Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Determination of Hydroxylamine Hydrochloride in Relugolix Using Pre-Column Derivatization HPLC Method
Funding: CAMS Innovation Fund for Medical Sciences (2019-I2M-5-020).
*Corresponding author: Mujun Zhang, Tianjin Institute of Pharmaceutical Research, China.
Received: July 04, 2024; Published: July 12, 2024
DOI: 10.34297/AJBSR.2024.23.003055
Abstract
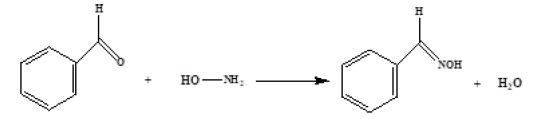
A pre-column derivatization in conjunction with HPLC-UV method was developed for determination of genotoxic impurity hydroxylamine hydrochloride content in Relugolix drug substance. The derivatization mechanism is that derivatization reagent benzaldehyde reacted with hydroxylamine to generate stable derivative benzaldoxime. Optimization of the derivatization method was conducted in terms of volume of the derivatization reagent solution, derivatization temperature and derivatization time. The optimized method was fully validated, which demonstrated it to be specific, sensitive, linear, accurate, precise and robust. The limit of quantitation was 12ppm, and the linear range was from 12 to 360ppm. The method was successfully applied to quantification of hydroxylamine hydrochloride in bulk Relugolix APIs. Finally, the method was characterized as easy operational procedure, low detection limit and good precision.
Keywords: Genotoxic impurity, Hydroxylamine hydrochloride, Pre-column derivatization, High performance liquid chromatography
Introduction
Hydroxylamine (HA) and its salt Hydroxylamine Hydrochloride (HA.HCl) are important reducing agents widely used in synthesis of pharmaceutical intermediates and drug substances [1,2]. It is also used as a tanning agent for leather manufacture and an antioxidant for fatty acids and soaps [3]. Hydroxylamine was reported to be mutagenic, cytotoxic and carcinogenic [4,5]. Hydroxylamine caused hematotoxicity mainly, leading to methemoglobinemia and hemolytic anemia [6]. Therefore, developing a reliable method to determine trace hydroxylamine in drug substances is very important. Numerous methods have been reported for the determination of hydroxylamine, including direct measurement methods and derivatization methods. Direct measurement methods like electrochemical detection method [7,8], Ion chromatographic detection method [9]. However, most direct methods were used for waste-water samples or environmental water samples and may not be applicative for pharmaceutical analysis with a complex matrix. Considering that hydroxylamine has no UV absorption and no carbon atoms, it is hard to determine it using conventional HPLC or GC methods. So, pre-column derivatization coupled with HPLC or GC detection are often used to determine hydroxylamine [10-13].
Relugolix is an oral small molecule Gonadotropin-Releasing Hormone (GnRH) receptor antagonist, indicated for the treatment of prostate cancer [14,15], uterine fibroids [16,17] and endometriosis-associated pain [18]. Hydroxylamine hydrochloride was used in Relugolix synthetic process and there may be potential impurity residue. According to the literature [19], the Permissible Daily Exposure (PDE) of hydroxylamine is 23μg/day. The maximum daily dose of Relugolix is 360mg. On this basis, the control limit of hydroxylamine hydrochloride calculated is 134ppm. However, in this case, a more stringent limit of 120ppm for hydroxylamine hydrochloride was considered with respect to test concentration. In this study, a simple derivatization method coupled with HPLC detection was described. As shown in Figure 1, the derivatization mechanism is that derivatization reagent benzaldehyde reacted with hydroxylamine to generate stable derivative benzaldoxime. The proposed method was optimized and validated according to the ICH guidance. The method was successfully applied to quantification of hydroxylamine hydrochloride in bulk Relugolix APIs. Finally, the method was characterized as easy operational procedure, low detection limit and good precision.
Experimental
Materials and Apparatus
Materials: All the chemicals used were analytical reagent grade or better. Freshly prepared ultrapure water was used throughout the study. The following chemicals were purchased from Aladdin Reagent Co., Ltd. (Shanghai, China): Potassium Dihydrogen Phosphate (KH2PO4), Hydroxylamine Hydrochloride (NH2OH·HCl), triethylamine and benzaldehyde. Phosphoric acid was obtained from Macklin Biochemical Co., Ltd. (Shanghai, China). Acetonitrile was supplied by Concord Technology Co., Ltd. (Tianjin, China). Methanol was obtained from Hipure Chem (NY, USA).
