrombosis miniplasmin has provided faster and higher reperfusion rates over alteplase almost without bleeding complications [37]. Authors of this work have demonstrated that reperfusion rates in the animal groups treated with 3.0 and 1.5 mg/kg doses of Mini-Plm were 100%. Time to reperfusion was 3.3± 1.0 and 7.0± 2.3 min, respectively, which was shorter than in alteplase-treated animals. None vessel in the miniplasmin groups was reoccluded, unlike alteplase-treated group, where 20% of vessels were clotted again. No hemorrhages were observed in Mini-Plm-treated group within 2hrs. The authors suggested that miniplasmin can overperform alteplase for the thrombolysis needs.
Microplasmin
Microplasmin (μ-Plm) is even shorter derivative of Plm than miniplasmin, which is produced in alkali conditions by cleavage between Arg529 and Lys530 residues [Figure 1; [38]]. Due to deletion of the kringle domains it does not have any affinity towards fibrin, but still can be inhibited by α2-antiplasmin and α2-macroglobulin [35,39]. Similar to miniplasmin, μ-Plm has provided good reperfusion rates in animal models with lesser bleeding complication level in comparison with alteplase treatment [Nagai et al., 2003]. Authors of this work have used mice and rabbit models of ischemic stroke. Microplasmin was produced in Pichia pastoris cells. In mice with middle cerebral artery (MCA) occlusion model, infarct size at 24hrs was reduced from ~20 to ~9.1 mm3 with 5.0mg/kg μ-Plm intravenous bolus, and to ~11 mm3 with 4.0mg/kg alteplase. Unlike alteplase, in the case of μ-Plm infarct reduction was still observed after 10 hrs. In rabbits with radiolabelled clots in an extracorporeal arteriovenous loop, infusion of 2.5 mg/kg μ-Plm over 2hrs induced ~51 % clot lysis, whereas alteplase - just ~23 %. In the rabbit model alteplase application was associated with extensive rebleeding, whereas in the case of μ-Plm was not. It is curious that thrombolysis efficiencies of μ-Plm and native Plm were comparable in both models. Higher μ-Plm safety over alteplase has been also confirmed in a rat photothrombotic stroke model [41]. Using multiparametric magnetic resonance imaging, authors of this study noninvasively controlled effects of microplasmin and alteplase on a clot reduction in rats: both thrombolytic agents had similar capacities.
In a multicenter, double-blind, randomized, placebo-controlled phase II clinical trial μ-Plm was found to be well tolerated by ischemic stroke patients with good safety profile and encouraging imaging findings, but further trials were recommended [42]. Another clinical study has evaluated intra-catheter microplasmin bolus administration safety and efficiency [43]. Complete restoration of catheter function was observed in 50% and 66% patients treated with 5 mg and 8 mg of μ-Plm, respectively. Second administration of the drug increased the restoration rate up to 86%. In this study neither bleeding complications nor other deleterious effects were observed after μ-Plm application.
Microplasmin has already occupied a therapeutic niche in ophthalmology for detachment of the vitreous cortex from the retina without surgical interventions. Enzymatic vitreolysis was shown to be less invasive method than surgical vitrectomy, but still effective for vitreoretinal traction relieving via induction of posterior vitreous detachment [44]. Microplasmin commercial form Jetrea (ocriplasmin, ThromboGenics) was approved by FDA in 2012 for the vitreolytic applications.
Another perspective application of μ-Plm is Alzheimer’s disease treatment. Previously it was demonstrated that Plm can directly degrade Aβ peptide polymers, one of the main culprits of AD, in both monomeric and fibrillar forms [45,46]. A number of studies have also shown possible therapeutic effect from increased Plm activity both in vitro and in vivo [45-48]. Due to instant Plm and μ-Plm inhibition by α2-antiplasmin (α2-AP) it was proposed to use their mutant forms with decreased α2-AP binding. Authors have applied a structure-based mutagenesis approach to engineer α2-AP escaping μ-Plm mutants toward a μ-Plm-based AD therapeutics [39]. Out of 52 Alanine scanning mutants tested, F587 residue was shown to make a critical contact, with F587A and F587R mutants being the most promising. Following this work direction, a new efficient, nontoxic and inexpensive AD therapeutic agent can be developed in the near future.
