Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
HRV Analysis of Autonomic Function in Aging and Disease: Insights from Large Population Samples
*Corresponding author: Jonathan R T Lakey, 1Department of Surgery and Biomedical Engineering, University of California Irvine, California, USA.
Received: July 25, 2024; Published: July 30, 2024
DOI: 10.34297/AJBSR.2024.23.003079
Abstract
The autonomic nervous system (ANS) regulates involuntary bodily functions through a delicate balance between sympathetic and parasympathetic activity, known as autonomic tone, which profoundly influences cardiovascular health and mortality risks. Key physiological measures such as heart rate (HR), blood pressure (BP), and heart rate variability (HRV) serve as indicators of this equilibrium. HRV, in particular, plays a pivotal role as a biomarker in assessing autonomic function across both normal aging and diseased conditions. This study examined HRV analysis encompassing time domain and frequency domain indices, revealing a consistent decline in HRV with age across a range from 20 to 90 years old, observed in both men and women (n=328,591). Parameters such as SDNN, RMSSD, LF, HF, and LF/HF ratio exhibited an age-dependent decrease, with this trend more pronounced than gender differences. HR, reflecting autonomic tone, also demonstrated an age-related decline. Notably, similar declines in autonomic tone were observed in patients with cancer and Type 2 diabetes, aligning their physiological profiles with those of older adults aged 80- 90 years. Using the Valsalva maneuver, deep breathing, and tilt table tests revealed declines in BP and HR measurements across the age spectrum, emphasizing aging’s significant influence on autonomic regulation irrespective of sex. Advancements in HRV measurement technology enhance its utility as a non-invasive tool for early detection and management of autonomic dysfunction in chronic diseases. This underscores the importance of continued research to refine HRV assessment methods, ultimately improving diagnostic accuracy and therapeutic approaches across diverse patient populations.
Keywords: HRV, Heart rate variability, Heart rate, Blood pressure, Autonomic disorders, Autonomic nervous system, Parasympathetic nervous system, Sympathetic nervous system, Aging, Cancer, Type 2 diabetes, Alcohol toxicity
Introduction
Autonomic disorders affect the autonomic nervous system (ANS), governing vital bodily functions like heart rate (HR), blood pressure (BP), digestion, respiration, and more at a subconscious level [1-3]. Autonomic dysfunction, or dysautonomia, arises from ANS nerve damage, disrupting regulation and causing symptoms such as fainting, palpitations, sense of weakness, nausea, vomiting, shortness of breath, pallor, vertigo, chest pain, burning sensation, stabbing pain, numbness, lightheadedness, visual loss, neck pain, diarrhea, constipation, abdominal pain, erectile dysfunction, hair loss, excessive/loss of sweating, cold/hot intolerance, pressure sore, heartburn, bladder incontinence, sleep disturbances, migraine, sensory loss, brain fog, and more [4]. Autonomic disorders may occur independently or due to conditions like Parkinson's disease, cancer, celiac disease, Sjogren syndrome, Churg-Strauss syndrome, Guillain-Barre syndrome, sarcoidosis, vaculitis, rheumatoid arthritis, alcohol abuse, lupus, psoriatic arthritis, dyslipidemia, amyloidosis, paraneoplastic syndromes, metabolic syndrome, porphryria, connective tissue disorders, mast cell disorders, multiple sclerosis, or diabetes [4]. Stress adaptation also involves the ANS, characterized by heightened sympathetic activity and diminished parasympathetic activity. These changes are observed in numerous common conditions, including depression, schizophrenia, active ulcerative colitis, obesity and metabolic syndrome, myocardial infarctions, high blood pressure, and smoking [5-11].
Autonomic disorders are categorized into primary types like orthostatic intolerance syndromes and small fiber neuropathies, and secondary types linked to specific medical conditions, including diabetes mellitus, alcohol toxicity, and spinal cord injuries [4,12]. Dysautonomia, comprising primary and secondary forms, leads to varied symptoms, from mild to severe, affecting multiple organ systems [4]. Common manifestations include chest pain, mood swings, fatigue, insomnia, headaches, and dizziness, associated with conditions like multiple system atrophy and Parkinson's disease [13,14]. Symptoms can be orthostatic (related to posture changes), non-orthostatic (like bladder problems), or diffuse (affecting multiple functions) [4]. Common autonomic disorders include orthostatic hypotension, Postural Orthostatic Tachycardia Syndrome (POTS), syncope, baroreflex failure, multiple system atrophy, and autonomic neuropathy. Orthostatic intolerance syndromes encompass conditions such as orthostatic hypotension, POTS, syncope, and baroreflex failure.
Orthostatic hypotension, a form of orthostatic intolerance, occurs when BP drops suddenly upon standing, potentially causing dizziness or fainting. It typically results from autonomic nervous system dysfunction, seen commonly in conditions like Parkinson's disease and small fiber neuropathies, and exacerbated by factors like dehydration or prolonged standing [4].
POTS involves an abnormal increase in HR upon standing, leading to symptoms like dizziness, fatigue, and palpitations, while syncope refers to sudden temporary loss of consciousness due to inadequate blood flow to the brain upon standing [4]. POTS, one of the most common forms of orthostatic intolerance, affects more than 1 million Americans and predominantly women (women:men ratio of 5:1) aged 15-50 [15]. It can stem from various causes including neuropathy and hypovolemia. Treatment involves education and a range of non-pharmacological approaches, with medications reserved for severe cases [15-17].
Neurally-mediated syncope refers to a transient loss of consciousness triggered by reflexes not yet fully understood, resulting in systemic hypotension and reduced cardiac output [18,19]. Evaluation distinguishes it from cardiac syncope due to differing treatments and prognosis, with neurally mediated syncope generally having a favorable outlook [16,18-21].
Baroreflex failure occurs when the body's baroreceptors, responsible for regulating BP, malfunction. This condition can lead to dangerous fluctuations in BP, potentially causing severe hypertension or hypotension. Causes include neurological disorders or medication effects. Management focuses on controlling BP through medications and lifestyle adjustments [4].
Multiple system atrophy is a rare neurodegenerative disorder affecting movement and autonomic functions. It progresses rapidly over 5 to 10 years, primarily impacting individuals in their 50s [4]. There are two main types: cerebellar, characterized by coordination issues, and Parkinsonian, resembling Parkinson's disease symptoms like tremors and stiffness. Treatment aims to manage symptoms as the disease progresses.
Autonomic neuropathy is damage to the ANS's nerves that can stem from conditions such as diabetes, autoimmune diseases, or infections. Small fiber neuropathy, affecting small nerve fibers often alongside autonomic fibers, manifests with symptoms like burning pain in hands or feet, and less commonly, sharp or tingling sensations [22,23]. This prevalent condition affects over 4 million Americans and can be either idiopathic (in 20-50% of patients) or secondary to other disorders, notably diabetic neuropathy, the most common form of neuropathy in developed countries [22,24-28].
Diabetic neuropathy involves both sensory and autonomic nerves, causing symptoms ranging from burning pain (in 10-20% of diabetic patients) to numbness and weakness [4,29]. Autonomic complications of diabetes include cardiovascular issues like orthostatic hypotension and gastrointestinal problems such as gastroparesis [30-32]. Treatment focuses on symptom management and addressing underlying conditions to enhance the quality of life for affected individuals.
Overall, these conditions highlight dysregulation of the ANS's ability to maintain BP and HR in response to changes in posture or nerve fibers, impacting daily life and requiring management tailored to each disorder's specific characteristics. Management focuses on symptom relief through lifestyle adjustments, medications, and supportive therapies to enhance quality of life [4,21].
Diagnosis of autonomic disorders involves specialized autonomic function tests. In the 1970s and early 1980s, Ewing, et al. devised and introduced a set of five straightforward non-invasive tests to diagnose autonomic dysfunction effectively [33]. These tests assess various aspects of cardiovascular reflexes: HR response to the VM, HR response to deep breathing, and HR response to standing evaluate parasympathetic functions, while systolic BP response to standing and diastolic BP response to sustained handgrip assess sympathetic functions. Known as Ewing's battery of autonomic function tests, these methods have become widely utilized for diagnosing peripheral autonomic disorders, such as autonomic neuropathy in diabetic patients.
Heart Rate Variability (HRV) is considered a valuable marker in detecting autonomic disorders and assessing cardiovascular health. Its clinical significance was recognized from studies dating back to the 1960s, first by Hon and Lee and later by Wolf, et al. as well as others respectively, showing HRV’s predictive value in conditions like fetal distress and post-infarction mortality [12,33-35]. In 1981, Akselrod, et al. introduced the power spectral density (PSD) analysis within the frequency domain method of HRV [36]. This approach enabled the quantitative analysis of HR fluctuations and the evaluation of beat-to-beat cardiovascular control. HRV analysis has proven valuable in cardiology, predicting outcomes in conditions like myocardial infarction and heart failure [37-42]. Techniques for analyzing HRV have evolved over the years, contributing to its widespread use in medical diagnostics and risk assessment [12].
HRV refers to the natural fluctuations in the time intervals between consecutive heartbeats, known as interbeat intervals (IBIs), and is widely utilized as a non-invasive method to assess autonomic tone [43]. The oscillations of a healthy heart are complex and constantly adjusting, enabling the cardiovascular system to swiftly respond to physical and psychological challenges to maintain homeostasis [44,45]. HRV serves as a metric of the ANS influence on the heart, specifically reflecting the balance between the sympathetic nervous system (SNS) and parasympathetic nervous system (PNS), both critical in regulating many involuntary bodily functions, including HR and cardiovascular function [46]. The ANS, as part of the peripheral nervous system, regulates organ functions through visceral reflexes [47]. It consists of three main branches: sympathetic, parasympathetic, and enteric. HRV primarily assesses the sympathetic and parasympathetic branches; the enteric branch, responsible for gastrointestinal function, operates independently and is not directly reflected in HRV measurements due to its responsiveness to local stimuli. The sympathetic and parasympathetic branches integrate centrally in the hypothalamus, brain stem, and spinal cord to control activities throughout the body, including the heart and blood vessels. Sympathetic nerve fibers typically increase HR, conduction velocity, and myocardial contractility, preparing the body for action in response to stress or stimuli [47]. In contrast, parasympathetic nerve fibers generally decrease HR and slightly reduce the strength of heart contractions, promoting relaxation and restoration during rest periods. There is a dynamic interplay between these two systems, with parasympathetic activity predominating during rest, influencing resting HR primarily through vagal nerve tone [48].