Apparatus: An Agilent 1260 Infinity Ⅱ HPLC (California, America) coupled with UV detector under the control of OpenLab CDS workstation (Version 3.4) was used for the quantitative analysis of the derivatives. A YMC-Pack ODS-A column (150×6mm, 5μm) was used as the HPLC analytical column. A Mettler Toledo S210 pH meter (Zurich, Switzerland) was used for pH adjustment. A MMS8-Pro magnetic stirrer (JOANLAB, Zhejiang, China) and a WN-060S ultrasonic cleaner (UL, Jiangsu, China) were used for the sample pretreatment. A Mettler Toledo XSR105/A Electronic balance was used throughout the study.
Chromatographic Conditions
The column used for HPLC was a YMC-Pack ODS-A column (150×4.6mm, 5μm). The mobile phase was the mixture of acetonitrile and phosphate buffer solution (0.01mol/L KH2PO4 aqueous solution was adjusted to pH 2.3) (35:65, v/v). The flow rate was 1.0 ml/min. The temperature of column oven was 40℃. A UV detector was used, and the detection wavelength was set at 254nm. The injection volume was 10μl. The acquisition time was 30 minutes.
Solution Preparation
Derivatization Reagent Solution: Measured accurately 1.0ml of benzaldehyde to a 10ml volumetric flask, dissolved and diluted to volume with methanol, and mixed well. Transferred 0.5ml above solution into a 50ml volumetric flask, dissolved and diluted to volume with water, and mixed well as derivatization reagent solution.
Standard Stock Solution: Weighed accurately 15mg of hydroxylamine hydrochloride standard into a 50ml volumetric flask, dissolved and diluted to volume with water, and mixed well. Transferred 1.0ml of above solution, accurately measured, into a 50ml volumetric flask, diluted to volume with water and mixed well as standard stock solution.
Blank Derivative Solution: 3ml of derivatization reagent solution and 20μl of triethylamine were transferred into a 10ml volumetric flask, diluted to volume with water and mixed well.
Standard Solution: Transferred 1.0ml of the standard stock solution, accurately measured, into a 10ml volumetric flask, 3ml of derivatization reagent solution and 20μl of triethylamine were added successively, diluted to volume with water and mixed well. Shaken well and reacted at room temperature for 0.5h.
Sample Solution: 100mg of drug substance sample was taken into a 20ml penicillin bottle and added 10ml of water, mixed well and ultrasonic extraction for 15 minutes, then filtered. Transferred 5ml of the filtrate into a 10ml volumetric flask, 3ml of derivatization reagent solution and 20μl of triethylamine were added successively, diluted to volume with water and mixed well. After reacting at room temperature for 0.5h, the sample solution was obtained.
Spiked Sample Solution: 100mg of drug substance sample was taken into a 20ml penicillin bottle and added 2ml of standard stock solution and 8ml of water, mixed well and ultrasonic extraction for 15 minutes, then filtered. Transferred 5ml of the filtrate into a 10ml volumetric flask, 3ml of derivatization reagent solution and 20μl of triethylamine were added successively, diluted to volume with water and mixed well. Reacted at room temperature for 0.5h, the spiked sample solution was obtained.
Results and Discussion
Optimization of Derivatization Condition
Effect of Volume of the Derivatization Reagent Solution: The effect of volume of the derivatization reagent solution was investigated by varying the volume from 0.05ml to 5.0ml. The results are shown in Figure 2. In the range of 0.05~0ml, the peak area increases with the increase of the derivatization reagent solution volume. In the range of 2.0~5.0ml, the peak area does not change with the increase of derivatization reagent solution volume. Thus, 3ml derivatization reagent solution was added to the reaction system in the rest of the experiments.
Effect of Derivatization Temperature: The derivatization reactions were conducted at 25, 35, 45, 55 and 65℃ respectively for 1 hour. The detected derivative peak area demonstrated that there is very little difference observed from 25℃~65℃, as shown in Figure 3. Therefore, the reaction was conducted at ambient room temperature.
Effect of Derivatization Time: To study the effect of derivatization time, the derivatization reactions were conducted for 5min, 10min, 30min, 60min, 90min and 120min respectively. It can be seen from Figure 4 that the reaction of hydroxylamine hydrochloride and benzaldehyde to produce benzaldoxime is very quick, and the reaction can reach the end point after 5min. The peak area of derivative is nearly unchanged from 5min to 120min. Thus, 30min was selected as the appropriate derivatization time.