Deltaplasmin
Another Plm derivative carrying KR1 and SP domains called deltaplasmin (δ-Plm; Figure 1) has been generated by Talecris Biotherapeutics. This truncated variant has exhibited fibrinolytic properties similar to the native plasmin. In the body it can be effectively inhibited by α2-antiplasmin and α2- macroglobulin [49]. The deleted non-glycosylated δ-Plm can be purified from bacterial cells, but it almost retains kinetic parameters of the native Plm: Km values were estimated 268.78±19.12 and 324.90±8.43 μM; and Kcat values were 770.48 ± 41.73 and 778.21 ± 1.51 min-1 for plasmin and deltaplasmin, respectively [50].
Thrombolysis by Plasmin Versus Plasminogen Activators
Historically it was plasmin, at the time called ‘fibrinolysin’, that was attempted for thrombolysis in the middle of the last century long before tPA was cloned and purified [10]. These first attempts were completely unsuccessful because systemically administered Plm was instantly inactivated by α2-antiplasmin in patients’ blood. But already in 1960 plasmin was shown to recanalize an occluded artery in a canine model, when administered locally, but not intravenously [51]. The first thrombolytic effect of plasminogen activators was achieved in 1952, when it was clearly shown that a rabbit ear vein clot could be dissolved by IV-administered streptokinase [52]. Almost from the same time the choice of agent (plasminogen activator vs. plasmin) is in the focus of the thrombolysis research [10].
From the other hand, the first clinical applications of tPA and its recombinant form alteplase in the last quarter of 20th century, have resulted in promising outcomes [4]. Since this time clinical thrombolysis practice has mainly focused on its systemic intravenous administration. Alteplase has obtained the FDA approval for the management of acute myocardial infarction in 1987, acute massive pulmonary embolism in 1990, and acute ischemic stroke in 1996 [1]. But if desirable PAs administration effect is ad hoc Plg conversion to Plm, it seems to be reasonable that targeted Plm delivery to a clot could have at least the same efficiency. It is not surprisingly that plasmin has demonstrated good preclinical and clinical outcomes.
Plasmin has overperformed tPA in an in vitro model of catheter-assisted thrombolysis [53]. In the rabbit distal abdominal aorta thrombosis model plasmin 4 mg kg-1 and alteplase 2 mg kg-1 have shown similar efficiencies, 91 and 84 %, respectively, in conditions with free plasminogen supply. If Plg supply was restricted, plasmin was almost twice superior to alteplase in the clot lysis, 93 vs. 49 %, and in reperfusion, 3/4 vs. 0/4 animals [54]. In this work authors observed rebleeding in 9/10 tPA-treated animals, and in 0/10 Plg-treated. In a rabbit model of the thrombin-induced MCA occlusion, plasmin 1-3 mg added into the downstream internal carotid artery provided rapid reperfusion within 10 min [55].
In 1996, in a clinical study on the thrombosed anterior cerebral artery of 20 patients with AIS, it was demonstrated higher thrombolysis and better functional recovery in a group receiving tPA or uPA with Lys-Plg than in a group treated with PAs only [56]. This combination could be considered as an equivalent of plasmin infusion. Full-length plasma-derived plasmin has been assessed for safety in 30 patients with thrombosed hemodialysis shunts [57]. Patients receiving the highest dose 24 mg infused over 30 min via criss-crossed pulse-spray catheters aft had over 75% lysis of the thrombosed grafts. Authors did not observe dose-related changes in α2-antiplasmin or fibrinogen concentrations, and rate of the bleeding events were attributable to heparin. In a porcine model plasmin was shown to have higher capacity over tPA in dissolving arteriovenous graft clots [58]. The grafts were induced bilaterally between the carotid artery and jugular vein by clamping for 1 hr. In this model overall thrombolytic activity of plasmin measured by residual clot plus released clot mass was ~1.5 times higher than that of tPA: 193 mg ± 25 vs 310 mg ± 35.
Thus, plasmin and its derivatives have demonstrated their efficiencies and in some conditions have overperformed alteplase in efficiency and safety of the thrombolysis. Targeting to a clot increases their potency. Due to pleiotropic roles of Plm in the body (Figure 2) its dosages and side effects should be carefully determined. We believe that plasmin(ogen) administration alone or in combination with other factors can find its clinical application for treatments of thrombosis or other pathologies.