HRV analysis provides valuable insights into the dynamic interaction between the sympathetic and parasympathetic branches of the ANS in regulating cardiovascular function, reflecting responses like "fight-or-flight" (SNS) and "rest-and-digest" (PNS) processes. While the PNS predominates at rest, slowing the heart to rates as low as 20-30 beats per minute, SNS activity increases HR and contractility [49]. This balance between SNS and PNS activity, known as autonomic tone, plays a crucial role in influencing cardiovascular health outcomes, including mortality risks [50]. Key metrics like HR, BP, and HRV reflect this equilibrium. Sympathetic stimulation elevates HR, whereas parasympathetic stimulation lowers it [51]. Resting HR is correlated with cardiovascular prognosis: higher rates indicate poorer outcomes, while lower rates may predispose to conditions like atrial fibrillation [52,53]. Similarly, BP regulation is influenced by ANS activity, impacting conditions such as hypertension and orthostatic hypotension [54-58]. The interplay between these systems, affected by factors like stress and physical activity, determines HRV patterns. The relationship between PNS and SNS activity is dynamic and can vary nonlinearly, highlighting the complexity of autonomic control over cardiovascular function [59].
HRV holds considerable potential in evaluating ANS fluctuations in both healthy individuals and patients with diverse cardiovascular and non-cardiovascular disorders. Changes in HRV can often manifest as early indicators of underlying conditions, providing insights into disease progression and the effectiveness of treatments. The use of HRV as a biomarker is particularly beneficial in evaluating secondary autonomic neurological disorders. Through detailed analysis of frequency and time-domain metrics, HRV elucidates the dynamics of autonomic function, offering profound insights into cardiovascular health. Autonomic tone, reflected in HRV measurements, serves as a critical physiological marker linked to various cardiovascular outcomes. Therefore, there is a pressing need for comprehensive assessment and management strategies to optimize the clinical utility of HRV in healthcare settings.
Developed by Medeia Inc., the VitalScan-ANS system (Figure 1) is meticulously designed to streamline the recording and analysis of HRV responses using portable Electrocardiography (ECG) devices. It delivers automated, standardized, and clinically intuitive results. VitalScan-ANS has received FDA 510K clearance (K191266), integrating advanced technologies to capture, store, and analyze data encompassing ECG, Pulse Wave Velocity (PWV), BP, and HRV.
Figure 1: This is an image of the VitalScan-ANS System developed by Medeia Inc. performing the tilt table test. The VitalScan-ANS System is portable, easy-to-use, and non-invasive.
The neuroanalytic VitalScan-ANS Platform incorporates discriminant ANS databases tailored for various autonomic disorders, leveraging patient data to establish clinical profiles. In the current study, Medeia Inc. employs VitalScan-ANS’s ECG, PWV, and HRV technologies to investigate HRV norms across age and sex subgroups within neurological patient populations participating in VitalScan-ANS assessments for ANS database construction. Given the close correlations among HRV measures, the current study focuses on two time-domain indices (RMSSD, SDNN) and four frequency-domain indices (LF, HF, LF/HF, total power) [12]. These indices are used to analyze short-term changes and patterns in HRV, providing valuable insights into ANS activity and overall HR patterns at rest. The study also assesses outcomes from tests such as the Valsalva Maneuver, deep breathing, and tilt table, enriching the understanding of cardiovascular responses in these patient populations.
Furthermore, this study delves into autonomic tone across the entire age spectrum in both sexes, with a particular focus on analyzing the impact of medical conditions such as cancer, Type 2 Diabetes Mellitus (T2DM), and alcohol toxicity on autonomic functions. Chronic alcohol consumption leads to a syndrome characterized by physiological, behavioral, psychological, and perceptual changes. Ethanol, a potent central nervous system depressant, damages autonomic nerve fibers, resulting in autonomic imbalance, arrhythmias, and cardiac complications [60]. According to the Global Burden of Disease study (2020), 59.1% of individuals aged 15-39 consume hazardous amounts of alcohol, with males comprising 76.9% of this group, contributing to 11.78 million deaths [61]. Alcohol stands as the leading risk factor for mortality among males aged 15-49 [61]. Alcohol intoxication adversely affects heart function, leading to reduced parameters of HRV, which is associated with a poorer prognosis [62]. Alcohol dependence (AD) also induces neurotoxic effects, including cardiac autonomic neuropathy (CAN), which increases the risk of cardiovascular diseases early in alcohol abusers [63]. Subclinical manifestations of CAN often go unnoticed in routine clinical exams, underscoring the critical role of HRV analysis for early detection and prognosis assessment [60].
Early detection is paramount in the management of CAN to mitigate associated cardiovascular complications. CAN in diabetes, specifically T2DM, refers to impaired autonomic regulation of the cardiovascular system, prevalent in 31% to 73% of affected individuals [64,65]. T2DM is a metabolic disorder characterized by elevated blood sugar levels due to impaired insulin secretion or action [66]. As of 2018, over 500 million people worldwide were estimated to have diabetes, with 90-95% falling under the category of T2DM [67,68]. This condition is linked to both acute and chronic complications, including microvascular and macrovascular issues [69].
Diabetic autonomic neuropathy (DAN), a microvascular complication, specifically affects autonomic nerves, particularly in the cardiovascular system, leading to CAN [32,70]. CAN arises from autonomic dysfunction that impacts the regulation of the heart and blood vessels, thereby altering cardiovascular hemodynamics. CAN is associated with serious outcomes such as cardiac arrhythmias, silent myocardial infarction, and sudden death in T2DM patients [71]. Risk factors for CAN include older age, longer diabetes duration, poor glycemic control, and complications like polyneuropathy, retinopathy, nephropathy, hypertension, obesity, and dyslipidemia [72]. CAN progresses from a subclinical phase marked by reduced HRV, an indicator of autonomic function, to clinical stages presenting symptoms such as resting tachycardia and orthostatic hypotension [32,73]. While subclinical CAN may be reversible, clinical CAN involves both parasympathetic and sympathetic denervation of the heart [73,74]. HRV, assessed through various electrocardiogram metrics, consistently shows reduced values in T2DM patients with CAN compared to those without, making it a crucial non-invasive diagnostic tool for early detection of autonomic dysfunction in diabetes [75,76]. Therefore, HRV serves as an essential diagnostic tool in identifying CAN in diabetic patients.
Time domain measures of HRV, traditionally utilized in cardiology and diabetes research, are increasingly being applied in cancer studies [77]. Cancer remains a leading cause of mortality despite advancements in medical science [78]. Over the past two decades, multiple studies have consistently demonstrated that HRV effectively predicts and monitors specific cancer-related outcomes [79-81]. Bijoor, et al. explored the correlation between autonomic function and cancer, noting signs of autonomic dysfunction such as irregular sweating patterns, orthostatic hypotension, and bladder and bowel disorders commonly observed in advanced cancer stages, suggesting a potential link between cancer pathogenesis and autonomic dysfunction [82,83].
Autonomic dysfunction is prevalent in patients with advanced cancer, possibly attributed to reduced physical activity, medications, or paraneoplastic processes [84-89]. However, the precise impact of autonomic dysfunction on clinical findings and prognosis in advanced cancer remains unclear [90]. Patients with advanced breast cancer exhibiting abnormal cardiovascular autonomic function tests were more likely to report symptoms like postural hypotension and chronic unexplained nausea [84,85,87]. Some studies have shown a correlation between autonomic dysfunction and shorter survival [91-94]. Further research is needed to elucidate the exact role of autonomic dysfunction in influencing outcomes in advanced cancer patients.
Despite the potential of HRV as a convenient biomarker for autonomic function, there is a notable absence of comprehensive population-based studies evaluating the influence of common variables such as age on HRV parameters [95-97]. This study by Medeia Inc. serves as a proof-of-concept to evaluate the effects of age and gender using a large sample size (n=328,591) on HRV parameters. Additionally, it aims to assess the impact of prevalent conditions such as alcohol toxicity, cancer, and T2DM on HRV parameters. This comprehensive analysis seeks to deepen understanding of how these factors, alongside age and gender, influence the ANS activity. It aims to potentially standardize HRV as a diagnostic marker within the VitalScan-ANS databases for neurological and neuropsychological disorders, expand the discriminant databases to include autonomic neuropathies, and inform personalized treatment approaches.
Materials And Methods
Subject and Variable Selection
Patient data acquisition occurred between 2014 and 2023 across multiple neurology offices. Patients who qualified for the VitalScan-ANS assessment were concurrently evaluated for their ECG/HRV data. The subject selection criteria for a VitalScan-ANS assessment are detailed below.
Inclusion/Exclusion Criteria, Demographics and Gender [98]: For subjects aged 4 to 18 years, parents completed a neurological history questionnaire for them, and psychometric evaluations were conducted. Adults (≥18 years) also completed a neurological questionnaire, and those deemed unhealthy were excluded based on questionnaire responses and/or physician comments. Physicians have access to the following questionnaires: GAD-7 (Anxiety Severity), DSM-5 Level 1 (Cross-Cutting Symptom Measures), PHQ-9 (Depression), PCL-C (PTSD Severity), and general neurological questionnaires. Inclusion required at least one questionnaire score below moderate and physician-verified health in that the patient was deemed healthy. Any patient records or previously known medical records with questionnaire score of ‘moderate’ or ‘severe’ were excluded from the VitalScan-ANS database, regardless of other information.
Demographic Characteristics [98]: It is crucial that the demographic mixture of males and females, various ethnic groups, and socioeconomic statuses be reasonably representative of the expected North American clientele. This diversity was derived from a large pool of subjects obtained from eight geographically dispersed sites, reflecting the North American demographics and addressing a wide range of ethnic and socioeconomic statuses found in the de-identified patient data before review.
Client-Based VitalScan-ANS Database [98]: Each client in the VitalScan-ANS database completed a DSM-based questionnaire. Regression analysis was utilized to remove any psychopathology-related variance from the ECG, PWV, BP, and HRV data. This process ensures that the variance in the ECG of ‘healthy’ subjects, which is explained by the variance in the questionnaire, is removed to create a ‘psychopathology-free’ ANS normative database or discriminant databases for various brain disorders.
Utilizing a client-based normative or discriminant database has its own set of advantages. Clients may harbor expectations distinct from those of 'healthy' subjects concerning ANS recordings. Given that it is common for clients to experience worry or stress during ANS sessions, research has demonstrated a significant correlation between anxiety levels and the power distribution of the frequency band spectrum. In essence, profound differences may exist in the resting state ANS recordings of clients compared to 'healthy' subjects, differences unrelated to the psychological complaints of the clients. Therefore, comparing a client's ANS (ECG, PWV, BP, and HRV data) with a normative database comprising 'healthy' subjects without accounting for the aforementioned variations might lead to incorrect conclusions and render the treatment ineffective.
Patients were prepared for a VitalScan-ANS assessment, where ECG/HRV data were collected concurrently.