Method Validation
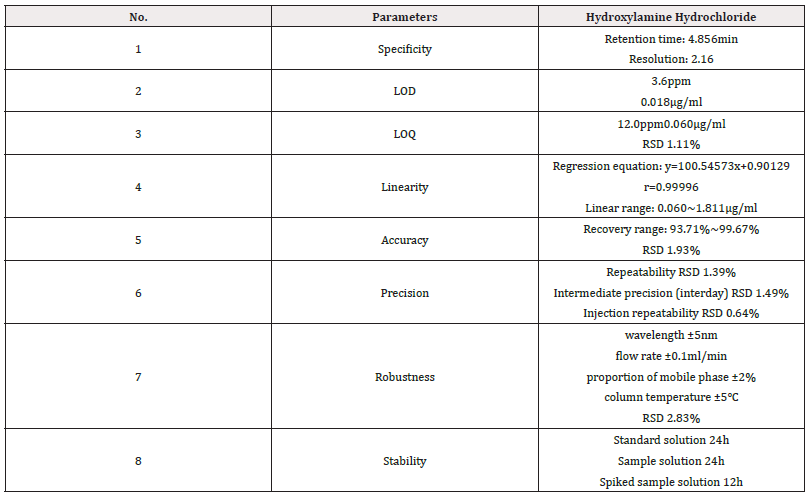
To establish the suitability of the presented method for determining traces of hydroxylamine hydrochloride in a drug substance, the validation parameters like specificity, limit of detection, limit of quantification, linearity, accuracy, precision, robustness and stability were investigated. The summary of analytical method validation is presented in Table 1.
Specificity: The blank derivative solution, standard solution, sample solution and the spiked sample solution were precisely measured and injected into the HPLC system. The result shows that no interference of blank derivative solution was observed. Hydroxylamine hydrochloride was not detected in the sample solution. The resolution of the derivatized product in the spiked sample solution meets the requirement. The retention time of the derivative in spiked sample solution corresponded to the retention time of the derivative from standard solution. The typical chromatograms are shown in Figure 5. The method has good specificity.

Figure 5: Chromatograms of specificity test: (a) blank derivative solution, (b) standard solution, (c) sample solution, (d) spiked sample solution.
Limit of Detection and Limit of Quantification: The Limit of Detection (LOD) was determined to be 0.018μg/ml with a signal-to-noise (S/N) ratio of about 7, which was equivalent to 3.6ppm (w/w) of API. The Limit of Quantification (LOQ) was determined to be 0.060μg/ml with a signal-to-noise (S/N) ratio of about 18, which was equivalent to 12ppm (w/w) of API. The RSD of peak area of LOQ solutions in 6 replicates was 1.11%. The sensitivity obtained from this analytical method was adequate for determining hydroxylamine hydrochloride in the API.
Linearity: Linearity was evaluated at seven different concentrations from 0.060 to 1.811μg/ml (12~360 ppm). The correlation coefficient (r) of the calibration curve was greater than 0.990. A good correlation was found between the concentration range and the measured responses.
Accuracy: To determine the accuracy of the proposed method, three concentration levels (50%, 100% and 150%) of standard analyte were added to the sample solution, and the recovery was evaluated. The recovery obtained of three concentration levels was within the ranges of 93.71%~67% and with an overall RSD of 1.93%. These results indicated that the recovery was reproductive and consistent.
Precision: The precision of the analytical method was studied using two different measures: repeatability and intermediate precision (interday) in terms of RSD of spiked sample solution of 6 replicates. The RSD of repeatability and intermediate precision was 1.39% and 1.49%, respectively. Injection repeatability was determined by injecting the standard solution into HPLC six times and evaluated in terms of peak area. The RSD of peak area was 0.64%.
Robustness: To assess the robustness of the method, various factors were slightly changed in the experimental. The study was carried out with respect to wavelength 254nm±5nm, flow rate 1.0±1ml/min, proportion of mobile phase ±2% and column temperature 40℃±5℃. There is not much variation observed in the content of hydroxylamine hydrochloride in the spiked sample solution obtained at different conditions. Hence, it concludes that the test method is robust for all varied conditions.
Stability: The stability of the derivative product was studied at room temperature. The peak area changes rate of the standard solution placed at room temperature for 24hours is less than 4%, therefore it is considered stable within 24hours. Within 24hours, no hydroxylamine hydrochloride was detected in the sample solution. Therefore, it is believed that the sample solution is stable within 24hours. The peak area change rate of the spiked sample solution is less than 4% for 12hours. Therefore, it is considered that the spiked sample solution is stable within 12hours.
Application of the Method in Drug Substance
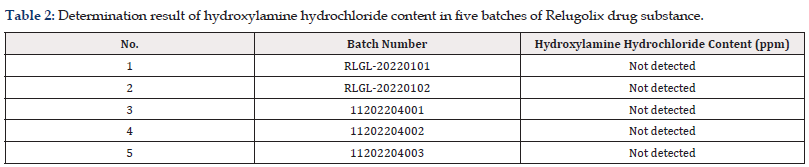
The proposed method was applied to the assessment of hydroxylamine hydrochloride content in five batches of Relugolix drug substance. As shown in Table 2, there was detected in none of the batches.