Plasmin and Nanocarriers
Liposomes, self-assembled membrane-like spherical vesicles, are non-toxic biocompatible nanoparticles approved by FDA and EMA for the application in human medicine with a considerable potential as diagnostic and theranostic carriers applicable for improving drug targeting as well as imaging techniques such as Computed Tomography (CT) or magnetic resonance imaging (MRI). Recently, we demonstrated targeting of nanoliposomes towards fibrin fibres in thrombi by specific binders derived from a small protein domain scaffold developed by ribosome display technology [59]. An example of liposomes targeted by specific binders towards fibrin fibres is in Figure 3. Targeting of liposomes carrying thrombolytic drugs to thrombi represents another step to improving efficacy of treatment.

Figure 3: Binding of nanoliposomes onto surface of fibrin fibres.
Note*: Binders with selectivity to fibrin were bound onto the surface of nanoliposomes. The fibrin clot was incubated with nanoliposomes targeted by selective binders. Picture was obtained by scanning electron microscopy. Liposomes - white arrows; fibrin fibres - Black arrows (details see in [Petroklova, et al, 2018][59]).
Current efforts to solubilize a thrombus by application of conventional thrombolytic therapy are limited owing to a low efficacy of present formulations of plasmin activators and thrombolytic enzymes used for therapy. It is caused by rapid degradation of thrombolytic enzymes. The side effects are induced by spreading out of enzymes within the circulation when introduced as free proteins (Figure 3).
Application of biocompatible nanocarriers like liposomes offers possibility to improve thrombus targeting and hence increasing the selectivity of thrombolytic enzyme action, because they will demonstrate their proteolytic activities in the vicinity to clots, but not in the whole blood. Long circulating liposomes with surface bound thrombolytic enzymes will improve their stability while increasing their local concentration in thrombi owing to increasing avidity of liposomal formulation via high number of surface linked molecules of enzyme. Some conceptual approaches were reviewed recently [9] and preparation of long circulating liposomes stabilised by biocompatible polymers was described [60]. Various derivatives of plasmin prepared by recombinant technology can be designed and engineered for binding to long circulating nanoliposome to improve their trhrombolytic therapeutic effect.
Conclusions
The development of protein engineering and biotechnological processes for the preparation of recombinant proteins provides a prerequisite for the development of effective and safe thrombolytics for the treatment of strokes and other diseases caused by blood clots. From one hand, appropriate design of recombinant plasmin-derived proteins can increase their stability, activity and thus efficiency, while decreasing undesirable side effects. From the other hand, suitability of nanocarrier-based preparations with long circulation properties can help enhancing their positive effects. Such complex functionalised nanocarriers with engineered thrombolytics inside can circulate in blood stream and find the thrombi, diffuse into their fibrous structure, bind to fibrin fibres and start rapid fibrolytic action, while leaving unattached off target tissue. Therefore, nanocarrier targeting can prevent bleeding which is the common side effect observed with application of thrombolytic formulated if used as free enzyme. There are technologies for development and GMP production of high quality recombinant plasmin-based enzymes in high-expression cell line. Long circulating biocompatible nanoliposomal carriers are available for construction of complexes with plasmin derivatives to improve their therapeutical potential.
Acknowledgements
The authors would like to express their thanks to the Ministry of Health of the Czech Republic, AZV grant nr. NU21-08-00510 (J.T,.R.M.) Ministry of Education, Youth, and Sport, Czech Republic grant OPVVV CEREBIT CZ.02.1.01/0.0/0.0/16_025 /0007397 (J.T.) and C2P NEXARS internal research project.
Conflicts of Interest
The authors declare that they have no conflict of interests.
References
- Sila CA, Furlan AJ (1996) Therapy for acute ischemic stroke: the door opens. Interpreting the NINDS rt-PA stroke study. Cleve Clin J Med 63(2): 77-79.
- Bivard A, Huang X, Levi CR, Spratt N, Campbell BCV, et al. (2017) Tenecteplase in ischemic stroke offers improved recanalization: Analysis of 2 trials. Neurology 89(1): 62-67.