To Prepare the Patient for a VitalScan-ANS Assessment [98]: To perform a reliable VitalScan-ANS assessment, it is essential to observe the following patient preparations: patients should abstain from consuming caffeine at least 2 hours before the assessment, avoid taking any new medications or supplements unless directed by a healthcare provider, and refrain from using alcohol, marijuana, or other recreational drugs at least 6 hours prior to the assessment. Patients with pacemakers should not undergo testing during the visit and are required to complete a brief neuropsychological questionnaire about their symptoms before testing. During the testing, ensure the patient is comfortably seated in a chair while brain behavioral measurements and activities are recorded.
Brief Guide to Operate the VitalScan-ANS System for Patient Assessment [98]: The VitalScan-ANS System comprises a workstation with three BP Cuffs, three photoplethysmography (PPG)/ SpO2 finger sensors, three-lead ECG sensors, and a response button for visual, and motor tests.
To operate the VitalScan-ANS System, follow these steps: Turn on your laptop, open the VitalScan-ANS software, and ensure that the ECG amplifier device's USB is properly connected. To confirm the connection, click on the settings button and press "Check Device Connection." Position the patient comfortably in a chair facing the laptop screen at eye level.
For patient preparation, apply the three-lead ECG sensors-place the red lead under the right clavicle, the black lead under the left clavicle, and the yellow lead below the last left rib. Attach the pulse Ox SpO2 finger sensors, along with three BP Cuffs - one on the right arm, and one each on the left and right ankles. In the software, select "New Measurement" and then "ANS Response Test." Choose options for Resting, Deep Breathing, Valsalva Maneuver, and Tilt Test.
Proceed to the patient information section, select "New" or "Existing Patient," and enter the patient's details, including name, date of birth, gender, weight, height, medications, symptoms, or previous diagnoses. Progress to the patient questionnaire, guiding the patient through detailed answers-an essential step. In the pre-test screen, check signal quality.
After a successful test, view the results on the overview page and disconnect the patient. The software results, starting with the neurofunctional test option, provide a general summary with scales ranging from red (abnormal) to green (healthy), helping diagnose and assess the patient's cardiovascular health. Light green is borderline, while yellow and orange indicate areas of concern.
Autonomic Function Tests
Standardized quantitative testing of autonomic function is crucial for both clinical diagnosis and research. While there are various autonomic tests available, only a few have been clinically validated and provide quantitative results. For quantitative autonomic testing, the following protocol, based on methodology developed primarily by Dr. P. Low and colleagues, and referenced by Dr. Peter Novak, is used to evaluate three key aspects of autonomic function: heart rate variability (cardiovagal), responses to stress (adrenergic), and sweat gland activity (sudomotor) [99,100]. Tests such as deep breathing, the Valsalva Maneuver, head-up tilt, and the quantitative sudomotor axon test (QSART) are used to assess these domains. Proper data collection, accurate parameter measurement, and unbiased interpretation of autonomic signals are emphasized. Several challenges related to data quality can impact various measurements. For instance, insufficient cleaning of skin can introduce noise in ECG signals, making it hard to detect R waves accurately. Dr. Novak also noted the incorrect positioning of blood pressure sensors can result in inaccurate high or low readings. Software used for data processing may struggle to distinguish artifacts in BP readings, especially in patients with conditions like Parkinson's disease characterized by tremors. In the current study, the following tests were performed to measure HR responses, while acknowledging the pitfalls of each test [99].
Deep Breathing Test [99]: The purpose of the test is to evaluate cardiac parasympathetic (cardiovagal) function, also referred to as cardiovagal testing. Deep Breathing Test (DBT) is conducted with the patient in a supine position. During inhalation, the diaphragm contracts, lowering intrathoracic pressure to facilitate air intake. Reducing arterial blood pressure deactivates baroreceptors and diminishes vagal tone, leading to increasing HR. Conversely, during exhalation, as the diaphragm relaxes, intrathoracic pressure rises to expel air from the lungs. This elevation in BP activates baroreceptors, enhancing vagal tone and consequently reducing HR. Thus, HRs rise during inhalation and fall during exhalation in healthy individuals.
Procedure:
1. Baseline: Record 5 minutes of resting HR to establish baseline.
2. Instructions: Instruct the subject to breathe deeply and regularly: inhale for 5 seconds and exhale for 5 seconds, repeating this cycle six times (totaling 1 minute).
3. Monitoring: Ensure breathing is done through the nostrils to monitor end-tidal CO2 levels and avoid hyperventilation. Additionally, patient should avoid breath holding and sudden inhalations or exhalations. ECG recordings were made throughout the test. The mean difference between the maximum and minimum HR was calculated for analysis.
4. Measurement: Calculate Respiratory Sinus Arrhythmia (RSA), which is the difference in HR between the end of expiration (or exhalation) [(longest RR interval (RRmax)] and end of inspiration (or inhalation) [(shortest RR interval (RRmin)] averaged over 6 respiratory cycles. HR response is quantified as the difference (E - I) or ratio (E / I):
i. E - I = RRmax - RRmin
ii. E / I = RRmax / RRmin
Pitfalls:
1) Anxiety and abnormal respiratory patterns can affect DBT results. The subject should be relaxed during testing. A key indicator of anxiety is HR; ensure it is stable before starting the test. The resting respiratory pattern should be normal with no hyperventilation or tachypnea (respiratory rate above 11 per minute).
2) The DBT test may not be useful in certain cardiac dysrhythmias such as atrial fibrillation or when the HR is controlled by a pacemaker. This is because vagal nerve modulation, which affects HR during deep breathing, can be overridden by the heart's intrinsic rhythm or a pacemaker. Understanding cardiac rhythm abnormalities is important for interpreting test results.
3) Errors in DBT results can occur due to improper detection of R waves from ECG signals. This can happen if R waves are too small to be detected reliably. Poor skin contact can create noise in the ECG background, which can lead to inaccurate test outcomes.
Valsalva Maneuver [99]
This test assesses autonomic function, particularly sympathetic (adrenergic) and parasympathetic (cardiovagal) responses. During the Valsalva Maneuver (VM), HR initially increases due to heightened sympathetic activity triggered by a fall in aortic BP caused by elevated intrathoracic pressure, which diverts blood away from the heart. Subsequently, HR continues to rise momentarily due to both the reflexive response to take a deep breath (vagal inhibition) and a delayed sympathetic response. Following this, HR decreases as BP rises, activating baroreceptors and enhancing parasympathetic cardiac activity. This increase in BP results from blood returning to the heart as intrathoracic pressure normalizes and from persistent sympathetic activity leading to peripheral vasoconstriction.
The test provides two key measurements: the Valsalva ratio, which assesses HR changes, and the Valsalva response, which evaluates BP changes. Monitoring BP changes during phases 2 (recovery) and 4 (overshoot) is particularly important for detecting sympathetic dysfunction.
Procedure:
1) Preparation: Practice a VM briefly with the subject, in a supine position, to ensure comfort.
2) Baseline: Allow approximately 5 minutes of relaxation before beginning the VM.
3) Instructions: Instruct the subject to take a deep breath and blow into a mouthpiece to maintain an expiratory pressure of around 40 mm Hg for 15 seconds.
4) Repetitions: Wait for 30 seconds between each VM and repeat the VM three times. Subjects were instructed to strain for at least 15 seconds. Continuous ECG recording was conducted throughout the procedure and for an additional 60 seconds thereafter. The Valsalva ratio was calculated during straining as the ratio between the longest mean RR intervals and the shortest mean RR interval observed.
5) Parameters: Evaluate several parameters:
a) Valsalva ratio is defined as the maximum HR during the VM divided by the lowest HR obtained within 30 seconds of the peak HR (ratio of longest RR interval after to shortest RR interval during VM).
b) Maximal drop in mean blood pressure during phase 2.
c) Peak of mean BP at the end of late phase 2 (recovery).
d) Overshoot in phase 4.
e) Maximal pulse pressure drop during phase 2.
Pressure recovery time.
Pitfalls:
1) Performing the VM requires significant cooperation from subjects. Many elderly or frail patients may struggle to perform it correctly due to weakness in the muscles around the mouth, leading to air leaks during the strain.
2) Evaluation of adrenergic functions cannot be conducted using a square-wave variant of the VM (Figure 2). This type of response is uncommon but may occur in healthy individuals or patients with congestive heart failure. Therefore, alternative methods or maneuvers may be needed for assessing adrenergic function in these cases.
Tilt Table Test [99]
The Tilt Table Test (TTT) predominantly evaluates adrenergic functions by simulating orthostatic stress by moving the subject lying on a table from a supine position to an upright tilt in a controlled manner (‘passive tilt’). In a supine position, the body is at rest, and the parasympathetic tone of the ANS predominates. This results in a low HR and high HRV during rest.
When transitioning to a head-up tilt position, there is a significant redistribution of blood from the upper body to the abdomen and lower extremities. This reduces blood volume in the upper body, leading to a decrease in cardiac output and BP. To counteract this sudden drop in BP, the SNS is activated, leading to an increase in HR and peripheral vasoconstriction. Normal HR responses involve an increase of 10 to 30 beats per minute, with a maximum HR of less than 120 beats per minute. BP responses are considered normal if the systolic BP decreases by less than 30 mm Hg or if the mean BP decreases by less than 20 mm Hg. Immediately after the tilt, HR gradually rises, reaching its peak approximately 15 seconds after the postural change. As the body adjusts to the new position, a new baseline is established where sympathetic and parasympathetic activities reach a stable balance. In the upright position, the average HR is higher compared to the supine position. Additionally, HRV decreases, particularly in indices sensitive to PNS activity. This shift reflects the altered autonomic balance in response to the postural change, with sympathetic activity playing a more dominant role.
Procedure:
1) et a baseline BP read from the brachial artery for 5 minutes before starting the tilt test.
2) Then tilt the subject upright at a 70-degree angle and monitoring changes in BP and HR for about 5 to10 minutes.
3) Monitor the subject closely for any signs of discomfort, chest pain, shortness of breath, dizziness, lightheadedness, and syncope that may require prematurely terminating the test. After lying down for 5 minutes, the subjects were instructed to stand up as quickly as possible. ECG recording began immediately upon standing and continued for 60 seconds thereafter. The 30:15 ratios, which represent the mean ratio between the RR interval of the 30th beat and the 15th beat, were estimated during this procedure.
Pitfalls:
i. The tilt test can be challenging to interpret accurately when a patient is taking multiple medications. Discontinuing medications before testing is not always feasible or practical, which can complicate the interpretation of the test results.
ii. Obtaining high-quality recordings during the tilt test can be difficult in patients with movement disorders, such as Parkinson's disease with tremors. These tremors can introduce noise or artifacts into the recordings, potentially affecting the reliability and interpretation of the test outcomes.