Conclusions
A pre-column derivatization in conjunction with HPLC-UV method was developed for determination of hydroxylamine hydrochloride content in Relugolix drug substance. The method was optimized in terms of volume of the derivatization reagent solution, derivatization temperature and derivatization time. The results of various validation parameters confirmed that the method is specific, sensitive, linear, accurate, precise and robust. The method was applied in Relugolix drug substance, and there was no hydroxylamine hydrochloride detected in the drug substance. Overall, easy operational procedure, low detection limit and good precision are dominant advantages of the proposed method.
Highlights
i. A pre-column derivatization in conjunction with HPLC-UV method was developed for determination of genotoxic impurity hydroxylamine hydrochloride.
ii. The method was fully validated in several parameters, which demonstrated to be specific, sensitive, linear, accurate, precise and robust.
iii. The method was successfully applied to the assessment of hydroxylamine hydrochloride content in Relugolix drug substance.
Acknowledgements
None.
Conflict of Interest
None.
References
- J Li (1989) Improvement of synthesis process of sulfamethoxazole intermediates. Guangdong Chemical Industrial(4): 26-27.
- VA Khripach, VN Zhabinskii, AI Kuchto, Galina P Fando, Yuliya Y Zhiburtovich, et al., (2004) Reaction of (13S)-13-iodo-6beta-methoxy-3alpha,5-cyclo-13,14-seco-5alpha-androstane-14,17-dione with hydroxylamine and its application to the synthesis of new 13,14-seco steroids. Steroids 69(7): 511-514.
- CT Evelo, AA Spooren, RA Bisschops, LG Baars, JM Neis (1998) Two mechanisms for toxic effects of hydroxylamines in human erythrocytes: involvement of free radicals and risk of potentiation. Blood Cells Mol Dis 24(3): 280-295.
- H Riemann (1950) On the toxicity of hydroxylamine. Acta Pharmacol Toxicol (Copenh) 6(3): 285-292.
- P Gross (1985) Biologic activity of hydroxylamine: a review. Crit Rev Toxicol 14(1): 87-99.
- JM DeSesso, GC Goeringer (1990) Developmental toxicity of hydroxylamine: an example of a maternally mediated effect. Toxicol Ind Health 6(1): 109-121.
- C Zhao, JF Song (2001) Flow-injection biamperometry for direct determination of hydroxylamine at two pretreated platinum electrodes. Analytica Chimica Acta 434: 261-267.
- HM Moghaddam, H Beitollahi, S Tajik, Mohammad Malakootian, Hassan Karimi Maleh (2014) Simultaneous determination of hydroxylamine and phenol using a nanostructure-based electrochemical sensor. Environ Monit Assess 186(11): 7431-7441.
- PN Fernando, IN Egwu, MS Hussain (2002) Ion chromatographic determination of trace hydroxylamine in waste streams generated by a pharmaceutical reaction process. J Chromatogr A 956(1-2): 261-270.
- F Lombardi, T Crolla (1998) Determination of hydroxylamine traces in propionohydroxamic acid bulk drug and pharmaceutical preparations by capillary gas chromatography. J Pharm Sci 77(8): 711-714.
- S Prabhune, K Darsi, M Gangrade, et al., (2017) Quantitative measurement of trace levels of residual hydroxylamine hydrochloride by a simple gas chromatographic method and its application in drug substance. World Journal of Pharmaceutical Research 6(2): 758-769.
- WD Korte (1992) Determination of hydroxylamine in aqueous solutions of pyridinium aldoximes by high-performance liquid chromatography with UV and fluorometric detection. Journal of Chromatography 603: 145-150.
- M Song, S Wu, Pb Lu, et al., (2016) A selective and sensitive pre-column derivatization HPLC method for the trace analysis of genotoxic impurity hydroxylamine in active pharmaceutical ingredients. Analytical Methods 8(47): 8352-8361.
- A Markham (2019) Relugolix: First global approval. Drugs 79(6): 675-679.
- M Shirley (2023) Relugolix: A review in advanced prostate cancer. Target Oncol 18(2): 295-302.
- Y Osuga, K Enya, K Kudou, Masataka Tanimoto, Hiroshi Hoshiai (2019) Oral gonadotropin-releasing hormone antagonist relugolix compared with leuprorelin injections for uterine leiomyomas: A Randomized Controlled Trial. Obstet Gynecol 133(3): 423-433.
- AA Hendy, AS Lukes, AN Poindexter, Roberta Venturella, Claudio Villarroel, et al., (2021) Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med 384(7): 630-642.
- HA Blair (2024) Relugolix/Estradiol/Norethisterone Acetate: A review in endometriosis-associated Pain. Drugs 84(4): 449-457.
- JP Bercu, SM Galloway, P Parris, et al., (2018) Potential impurities in drug substances: Compound-specific toxicology limits for 20 synthetic reagents and by-products, and a class-specific toxicology limit for alkyl bromides. Regul Toxicol Pharmacol 94: 172-182.






 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.