- Yaghi S, Willey JZ, Cucchiara B, Goldstein JN, Gonzales NR, et al. (2017) Treatment and outcome of hemorrhagic transformation after intravenous alteplase in acute ischemic stroke: A scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 48(12): e343-e361.
- Collen D, Lijnen HR (2009) The tissue-type plasminogen activator story. Arterioscler Thromb Vasc Biol 29(8): 1151-1155.
- Albers GW, MM, Kemp S, Christensen S, Tsai JP, et al. (2018) Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med 378(8): 708-718.
- Bivard A, Lin L (2013) Parsons M. Review of stroke thrombolytics. J Stroke 15(2): 90-98.
- Mican J, Toul M, Bednar D, Damborsky J (2019) Structural Biology and Protein Engineering of Thrombolytics. Comput Struct Biotechnol J 17: 917-938.
- Nikitin D, Choi S, Mican J, Toul M, Ryu W S, et al. (2021) Development and testing of thrombolytics in stroke. J Stroke 23(1): 12-36.
- Koudelka S, Mikulik R, Masek J, Raska M, Knotigova P, et al. (2016) Liposomal nanocarriers for plasminogen activators. J Contr Release 227: 45-57.
- Marder VJ (2011) Historical perspective and future direction of thrombolysis research: the re-discovery of plasmin. J Thromb Haemost 9: 364-373.
- Law R, Caradoc Davies T, Cowieson N, Horvath A, Quek A, et al. (2012) The X-ray Crystal Structure of Full-Length Human Plasminogen. Cell Rep 1(3): 185-190.
- Kim P, Tieu L, Stafford A, Fredenburgh J, Weitz J (2012) A High Affinity Interaction of Plasminogen with Fibrin Is Not Essential for Efficient Activation by Tissue-type Plasminogen Activator. J Biol Chem 287(7): 4652-4661.
- Hunt J, Petteway S, Scuderi P, Novokhatny V (2008) Simplified recombinant plasmin: production and functional comparison of a novel thrombolytic molecule withplasma-derived plasmin. Thromb Haemost 100(3): 413-419.
- Barthel D, Schindler S, Zipfel P (2012) Plasminogen is a complement inhibitor. Journal Biol Chem 287(22): 18831-18842.
- Agarwal V, Talens S, Grandits A, Blom A (2015) A Novel Interaction between Complement Inhibitor C4b-binding Protein and Plasminogen That Enhances Plasminogen Activation. Journal Biol Chem 290(30): 18333-18342.
- Singh B, Al Jubair T, Voraganti C, Andersson T, Mukherjee O, et al. (2015) Moraxella catarrhalis Binds Plasminogen To Evade Host Innate Immunity. Infect Immun 83(9): 3458-3469.
- Liu K, Shih N (2007) The role of enolase in tissue invasion and metastasis of pathogens and tumor cells. J Cancer Mol 3: 45-48.
- Huang M, Gong Y, Grondolsky J, Hoover Plow J (2014) Lp(a)/apo(a) modulate MMP-9 activation and neutrophil cytokines in vivo in inflammation to regulate leukocyte recruitment. Am J Pathol 184(5): 1503-1517.
- Chen Z, Strickland S (1997) Neuronal death in the hippocampus is promoted by plasmin-catalyzed degradation of laminin. Cell 91(7): 917-925.
- Flick M, and Bugge T (2017) Plasminogen-receptor KT: plasminogen activation and beyond. J Thromb Haemost 15(1): 150-154.
- Ceremuga I, Seweryn E, Bednarz Misa I, Pietkiewicz J, Jermakow K, et al. (2014) Enolase-like protein present on the outer ´ membrane of Pseudomonas aeruginosa binds plasminogen. Folia Microbiol 59(5): 391-397.
- Collen D (1998) Staphylokinase: a potent, uniquely fibrin-selective thrombolytic agent. Nat Med 4(3): 279-284.
- Berri F, Rimmelzwaan G, Hanss M, Albina E, Foucault Grunenwald M, et al. (2013) Plasminogen controls inflammation and pathogenesis of influenza virus infections via fibrinolysis. PLoS Pathog 9(3): e1003229.
- Hong Long J, Zhao R, Matalon S, Matthay M (2020) Elevated Plasmin(ogen) as a Common Risk Factor for COVID-19 Susceptibility. Physiol Rev 100(3): 1065-1075.