The above battery of tests helps to diagnose and classify autonomic disorders, providing insights into the extent and nature of dysfunction. In summary, cardiovagal function is derived from the DBT and VM results. Adrenergic function is assessed based on the VM and TTT outcomes. Results will guide treatment decisions and interventions aimed at improving autonomic regulation and patient outcomes. In clinical practice, these autonomic function tests are crucial for assessing autonomic health comprehensively, aiding in the diagnosis and management of conditions affecting the ANS.
Assessment of Heart Rate (HR)
Resting HR was assessed by counting the total number of RR intervals observed in a one-minute electrocardiogram recorded in lead II, with the patient in a supine position and completely relaxed. During measurement, patients were seated upright, nearly motionless, and breathing at a normal rate for a duration of 3 to 5 minutes. HRV was quantified using three consecutive minutes of ECG recordings. The ECG sampling rate employed was 100 Hz, chosen to provide sufficient bandwidth for detecting QRS peaks, which were bandpass filtered between 8 to 15 Hz [101].
HRV Measurements: Time and Frequency Domains
The Task Force of the European Society of Cardiology and North American Society of Pacing Electrophysiology (thereafter refer to as ‘Task Force’) established HRV standards in 1996, which were reviewed by Shaffer and Ginsberg in 2017 [12,102]. When evaluating HRV, ECG equipment must adhere to voluntary industrial standards concerning signal-to-noise ratio, common mode rejection, bandwidth, and other factors [12]. To standardize physiological and clinical studies, two types of recordings are typically used: a) 5-minute short-term recordings, under stable physiological conditions, processed by frequency domain methods, and b) nominal 24-hour recordings processed by time-domain methods [12].
Time-domain Measures: The time domain method of HRV assesses intervals between successive QRS complexes in ECG recordings, termed normal-to-normal (NN) intervals. These intervals are used to compute several HRV time-domain indices that quantify variability over periods ranging from less than a minute to over 24 hours. Key indices applied in this study are RMSSD and SDNN [102]. SDNN measures the standard deviation of NN intervals and reflects overall HRV excluding abnormal beats like ectopic beats. It provides a measure of total variability over the recording period. RMSSD calculates the root mean square of successive differences between NN intervals (noise-free RR), emphasizing beat-to-beat variability primarily influenced by PNS activity [103]. It is more sensitive to vagal changes than SDNN and is calculated using successive NN interval differences [104].
Artifact contamination in even a short segment can significantly distort SDNN and RMSSD values [105]. RMSSD, widely used to estimate high-frequency HRV variations, is preferred for its robust statistical properties [12]. Comparing time-domain HRV measures across recordings of different durations is inappropriate due to their specific roles in assessing autonomic output, including parasympathetic and sympathetic activity [12,102,106].
Frequency-domain Measures: Frequency domain analysis utilizes methods such as Fast Fourier Transform (FFT) to categorize HRV into spectral bands: Very Low Frequency (VLF, ≤0033 Hz), Low Frequency (LF, 0.04-0.15 Hz), and High Frequency (HF, 0.15-0.40 Hz) [47]. These bands reflect different oscillatory rhythms of HR influenced by ANS activity [12]. VLF is typically assessed over longer periods (e.g., 24 hours), while LF and HF bands capture shorter-term variations influenced by respiration [102].
Short-term recordings (2 to 5 minutes) of HRV distinguish three main spectral components: VLF, LF, and HF components [12]. According to the Task Force, the distribution of LF and HF power and their central frequencies can vary with changes in autonomic modulation of heart period [12]. However, the interpretation of Very Low Frequency (VLF) components from short-term recordings (≤5 minutes) is less clear due to uncertainties in its physiological significance and should be cautiously interpreted in PSD analyses [12]. Total power sums the energy across ULF, VLF, LF, and HF bands over a 24-hour period or excludes ULF for short-term recordings [12].
Absolute power measures the energy within each frequency band (in ms²/Hz), while relative power (normalized units or as a percentage of total power) allows comparison between individuals by standardizing the power across LF and HF bands [102]. The LF/HF ratio, derived from LF and HF powers, provides an estimate of SNS to PNS nervous system activity [102].
In summary, HRV analysis encompasses comprehensive evaluation using both time domain and frequency domain methods, providing valuable insights into ANS function and its modulation of HR dynamics.
Results and Discussion
Resting HR average versus Age
Normal human aging is associated with changes in autonomic functions, in addition to those occurring in diseased states [107]. In this study, ECG, BP and PPG data from 328,591 participants, both male and female individuals aged 20 to 90 years, were collected during VitalScan-ANS assessments across multiple neurology offices. The study evaluated the cardiovagal and adrenergic domains of the ANS using HRV metrics to explore the impact of age and sex on autonomic function in neurological patients, including those with concomitant T2DM, cancer, and alcohol toxicity. Numerical data were summarized as mean and standard deviation (SD) for each sex across different age groups.
HRV declined steadily from ages 20 to 90 years, with decreases observed across all HRV metrics. Figure 3 illustrates the resting HR responses to age and sex as mean ± SD (standard deviation) for both males and females aged 20 to 90 (Figure 3a). Thus, resting HR serves as a marker reflecting the status of vagal nerve function, indicating the balance between sympathetic and parasympathetic influences on cardiac activity [48]. A gradual decline in resting HR was evident in both sexes. For males, there was a decline from 80.48±12.62 in the 20-25 years old to 66.56±10.39 in the 80-90 years old (Table 1). Similarly, resting HR in females trended downward from 82.48±12.19 to 68.78±10.06 over the same age range. Jha, et al. also found that females had a higher baseline HR at rest compared to males (84.37 vs. 78.43) in individuals aged between 18 and 25 years [108]. Thus, while there were no significant differences in HR responses between sexes, a consistent regression with age was observed. However, females tended to exhibit slightly higher resting HR responses across the age spectrum (Figure 3b). It's worth noting that all recorded HR values fell within the normal range of 60-100 beats per minute, as HR above 100 or below 60 beats per minute is considered abnormal [107]. The decreasing HR responses suggest an age-related decline in both sympathetic and parasympathetic functions, irrespective of sex. Unlike Umetani, et al., who found HRV to be significantly influenced by both sex and aging, the current study did show influence, albeit not significant [109]. Specifically, Umetani, et al. found that females under 30 years had lower HRV than males across all time-domain metrics, with no significant differences for those over 50 [109]. Gender differences decreased after age 30 and disappeared by age 50. Their 24-hour study included 260 healthy subjects (112 males and 148 females, aged 10 to 99), which is smaller than the current study, but both studies showed an age-dependent decline in HR and HRV metrics.
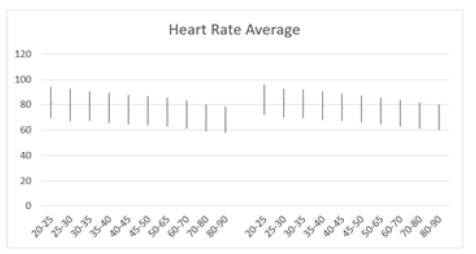
Figure 3a: Average resting HR (bpm) versus age, with error bars, shows a decrease with age in both men (left) and women (right) aged 20-90 years.
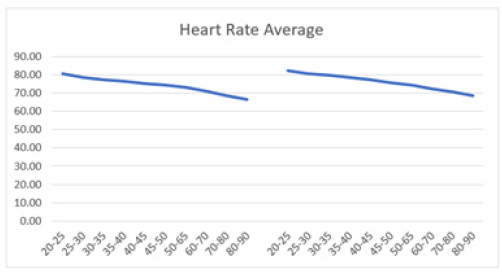
Figure 3b: Average resting HR (bpm) versus age shows a decline in both men (left) and women (right) aged 20-90 years, with both curves demonstrating a parallel downward trend.

Table 1: Average HR (bpm) and standard deviation (SD) of male and female patients, across ages 20-90 years.
Time-domain Metrics (SDNN, RMSDD) versus Age
Time-domain indices offer insights into HRV influenced by various physiological factors. These indices include mean NN interval, mean HR, as well as differences between the longest and shortest NN intervals, and variations between night and day HRs. The simplest measure is evaluating the intervals between QRS complexes in an ECG, known as normal-to-normal (NN) intervals or instantaneous HR. One key metric is the standard deviation of NN intervals (SDNN), which reflects the total cyclic components contributing to variability during a recording period [12]. SDNN, expressed in milliseconds, indicates the fluctuation of HRV around its mean value, providing insights into the ANS and overall HRV.
SDNN is considered the "gold standard" for assessing cardiac risk when measured over a 24-hour period [12,103]. Although typically calculated over 24 hours, SDNN can also be computed over shorter durations to assess HRV dynamics. SDNN measures both parasympathetic and sympathetic activity, and higher SDNN values are associated with reduced morbidity and mortality rates [110]. For instance, individuals with SDNN values over 100 ms have significantly lower mortality risks compared to those below 50 ms [42]. In clinical practice, SDNN values below 50 ms are indicative of poor health, values ranging from 50 to 100 ms suggest compromised health, while values exceeding 100 ms are considered healthy [42].
In this study, SDNN and RMSSD metrics were calculated in different age cohorts. SDNN was higher in men compared to women, ranging from 48.54±17.83 (20-25 years old) to 32.55±14.00 (80-90 years old) for men, and showing a similar decline with age in women, from 46.46±15.81 (20-25 years old) to 31.67±13.48 (80-90 years old) (Table 2). These SDNN values were below 50 ms, suggesting poor health and higher than normal mortality risks in the tested subjects. Since SDNN is typically measured over a longer period, conducting short-term recordings might affect the results, necessitating further examination of SDNN's appropriateness as a metric for this specific test subject population. Overall, both sexes exhibited a comparable linear decline in SDNN (Figure 4a and 4b).
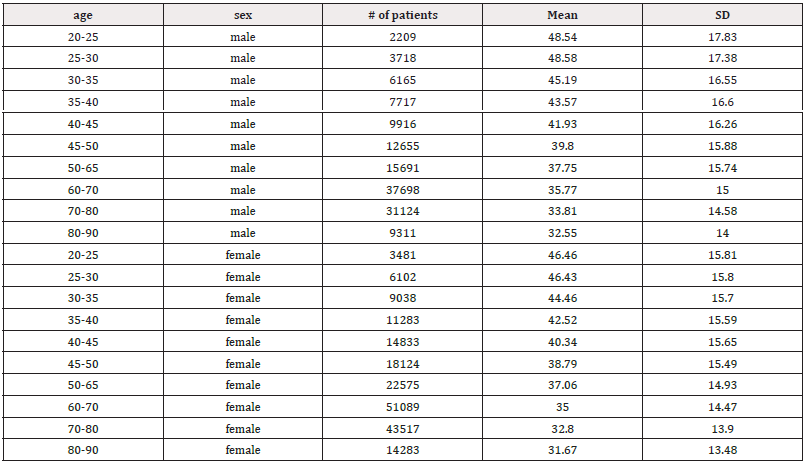
Table 2: Mean SDNN (ms) and standard deviation (SD) of male and female patients, across ages 20-90 years.
