Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Final Methodical Research in the Surface Hydrophobicity (SHP) of Native Casein Micelles in Milk
*Corresponding author: Barbel Lieske, 10407 Berlin Kniprodestr. 120, Germany.
Received: June 17, 2024; Published: June 25, 2024
DOI: 10.34297/AJBSR.2024.23.003039
Introduction
The development of this method goes back to1990/91 and was refined further until 2004. The initial interest was to know the interference of the non-ionic detergent Tween 80 in the Bio-Rad Protein Assay with a variety of proteins The detergent binding seemed to be a measure of hydrophobic areas of integral proteins and thus constituting the basis for an assay. First, the methodical research was turned to analyze only isolated proteins [1]. Further development of the SHP-method was necessary to meet the demands of natural casein micelles. At the beginning double distilled water was considered to be just right for that purpose, however, its efficiency was not lasting. It seemed that the purity of water remained one crucial point for the colloid-chemical analysis. Ongoing research into the causes was promising and integrated in the methodical description of the further developed method as described below.
Reagent and Sample Preparation
Conditioning of Water
500ml water (deionized) plus Phenylmethylsulphonyl- chloride (PMS-Cl), (Sigma, Deisenhofen, Germany) are heated at 115°C for 70min. Then stored closed in a refrigerator overnight. The final pH of the water should be 3,7-3,8. In our case 3,6 mg PMS-Cl are required being equal to that pH.
Detergent Reagent
0.25% Tween 80 (Sigma, Deisenhofen, Germany) is prepared with the conditioned water.
Dye Reagent
The dye reagent is purchased as a five-fold concentrate, which must be diluted and filtered through a large-pored filter prior to use.
Sample Preparation
Proteins are diluted to about 0.05-0.10% using buffer or deionized water. As a rule, the color intensity of 50uL diluted sample developed with 2.5mL dye reagent should not exceed A 595nm, 1cm = 0.500 vs. pure water or buffer.
Additional Items Required
i. Spectrophotometer: allowing measurements at 595nm.
ii. Water- bath: allowing the preparation of milk samples.
iii. Shaker: supporting the Tween 80 reaction.
iv. Polystyrene cuvettes: 10mm path length as semi-micro cuvettes.
v. Test tubes: polystyrene test tubes /13*64 mm) each fitted with a mixing spatula (Boehringer, Manheim, Germany).
vi. Dispenser and microliterpipets for precise dispensing the dye reagent resp. for adding the sample.
vii. Rack: test tube rack to store the test tubes containing samples and blanks.
Assay Procedure
The hydrophobicity of proteins and casein micelles is calculated from two different protein assays, which are developed at the same time. Triplicates for each single measurement are necessary.
a) 50µL protein (A sample) is placed on the bottom of a dry and clean test tube whereas 50µL deionized water or buffer are used as blank (A blank).
b) 50µL 0,25% Tween 80 is placed on the bottom of a dry and clean test tube and in addition to 50µL protein is placed onto the drop of Tween 80 (B sample) whereas deionized water or buffer and Tween 80 are prepared as blank (B blank).
c) Only the detergent-containing tubes (b) are shaken for 10 min (avoid foaming) to complete detergent binding at a temperature between 18-22°C.
d) Add 2.5mL diluted dye reagent to each test tube, insert mixing spatula by use of a pipette to avoid skin contact. Move spatula several times up and down without foaming.
e) Allow standing for 12min to develop the colour. The colour intensity of each tube is measured at 595nm vs. deionized water or buffer. Avoid any warming of cuvettes by prolonged standing inside the cuvette department of the photometer.
Calculation
Protein Hydrophobicity (PH) is defined as following (Nakai and LiChan)
PH= (nonpolar residues)/ (nonpolar residues) - (polar residues)
Using this definition on detergent binding according the proposed method, PH is calculated as
SHP (%) = (A sample- A blank) - (B sample- B blank) * 100: (A sample - A Blank)
For analysing the casein in its native status this method was modified to protect the micelle structure in the B samples from an early dissociation. Among some other things, the purity of water has always been one crucial point for the colloid-chemical analysis of natural casein micelles.
The modified method has been proved over 5 years taking 3 different herds of cows into account. Results obtained were just right to evidence both, the hygienic status of milk and inherent effects on milk processing.
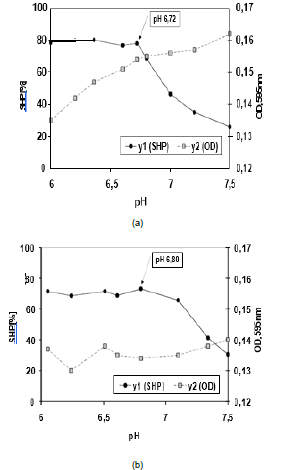
Figure 1a/b: Profiles of SHP and dye binding and OD, 585nm from good coagulating herd milk (1a) and very poor renneting quarter milk (b).
Both aspects are taken into account in the profiles of SHP and dye binding of good coagulating herd milk (Figure 1a) and of a poor renneting quarter milk sample (Figure 1b). The profile in Figure 1a is a classic example for first-class raw milk. In the courses of SHP and OD no hitch is seen. At the point of intersection (pH 6.7-6.8) the SHP falls off while the dye binding is increasing, both effects are an indication of micellar dissociation. The profile in Figure 1b was made with a poor renneting quarter milk sample. The low dye binding in that case points to a dense protein structure as a symptom of high oxidative stress on udder infections.
Foreign substances in raw milk (e.g. toxins of chemical and of microbial origin) are protein-bounded and thus are visible in the colloidal profiles [2,3].
Particularly, the glycosylated variants of Casein Macro Peptide (CMP) at the micellar surface are known to provide space for those toxins and both are released right at the outset of the micellar dissociation at pH 6.75 - 6.9. In the assay procedure presented here the discovery of toxins is always paralleled with both, an increased SHP and a decreased value of OD, 595nm.
Among the toxins (such as antibiotics and herbicides) there is one exception with mycotoxins. These are taken up by the micellar surface and thus evident at each pH of analysis. These toxins compete quickly with the nonionic detergent Tween 80 to dock with hydrophobic areas of casein on gentle warming in the cuvette department of the photometer and thus easily detectable.
The method has been tested in a long-term study of casein micelles. In this case it is to take note of identical working temperatures.
Acknowledgement
None.
Conflict of Interest
None.
References
- Lieske B, Konrad G, Faber W (1994) A new approach to estimate surface hydrophobicity of proteins. Milchwissenschaft 49: 663-666.
- Lieske B (1998) Effects of aging of raw milk on some structural properties of natural casein micelles. Milchwissenschaft 53: 562-565.
- Lieske B (2012) Complexation Between Casein Micelles and Whey Proteins by Indirect UHT-Processing of Milk / Influence of Surface Hydrophobicity and Dye Binding Characteristics of Micelles in Relationship with Their Physico- Functionality. Milk Protein, IntechOpen.com, Edited by Walter L. Hurley, (2012) Chapter 6, 159-170.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.