- Stie J, Fox D (2012) Blood-brain barrier invasion by Cryptococcus neoformans is enhanced by functional interactions with plasmin. Microbiology 158(pt 1): 240-258.
- Zaas A, Liao G, Chien J, Weinberg C, Shore D, et al. (2008) Plasminogen alleles influence susceptibility to invasive aspergillosis. PLoS Genet 4(6): e1000101.
- Gonzalez Miguel J, Valero M, Reguera Gomez M, Mas Bargues C, ´Bargues M, et al. (2019) Numerous Fasciola plasminogen-binding proteins may underlie blood-brain barrier leakage and explain neurological disorder complexity and heterogeneity in the acute and chronic phases of human fascioliasis. Parasitology 146(3): 284-298.
- Mehra A, Ali C, Parcq J, Vivien D, Docagne F (2016) The plasminogen activation system in neuroinflammation. Biochim Biophys Acta 1862(3): 395-402.
- Yepes M, Roussel B, Ali C, Vivien D (2009) Tissue-type plasminogen activator in the ischemic brain: more than thrombolytic. Trends Neurosci 32(1): 48-55.
- Qian Z, Gilbert M, Colicos M, Kandel E, Kuhl D (1993) Tissue plasminogen activator is induced as an immediate-early gene during seizure, kindling and long-term potentiation. Nature 361(6411): 453-457.
- Min K, Jou I, Joe E (2003) Plasminogen-induced IL-1beta and TNFalpha production in microglia is regulated by reactive oxygen species. Biochem Biophys Res Commun 312(4): 969-974.
- Miyazaki T, Kimura Y, Ohata H, Hashimoto T, Shibata K, et al. (2011) Distinct effects of tissue-type plasminogen activator and SMTP-7 on cerebrovascular inflammation following thrombolytic reperfusion. Stroke 42(4): 1097-1104.
- Lenglet S, Montecucco F, Denes A, Coutts G, Pinteaux E, et al. (2014) Recombinant tissue plasminogen activator enhances microglial cell recruitment after stroke in mice. J Cereb Blood Flow Metab 34(5): 802-812.
- Melchor J, Pawlak R, Strickland S (2003) The tissue plasminogen activator-plasminogen proteolytic cascade accelerates amyloid-beta (Abeta) degradation and inhibits Abeta-induced neurodegeneration. J Neurosci 23(26): 8867-8871.
- Marder V, Novokhatny V (2010) Direct fibrinolytic agents: biochemical attributes, preclinical foundation and clinical potential. J Thromb Haemost 8(3): 433-444.
- Moroz L (1981) Mini plasminogen: a mechanism for leukocyte modulation of plasminogen activation by urokinase. Blood 58(1): 97-104.
- Fu J, Ren J, Zou L, Bian G (2008) The thrombolytic effect of miniplasmin in a canine model of femoral artery thrombosis. Thromb Res 122(5): 683-690.
- Shi G, Wu H (1988) Isolation and characterization of microplasminogen. A low molecular weight form of plasminogen. J Biol Chem 263(32): 17071-17075.
- Yang D, Zhu W, Wang Y, Tan F, Ma Z, et al. (2020) Selection of mutant µplasmin for amyloid-β cleavage in vivo. Sci Rep 10(1): 12117.s
- Nagai N, Demarsin E, Van Hoef B, Wouters S, Cingolani D, et al. (2003) Recombinant human microplasmin: production and potential therapeutic properties. J Thromb Haemost 1(2): 307-313.
- Chen F, Suzuki Y, Nagai N, Sun X, Wang H, et al. (2007) Microplasmin and tissue plasminogen activator: comparison of therapeutic effects in rat stroke model at multiparametric MR imaging. Radiology 244(2): 429-438.
- Thijs V, Peeters A, Vosko M, Aichner F, Schellinger PD, et al. (2009) Randomized, placebo-controlled, dose-ranging clinical trial of intravenous microplasmin in patients with acute ischemic stroke. Stroke 40(12): 3789-3795.
- Verhamme P, Jerome M, Goossens G, Devis J, Maleux G, et al. (2009) A pilot trial of microplasmin in patients with long-term venous access catheter thrombosis. J Thromb Thrombolysis 28(4): 477-481.