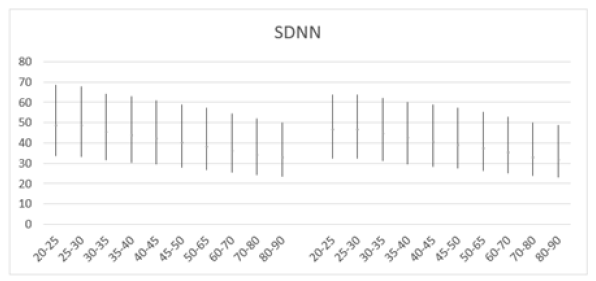
Figure 4a: Mean SDNN (ms) versus age, with error bars, shows a decline with age in both men (left) and women (right) aged 20-90 years.
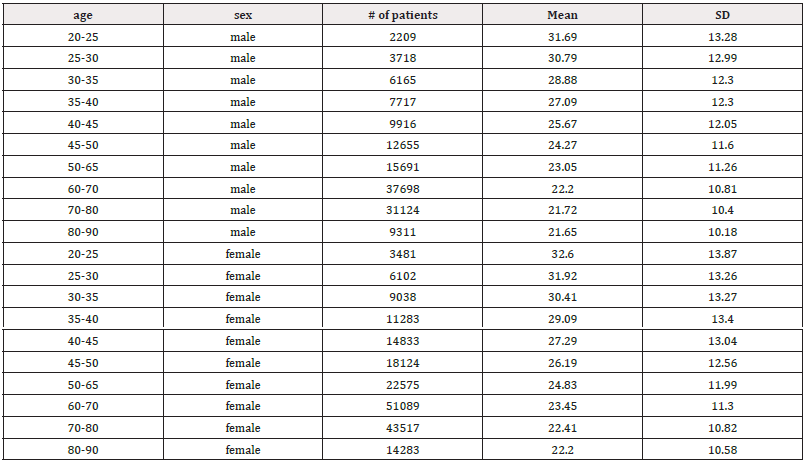
Table 3: Mean RMSSD (ms) and standard deviation (SD) of male and female patients, across ages 20-90 years.
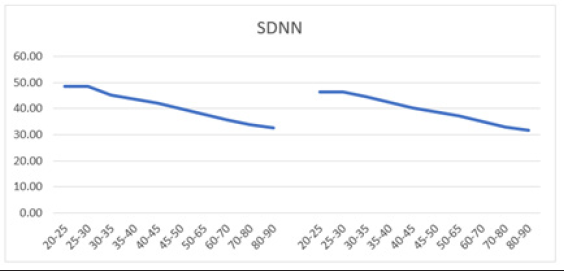
Figure 4b: Mean SDNN (ms) versus age shows a decline in both men (left) and women (right) aged 20-90 years, with both curves demonstrating a parallel downward trend.

Figure 5a: Mean RMSSD (ms) versus age, with error bars, shows a decline with age in both men (left) and women (right) aged 20-90 years.

Figure 5b: Mean RMSSD (ms) versus age shows a decline in both men (left) and women (right) aged 20-90 years, with both curves demonstrating a parallel downward trend.
An important feature of RMSSD is its ability to characterize short-term rapid changes in HR, which occur primarily under the influence of the PNS. RMSSD is considered the primary parameter for assessing PNS function, quantifying the short-term components of HRV and reflecting overall vagal modulation of HRs [111]. Across all age groups, mean RMSSD values were higher in women compared to men (Table 3). In the youngest age group (20-25 years), men exhibited a mean RMSSD of 31.69±13.28 compared to 32.60±13.87 in women. In the oldest age group (80-90 years), men also showed a lower mean RMSSD of 21.65±10.18, whereas women had a mean RMSSD of 22.20±10.58. While the difference in mean RMSSD between men and women across different age cohorts does not appear significant, women tend to start with slightly higher RMSSD values than men in their early twenties. Overall, mean RMSSD decreased with age (Figures 5a and 5b). Umetani, et al. also found that all HRV metrics decreased with age, similar to the current study, which showed declines in both SDNN and RMSSD [109]. However, unlike Umetani’s findings, the current study did not indicate that these metrics fell below levels associated with increased mortality risk for individuals over 65 [109]. Instead, SDNN values fell below the 50 ms mark starting at 20 years old for both males and females, continuing to decline through 90 years old, which is indicative of poor health and potentially high mortality risk.
Frequency-domain Metrics (LF, HF, LF/HF, Total Power) versus Age
Low-Frequency (LF) and High-Frequency (HF) components are typically reported in absolute values of power (milliseconds squared). Additionally, LF and HF can be expressed in normalized units, reflecting their proportion relative to total power minus very low frequency (VLF) [12]. Normalization underscores the balanced activity of autonomic nervous system branches and mitigates the impact of total power changes on LF and HF values. However, it is recommended to present both normalized units and absolute power values of LF and HF to comprehensively describe the distribution of spectral power components. The LF power spectrum reflects both sympathetic and parasympathetic modulation of the RR intervals, while HF serves as a surrogate marker for parasympathetic modulation [112]. Mean LF power decreased by 76.4% [(758-179)/758] in males and 73.5% [(645-171)/645] in females from age 20 to 90 years (Table 4), while HF power decreased by 66.4% [(342-115)/342] in males and 68.2% [(412-131)/412] in females (Table 5). This suggests a greater decline in sympathetic activities compared to parasympathetic functions.
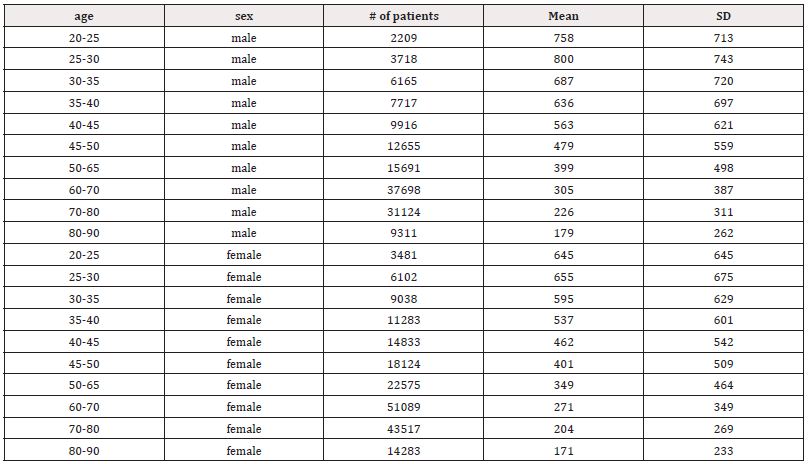
Table 4: Mean LF (ms2) and standard deviation (SD) of male and female patients, across ages 20-90 years.
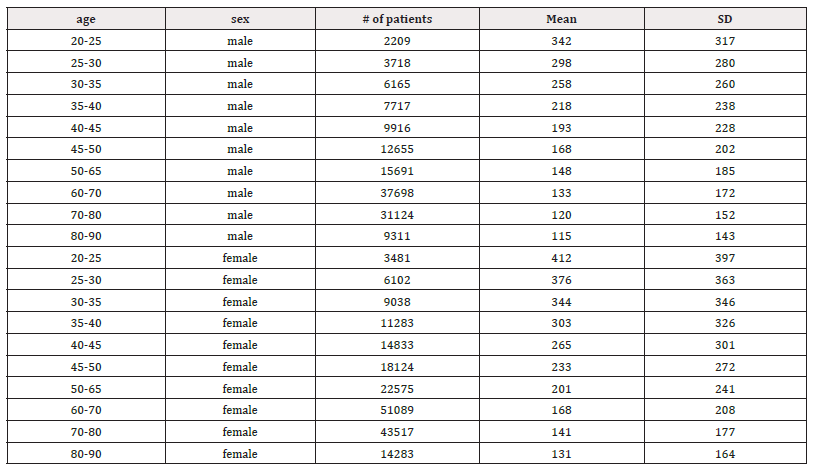
Table 5: Mean HF (ms2) and standard deviation (SD) of male and female patients, across ages 20-90 years.
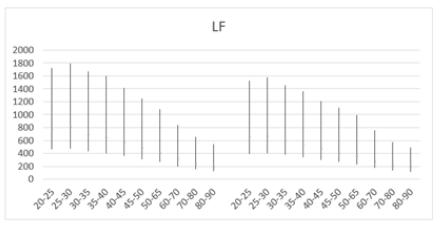
Figure 6a: Mean LF (ms2) versus age, with error bars, shows a decline with age in both men (left) and women (right) aged 20-90 years.
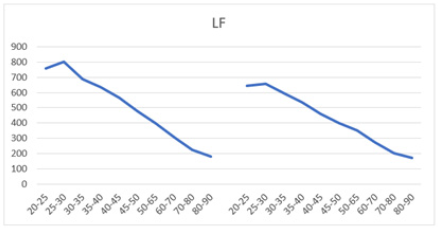
Figure 6b: Mean LF (ms2) versus age shows a decline in both men (left) and women (right) aged 20-90 years, with both curves demonstrating a parallel downward trend.
When comparing LF power between males and females across different age groups, males generally exhibited higher mean LF values (Table 4). Both sexes showed a slight peak in mean LF power from ages 20-25 to 25-30 before a subsequent decline (Figures 6a and 6b). For example, mean LF power rose from 758±713 to 800±743 in males and from 645±645 to 655±675 in females before declining after age 30. However, males experienced a greater magnitude of LF decline compared to females (76.4% vs. 73.5%), suggesting a higher sympathetic decline in males as they age. Moreover, higher SD values compared to the mean were observed across all age groups in males aged 30 (687±720) and older, and in females aged 25 (655±675) and older. These higher SD values suggest significant individual variation within each age and gender cohort within the population.
Similarly, higher SD values compared to the mean were also found in mean HF power for both males (258±260) and females (344±346) aged 30 and older (Table 5). However, unlike LF power, females (68.2%) exhibited a steeper decline in HF power compared to males (66.4%) (Table 5, Figures 7a and 7b). Moreover, females exhibited higher HF power than males across all age groups, with values of 412±397 to 131±164 compared to 342±317 to 115±143 in men within the same age category. The lowest HF power was observed in the oldest group (80-90 years), where females showed HF power of 131±164 versus 115±143 in their male counterparts. Additionally, the brief rise in power observed for LF in the 25-30 age group was absent for HF in both males and females. Both sexes showed a decline in both HF and LF power with age, suggesting a weakening ANS with advancing age. In a study conducted by Dietrich, et al., it was demonstrated that age exhibits an inverse correlation with HRV in both men and women [113]. This observation is attributed to a reduction in vagal tone as individuals age. Furthermore, total power represents the sum of VLF, LF, and HF bands in short-term recordings. Therefore, the decrease in total power aligns with the reductions observed in LF and HF bands with age (Table 6, Figures 8a and 8b).
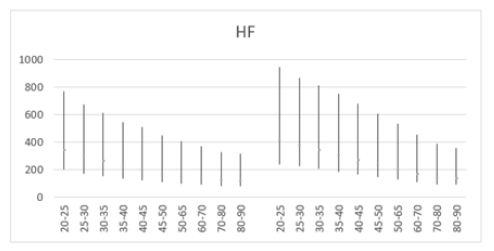
Figure 7a: Mean HF (ms2) versus age, with error bars, shows a decline with age in both men (left) and women (right) aged 20-90 years.
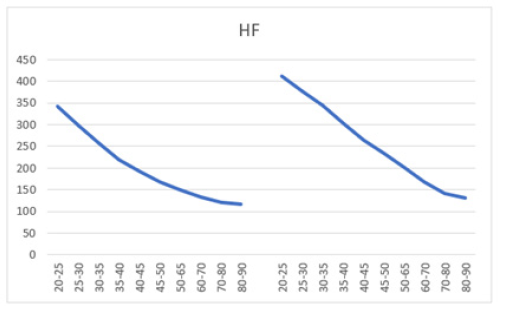
Figure 7b: Mean HF (ms2) versus age shows a decline in both men (left) and women (right) aged 20-90 years, with both curves demonstrating a parallel downward trend.
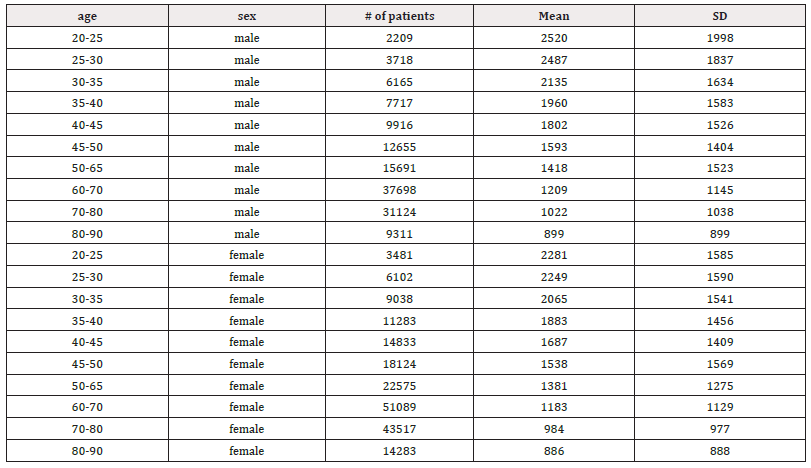
Table 6: Mean total power (ms2) and standard deviation (SD) of male and female patients, across ages 20-90 years.
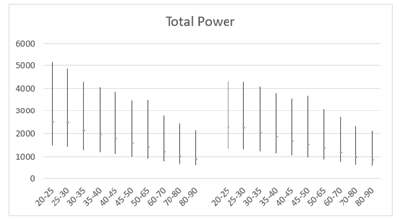
Figure 8a: Mean total power (ms2) versus age, with error bars, shows a decline with age in both men (left) and women (right) aged 20-90 years.
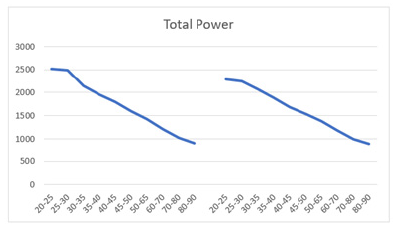
Figure 8b: Mean total power (ms2) versus age shows a decline in both men (left) and women (right) aged 20-90 years, with both curves demonstrating a parallel downward trend.
The PNS controls homeostasis, digestion, and resting bodily functions, while the SNS governs fight-or-flight responses. Vagal activity predominantly influences HRV, which can predict, diagnose, manage, and prevent many cardiovascular dysfunctions by assessing sympathovagal balance. The LF/HF ratio serves as an indicator of sympathovagal dominance and reflects the balance between SNS and PNS activity [114]. Normal values published for LF, HF, and LF/HF ratio are 591 ± 291 ms², 657 ± 777 ms², and 2.8 ± 2.6, respectively [102,115]. A higher LF/HF ratio indicates SNS dominance, while a lower ratio suggests PNS dominance, quantifying the overall autonomic balance [116] Billman, however, challenged the view that LF/HF ratio measures sympatho-vagal balance [114,117,118].
The LF/HF ratio at rest is higher in males than females, indicating elevated sympathetic modulation or dominance (Figures 9a and 9b) [119]. The most significant increase in ratio was observed between ages 35-50, with no comparable rise seen in females (Table 7). Compared to sedentary individuals aged 18-22 (LF/HF ratio: 2.6), the male ratios in this study ranging from 5.107 to 5.356 in the 35-50 age group illustrated markedly higher SNS dominance [120]. Although to a lesser extent, females aged 35-50 also showed elevated stress responses (LF/HF: 3.229-3.365) compared to sedentary individuals [120].
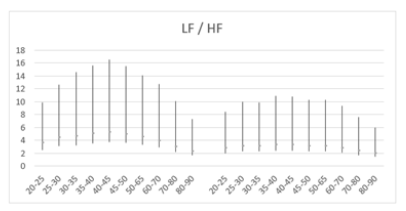
Figure 9a: Mean LF/HF ratio versus age, with error bars, demonstrates a decline starting from age 45+ years in both men (left) and women (right) aged 20-90 years.
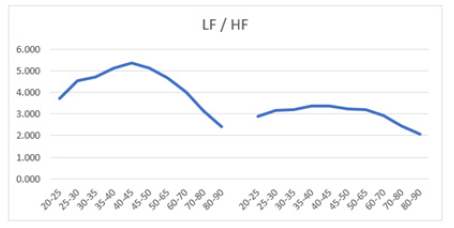
Figure 9b: Mean LF/HF ratio versus age shows a decline starting from age 45+ years in both men (left) and women (right) aged 20-90 years. The male curve consistently lies above the female curve, indicating higher LF/HF ratios in males across all ages.
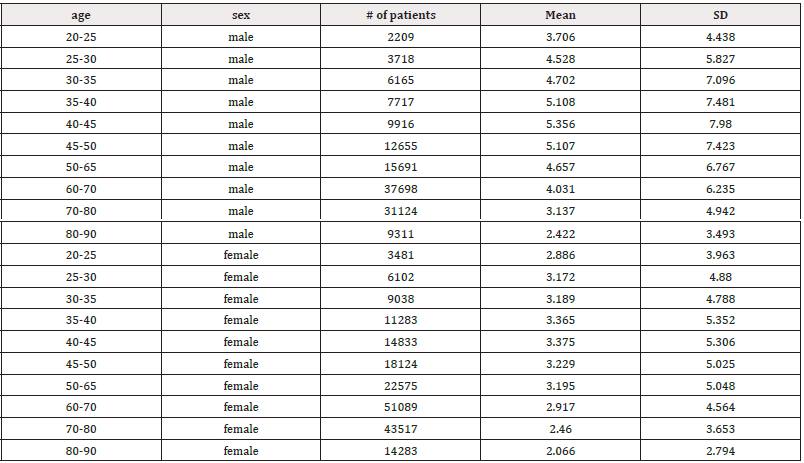
Table 7: Mean LF/HF ratio and standard deviation (SD) of male and female patients, across ages 20-90 years.
Frequency-domain Metrics (LF, HF, LF/HF, Total Power) and Impact of Alcohol Toxicity
Furthermore, individuals (n=34) under alcohol toxicity exhibited a tripled LF/HF ratio (LF/HF: 6.7±4.1) compared to sedentary individuals, indicating severe SNS dominance in these patients experiencing physical stress (Table 8) [120]. This mirrors the general adaptation syndrome described by Hans Selye, where any stressor, regardless of its origin, triggers an increased sympathetic drive and withdrawal of PNS activity [121]. Overall, females in this study exhibited higher HRs and lower values for SDNN, LF, LF/HF ratio, and total power at rest compared to males.
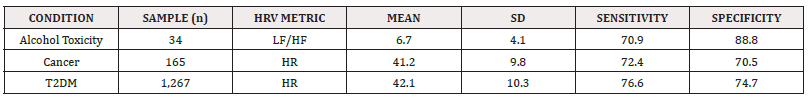
Table 8: The impact of individual conditions (toxicity, cancer, T2DM) on specific HRV metrics (mean and SD), with assessment of sensitivity and specificity levels.
Autonomic Tone Measured by HR versus Age
Conceptually, "autonomic tone" acts as a rheostat regulating ANS divisions [50]. Figures 10a and 10b illustrate a decline in autonomic tone with age, reflected particularly in the HR parameter. The HR, a marker of autonomic tone, decreased with age in both males and females across all age groups. Although the fall in HR was comparable between females and males, females consistently exhibited slightly higher HRs across all ages (Table 9).

Figure 10a: Reduction in autonomic tone with age, depicted by declining average HR (bpm) in males (left) and females (right), aged 20-90 years, with error bars.

Figure 10b: Reduction in autonomic tone with age, depicted by declining average HR (bpm) in males (left) and females (right), aged 20-90 years, with both curves demonstrating a parallel downward trend.
In cancer patients (n=165), autonomic tone resembled that of individuals aged 80-90 years. Despite being at different stages of disease progression, cancer patients exhibited a mean HR response of 41.2±9.8 (Table 8), closely aligning with male patients aged 80-90 (41.41±16.42). Similarly, T2DM patients (n=1267) showed a mean HR of 42.1±10.3 (Table 8), also falling within the range of both male (41.41±16.42) and female (43.01±16.20) patients aged 80-90. Although comparable, the test demonstrated higher sensitivity (Se: 76.6) and specificity (Sp: 74.7) for T2DM compared to cancer (Se: 72.4, Sp: 70.5). On the other hand, the LF/HF ratio exhibited very high specificity (88.8) for detecting alcohol toxicity, with sensitivity (70.9) similar to the HR measure. Nevertheless, any sensitivity or specificity score above 70% in a large cohort is considered significant.
Valsalva Maneuver versus Age
A Valsalva ratio value of 1.2 or higher in healthy individuals is considered normal [1,33,99,108,122,123]. In contrast, a ratio between 1.1 and 1.2 is deemed borderline, and a ratio of 1.1 or lower is abnormal [33]. In the current study, male and female patients aged 20-90 generally exhibited a healthy Valsalva ratio until reaching 80-90 years old, where the ratios became borderline (Figure 11). With advancing age nearing a century, it is expected that the ratio will drop below 1.2. Both sexes displayed the same age-dependent decrease in the Valsalva ratio, but there was no marked difference between the two curves.