- Chen W, Huang X, Ma X, Mo W, Wang W, et al. (2008) Enzymatic vitreolysis with recombinant microplasminogen and tissue plasminogen activator. Eye (Lond) 22(2): 300-307.
- van Nostrand W, Porter M (1999) Plasmin cleavage of the amyloid β-protein. Alteration of secondary structure and stimulation of tissue plasminogen activator activity. Biochemistry 38(35): 11570-11576.
- Tucker H, Kihiko Ehmann M, Wright S, Rydel R. Estus S (2000) Tissue plasminogen activator requires plasminogen to modulate amyloid-beta neurotoxicity and deposition. J Neurochem a 75(5): 2172-2177.
- Tucker H, Kihiko M, Caldwell J, Wright S, Kawarabayashi T, et al. (2000) The plasmin system is induced by and degrades amyloid-betaaggregates. J Neurosis 20(11): 3937-3946.
- Jacobsen J, Comery T, Martone R, Elokdah H, Crandall D, et al. (2008) Enhanced clearance of Abeta in brain by sustaining the plasmin proteolysis cascade. Proc Natl Acad Sci U S A 105(25): 8754-8759.
- Hunt J, Petteway S, Scuderi P, Novokhatny V (2008) Simplified recombinant plasmin: production and functional comparison of a novel thrombolytic molecule with plasma-derived plasmin. Thromb Haemost 100(3): 413-419.
- Alves N, Kline J (2015) Comparative study on the inhibition of plasmin and delta-plasmin via benzamidine derivatives. Biochem Biophys Res Commun 457(3): 358-362.
- Boyles P, Meyer W, Graff J, Ashley C, Ripic R (1960) Comparative effectiveness of intravenous and intra-arterial fibrinolysin therapy. Am J Cardiol 6: 439-440.
- Johnson A, Tillett W (1952) Lysis in rabbits of intravavascular blood clots by the streptococcal fibrinolytic system (streptokinase). J Exp Med 95(5): 449-464.
- Novokhatny V, Taylor K, Zimmerman T (2003) Thrombolytic potency of acid-stabilized plasmin: superiority over tissue-type plasminogen activator in an in vitro model of catheter-assisted thrombolysis. J Thromb Haemost 1(5): 1034-1041.
- Marder V, Landskroner K, Novokhatny V, Zimmerman T, Kong M, et al. (2001) Plasmin induces local thrombolysis without causing hemorrhage: a comparison with tissue plasminogen activator in the rabbit. Thromb Haemost 86(3): 739-745.
- Jahan R, Stewart D, Vinters H, Yong W, Vinuela F, et al. (2008) Middle cerebral artery occlusion in the rabbit using selective angiography: application for assessment of thrombolysis. Stroke 39(5): 1613-1615.
- Freitag H, Becker V, Thie A, Tilsner V, Philapitsch A, et al. (1996) Lys-plasminogen as an adjunct to local intra-arterial fibrinolysis for carotid territory stroke: laboratory and clinical findings. Neuroradiology 38: 181-185.
- Shlansky Goldberg R, Matsumoto A, Baumbach GA, Siegel J, Raabe R, et al. (2008) A first-in human phase I trial of locally delivered human plasmin for hemodialysis graft occlusion. J Thromb Haemost 6(6): 944-950.
- Hoefer I, Stroes E, Pasterkamp G, Levi M, Reekers JA, et al. (2009) Locally applied recombinant plasmin results in effective thrombolysis in a porcine model of arteriovenous graft thrombosis. J Vasc Interv Radiol 20(7): 951-958.
- Petroková H, Mašek J, Kuchar M, Vítecková Wünschová A, Štikarová J, et al. (2019) Targeting Human Thrombus by Liposomes Modified with Anti-Fibrin Protein Binders. Pharmaceutics 11(12): 642-663.
- Bartheldyova E, Effenberg R, Masek J, Prochazka L, Knotigova PT, et al. (2018) Hyaluronic acid surface modified liposomes prepared via orthogonal aminoxy coupling: Synthesis of nontoxic aminoxylipids based on symmetrically alpha-branched fatty acids, preparation of liposomes by microfluidic mixing, and targeting to cancer cells expressing cd44. Bioconjugate Chem 29(7): 2343-2356.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.