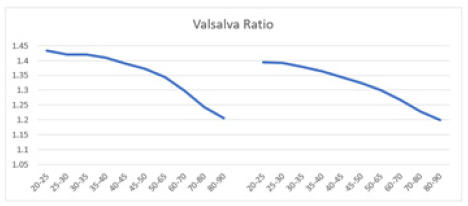
Figure 11: The graph displays the Valsalva ratio of HR response to VM. Mean Valsalva ratio versus age shows a decline in both men (left) and women (right) aged 20-90 years, with both curves demonstrating a similar downward trend.
In healthy women aged 20-80 years, Risk, et al. found that the average ratio linearly decreases with age, ranging from approximately 2.1 to 1.4 [123]. Similarly, in men of the same age range, the ratio decreases from about 2.1 to 1.3. This study observed a similar age-dependent decline in both men (1.44 to 1.20) and women (1.40 to 1.20), consistent with Risk’s findings [123]. However, the participants in Risk’s study began with a higher Valsalva ratio at age 20 [123]. Though not significant, Jha, et al. found that the Valsalva ratio tended to be higher in females (1.69 ± 0.54) than in males (1.59 ± 0.39), both aged 18-25 years [108]. Nonetheless, the men and women in the current study were relatively healthy based on the Valsalva ratio [33]. Although men aged 20-25 initially showed a higher ratio than their female counterparts, the decline does not appear to be gender-dependent. The downward trend in the Valsalva ratio between men and women is comparable, with the two curves mirroring each other.
Deep Breathing versus Age
As an index of cardiac parasympathetic activity, the expiration-to-inspiration ratio (E/I ratio) is measured during deep breathing at six respiratory cycles per minute [124]. The E/I ratio is significantly influenced by the resting HR. Lower resting HR values result in higher E/I ratios, even if the HR response (RRmax - RRmin) remains consistent. A HR response of 15 beats per minute (bpm) or higher in healthy adults is considered normal, 10-15 bpm is borderline, and less than 10 bpm is abnormal [1,33,125]. A typical resting HR during deep breathing should be below 100 bpm [60,107]. Despite a decline with age, the mean E/I ratio observed in this study in patients aged 80-90 was above 1.2 in both males and females, suggesting their HR response is comparable to that of young adults (Figure 12a). In young adults, the E/I ratio should be greater than 1.2 [1]. Smith, et al. demonstrated a decrease in the average E/I ratio from 1.62 to 1.20 in healthy adults aged 16-70 years [126]. A decrease in the E/I ratio was observed from ages 20-90 years in this study, consistent with findings in healthy adults aged 16-70 years in the study by Smith, et al. [126]. Males showed a decrease from approximately ~1.45 to ~1.22, while females decreased from ~1.43 to ~1.23. In the youngest age group [20-25], both males (E/I: ~1.45) and females (E/I: ~1.43) demonstrated E/I ratios much higher than 1.2 (1). The curves for males and females mirrored each other, showing a linear decline in HR response to deep breathing, indicating that gender does not significantly impact the HR response to deep breathing. In summary, the E/I ratio, and consequently the HR response, are influenced by age but apparently not significantly by gender.
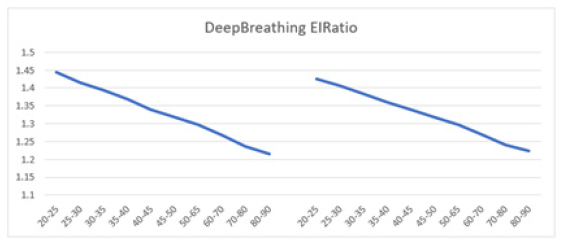
Figure 12a: The graph illustrates the E/I ratio of HR response to the DBT. Mean E/I ratio versus age shows a decline in both men (left) and women (right) aged 20-90 years, with both curves demonstrating a parallel downward trend.

Figure 12b: The graph illustrates the BP response to the DBT. Mean BP ratio versus age exhibits a decline starting from age 40+ years in males (left) and 35+ in females (right), aged 20-90 years. The female curve consistently remains above the male curve, indicating higher BP ratios in females across all ages.
Additionally, males and females exhibited a similar-shaped BP curve, trending downward from ages 20-90 (Figure 12b). However, notable differences include: 1) females consistently had higher BP across all ages, ranging from approximately -3.2 (aged 20-25) to -5.6 (aged 80-90), compared to males who ranged from about -5.0 to -7.5, and 2) BP peaked at approximately -1.9 (aged 35-40) in females and -3.4 (aged 40-45) in males, after which both curves declined.
BP regulation in the body is finely tuned by the ANS and cardiac centers [127]. Parasympathetic dominance induced by slow deep breathing reduces BP [127]. Garg, et al. supports the use of breathing exercises as an effective alternative method to control BP [128]. They reported reductions in both systolic (SBP) and diastolic (DBP) blood pressure in patients performing breathing exercises compared to control groups. Specifically, breathing exercises were found to significantly decrease SBP by -7.06 mm Hg and DSP by -3.43 mm Hg in their meta-analysis. Additionally, these exercises were associated with a significant reduction in HR by -2.41 bpm. Bernardi, et al.’s findings suggest that slow breathing not only improves oxygen saturation and exercise tolerance, but also enhances baroreflex sensitivity in both controls (from 9.4 to 13.8 ms/mm Hg) and chronic heart failure (CHF) patients (5.0 to 6.1 ms/mm Hg) [129]. The increase in baroreflex sensitivity was observed specifically with slow breathing (6 breaths/min), not with controlled breathing at a faster rate (15 breaths/min) similar to normal breathing. This indicates that the effect is dependent on the slower respiratory rate rather than simply the act of controlling breathing frequency. Furthermore, the decrease in BP seen during slow and deep breathing suggests that this effect is due to reduced afterload, possibly stemming from decreased sympathetic activity, rather than a decline in heart function. Overall, Bernardi’s findings suggest multiple beneficial effects of slow breathing in CHF patients [129]. Additionally, other commonly used HRV measures, such as RMSSD or SDNN, may also be employed to characterize HRV magnitude during the deep breathing challenge.
Tilt Table Test versus Age
The transition from supine to upright position alters autonomic function, as reflected in HRV parameters. While the supine position provides baseline HRV and HR during relaxed breathing, shifting to an upright posture typically reduces parasympathetic activity. This reduction is indicated by a decrease in the PNS index, which compares an individual’s resting HRV to normal values in adults. Standard time-domain and frequency-domain HRV metrics, such as the LF and HF components, assess changes in sympatho-vagal balance.
The 30:15 ratio relates to the HR dynamics after assuming an upright position, characterized by an initial peak around the 15th beat followed by relative bradycardia and a local minimum near the 30th beat [33]. This response is defined by the longest RR interval near the 30th beat compared to the shortest near the 15th beat. In healthy adults aged 16-69 years, an average 30:15 ratio of 1.29 is typical, with values of 1.05 or higher considered normal [1,33,122]. In the current study, there is an age-dependent decline in the HR response to tilting observed in both males and females, with males showing a slightly higher 30:15 ratio (1.29 vs. 1.27 in females aged 20-25, and 1.19 vs. ~1.18 in females aged 80-90). Both curves are similar, suggesting a comparable rate of decline in both sexes (Figures 13a).
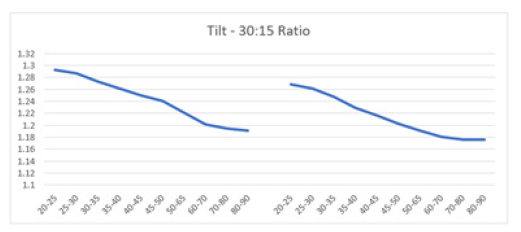
Figure 13a: The graph illustrates the 30:15 ratio of HR response to the TTT. Mean 30:15 ratio versus age shows a decline in both men (left) and women (right) aged 20-90 years, with both curves demonstrating a parallel downward trend.
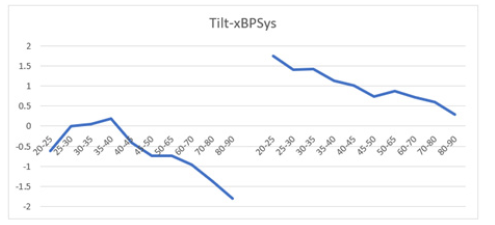
Figure 13b: The graph illustrates the BP response to the TTT. Mean BP ratio versus age exhibits a decline starting from age 40+ years in males (left) and 25+ in females (right), aged 20-90 years. The female curve consistently stays above the male curve, indicating higher BP ratios in females across all ages.
However, the BP ratio curves for males and females differ significantly in shape and magnitude. For instance, the mean BP ratio in females aged 20-25 is +1.75, which is much higher compared to -0.60 in males (Figure 13b). Regardless of gender, an age-dependent decline is observed in both sexes, with females exhibiting a higher mean BP ratio of +0.75 at age 80-90 compared to -1.90 in males. The BP ratio curve for females shows a notably higher shift upward compared to males during tilting. This analysis underscores how transitioning from supine to upright position affects autonomic function differently between genders, as observed through distinct HR and BP responses.
After the Task Force Report, Nunan, et al. reviewed normative data from short-term HRV studies, as detailed in (Table 10) (12,102,130). Their analysis covered 44 studies involving 21,438 healthy adult participants, encompassing three large populations aged 40. All time-domain (SDNN, RMSSD) and frequency-domain (LF, HF, LF/H) results fell within normative ranges established from several short-term normative studies listed in the table. However, older age cohorts exhibited greater deviations from the normative mean (SD). It is also possible that results from patient populations in Medeia Inc.’s study differed from normative values, such as RMSSD [42 vs. 31.69 (M) and 32.60 (F)] and HF [657 vs. 342 (M) and 412 (F)], due to distinct characteristics between healthy populations in normative studies and patients in Medeia Inc.’s study. The HRV measure ranges from the normative studies were broad, possibly reflecting differences in experimental conditions and subject variables. Despite these deviations from the mean norms, all results remained within normative ranges, suggesting normal autonomic function (Table 10).
In clinical practice, autonomic tone serves as a critical marker for cardiovascular health, integrating fundamental measurements like HR and BP with dynamic assessments of HRV spectra. Understanding these dynamics is crucial for managing cardiovascular risks and optimizing patient outcomes.
Discussion
HRV Limiting Factors
Understanding the contextual and subject-related variables is essential for accurate interpretation of HRV measurements, as they can significantly influence ANS activity, breathing patterns, and emotional states captured by HRV analysis [102]. The interpretation of HRV measurements hinges significantly on contextual factors related to recording conditions and subject variables. Factors such as the length of the recording period, method of detection, sampling frequency, artifact removal, respiration patterns, and the presence of paced breathing are crucial. Subject variables such as age, sex, HR, and health status also play a significant role. For instance, the length of the recording period impacts both time-domain and frequency-domain HRV measurements, with longer recordings generally showing increased HRV [131]. Comparisons between metrics calculated from epochs of different lengths are inappropriate due to these variations [12,132,133]. The choice of ECG or PPG methods can also affect HRV measures, with slight discrepancies observed between them [134].
Artifacts in HRV data, such as missed or spurious beats, can distort measurements significantly, affecting both time-domain and frequency-domain result [135]. Techniques to handle artifacts include selecting artifact-free epochs or manually editing affected RR intervals [136]. Proper sampling rates are crucial, with higher rates necessary for specific conditions like low RR interval variability [131,133,137].
Respiration patterns, including depth and rate, influence HRV measures, with deeper breaths generally increasing HRV (138-140). The E/I ratio during breathing also affects HRV metrics, though the exact impact remains uncertain and varies based on study conditions and participant characteristics [102].
Short-term HRV measured over 5 minutes (at rest) primarily reflects ANS control, particularly vagal tone, and sinus atrial stretch [141]. In contrast, 24-hour Holter ECG-derived HRV can be affected by concurrent illnesses, medications, and lifestyle factors such as exercise and stress, in addition to physiological factors [141]. HRV serves as a reliable indicator for detecting autonomic dysfunction, quantifying resting sympathetic and parasympathetic activity and their balance [73]. It is assessed through time domain and frequency domain analyses of ECG recordings, making it a precise tool for evaluating autonomic nerve function [75].
HRV time-domain and frequency-domain measurements exhibit age-related declines, as observed in the current study and studies across various age groups [44,142]. Almeida-Santos, et al. reported linear declines in SDNN with age, while RMSSD showed a U-shaped pattern, decreasing from ages 40 to 60 and then increasing after age 70 in a large cohort (n=1,743) [143]. A similar linear decline in SDNN with age was observed in the current study, but the RMSSD did not display a U-shaped pattern reflective of the sharp decrease from 40 to 60 years old then increase thereafter. The differences in the cohort characteristics, length of recording (long-term vs. short-term) and sample size (n=1743 vs. n=328,591) may be the reason for the U-shaped pattern.
A meta-analysis by Koenig et. al involving 296,247 healthy participants highlighted gender differences in HRV, with women generally exhibiting higher mean HR and lower SDNN values compared to men, especially in 24-hour studies [144]. With short-term recordings, patient sample (n=328,591) in this study also yielded similar results across the age spectrum (20 - 90 year old). Women also showed lower LF and total power, but greater HF power, indicating relative vagal dominance despite higher mean HR, whereas men exhibited relative SNS dominance despite lower HR. Overall, this study achieved similar results for the frequency-domain measures, despite different sample characteristics (healthy vs. patients).
The phenomenon of cycle length dependence explains that faster HRs reduce HRV by limiting the variability between successive heartbeats, whereas slower HRs increase HRV by allowing more variation in IBIs [43]. Elevated resting HRs (>90 bpm) are associated with increased mortality risk [145]. Conversely, increased aerobic fitness is correlated with higher HRV time-domain measurements, while decreased health generally correlates with reduced HRV [146-149].
In summary, HRV time-domain and frequency-domain measurements generally decline with age, vary by gender, are influenced by HR, fitness level, and health status, and serve as valuable indicators of autonomic function and health outcomes in both clinical and research settings.
HRV Analysis in Diseased Conditions - T2DM
Autonomic cardiac dysregulation plays a crucial role in various autonomic disorders such as diabetes, cancer, and alcohol toxicity. HRV has been established as a predictor of morbidities and mortality, reflecting its utility in assessing autonomic imbalance and overall health status across diverse conditions.
CAN in diabetes refers to impaired autonomic control of the cardiovascular system, predominantly affecting individuals with diabetes, with a prevalence as high as 73% in T2DM, and up to 90% in long-standing Type 1 diabetes [64,65,150]. Initially appearing as subclinical stages with reduced parasympathetic control and imbalanced sympatho-vagal activity, early signs include decreased HRV, detectable even in prediabetes [72,151]. Clinical CAN is diagnosed and assessed using five standard cardiovascular autonomic reflex tests (CARTs), which are considered the gold standard for CAN assessment: HR response to deep breathing, HR response to standing, VM, BP response to standing, and BP response to sustained handgrip [33,152]. Subclinical CAN is diagnosed based on sensitive indicators like changes in HRV, baroreflex sensitivity, and cardiac imaging showing increased left ventricular torsion [152]. Standard CARTs have limited sensitivity for detecting subclinical CAN [152]. Early detection of subclinical CAN is crucial for timely intervention on modifiable risk factors to prevent progression to severe forms of CAN and associated cardiovascular complications in diabetes [72,152].
Reduced HRV is an early sign of CAN in T2DM, indicating impaired sympathetic and parasympathetic activity before clinical symptoms appear [153]. Benichou, et al. confirmed decreased HRV across various variables in T2DM patients, reflecting reduced function of both ANS branches [97]. Benichou, et al. conducted a meta-analysis involving 25 case-control studies with 2,932 patients (1,356 with T2DM and 1,576 healthy controls), confirming decreased parasympathetic and sympathetic activities in T2DM patients [97]. They observed significant decreases in HRV parameters such as SDNN, RMSSD, total power, LF, and HF, whereas LF/HF ratio remains unchanged. The disease's metabolic nature affects both autonomic fiber types, although LF/HF ratio shows no significant difference due to similar LF and HF component changes. Both sympathetic and parasympathetic activities are diminished compared to non-T2DM patients, attributed to adverse metabolic effects of blood glucose levels on HRV [154]. Dyslipidemia and hypertension exacerbate HRV decline in T2DM patients. T2DM's impact on most HRV parameters underscores its role in cardiac autonomic dysfunction [155].
HRV is crucial for assessing CAN, often using Ewing's standard CARTs [33]. HRV measurement, considered one of the simplest and most reliable methods, quantifies the variation between consecutive heartbeats, with higher variability indicating greater parasympathetic activity and adaptability to environmental changes [156]. Conversely, low HRV serves as a marker for increased cardiovascular risk [157]. Despite various studies assessing HRV in T2DM, results are conflicting [158-160]. There is no consensus on reduced HRV levels in T2DM, despite its association with disease severity [97,155].
Goit, et al. reported that in T2DM, parasympathetic activity declines before sympathetic activity becomes affected [161]. The study aimed to compare cardiovascular autonomic function tests between 60 patients with type 2 diabetes mellitus (T2DM) and 30 controls. The results indicated that parasympathetic dysfunction was more pronounced than sympathetic dysfunction, which may reflect the severity and progression of the disease. Contrary to earlier beliefs, autonomic dysfunction can begin in the pre-diabetes stage and worsens with the progression to diabetes [162]. Hadad, et al.’s cross-sectional study reveals that HbA1c levels and age independently correlate with parasympathetic tone in individuals with pre-diabetes and well-controlled diabetes [163]. Participants with pre-diabetes and higher HbA1c levels exhibited higher 24-hour average HRs and lower SDNN, akin to elderly individuals (80-90 years old) in this study [163]. Similarly, Medeia Inc’s current study, too, showed the average HR of T2DM patients coincided with the 80-90 year olds in the populations. Moreover, Hadad’s results implied comparable impacts on CAN from both age and elevated HbA1c levels, suggestive of a cumulative effect of age and HbA1c exacerbating CAN effects [163]. While a causal relationship remains unestablished, Hadad’s study supports the notion that both age and hyperglycemia significantly influence parasympathetic tone [163].
Geijselaers, et al. demonstrated that diabetes correlates with impaired cognitive function, primarily influenced by hyperglycemia [164]. The study aimed to assess how differences in cognitive performance between individuals with varying glucose metabolism statuses could be attributed to hyperglycemia, insulin resistance, and BP-related factors. Using cross-sectional data from 2,531 participants (n=666 with T2DM), cognitive performance was evaluated using a neuropsychological test battery. Results showed that compared to individuals with normal glucose metabolism, those with type 2 diabetes exhibited poorer performance across all cognitive domains (memory, processing speed, executive function, and attention). Differences in processing speed and executive function were largely explained by hyperglycemia, and partially by blood BP variables for processing speed, but not by insulin resistance. The study suggests that early management of glycemic and BP control, even during the prediabetic stage, could potentially mitigate diabetes-related declines in cognitive function. Moreover, reduced parasympathetic tone contributes to increased inflammatory responses and elevated resting HRs, a hallmark of diabetes and a cardiovascular disease risk factor [165,166]. Overall, in individuals with diabetes and pre-diabetes, increasing HbA1c levels and age are critical factors associated with impaired parasympathetic function.
Age and male gender are associated with decreased sympathetic and parasympathetic activities, as well as reduced LF and HF components, although their influence on HRV parameters is less pronounced compared to T2DM-specific variables [95-97,111,167]. Studies utilizing short-term and 24-hour ECG recordings consistently demonstrate reduced HRV in these conditions [153]. In patients at increased risk for diabetes, reduced HRV compared to healthy controls worsens with increasing diabetes risk, even progressing further with chronic complications [168]. This reduction in HRV parameters reflects impaired parasympathetic and sympathetic activity, whether or not clinical signs of diabetes autonomic neuropathy are present. While no standard reference values exist for diagnosing CAN using HRV variables, Breder and Sposito propose criteria based on abnormal results in at least two of the following parameters derived from 24-hour Holter ECG recordings: SDNN < 50 ms, RMSDD < 15 ms, PNN50 < 0.75%, LF < 300 ms2, and HF < 300 ms2 [141,169]. Triverdi, et al. suggest that T2DM is a continuum, with studies consistently demonstrating impaired HRV from stress through to T2D, highlighting its role as a marker of stress-induced ANS imbalance and chronic disease [153]. Understanding the correlation between HRV and risk factors contributing to T2DM complications is crucial for further exploration and management strategies.
HRV Analysis in Diseased Conditions - Cancer
Other prevalent conditions such as depression, schizophrenia, multiple sclerosis, and cancer also correlate with reduced parasympathetic and increased sympathetic activities, as adaptation to stress heavily involves the ANS [5,6,170,171]. Indices like E/I ratio indicate efferent parasympathetic nerve damage, while SDNN reflects overall sympathetic and parasympathetic activity [172-174]. Bijoor, et al. found that resting HR (74.02±7.23 vs. 80.49±9.46 bpm) and E/I ratio (1.113±0.061 vs. 1.39±0.16) were significantly lower in cancer patients compared to controls, while no statistical significance was found between the two groups for SDNN [175]. This suggests reduced vagally mediated parasympathetic activity in cancer patients, although SDNN did not differ significantly between groups. Compared to young healthy adults, whose E/I ratio should be above 1.2 as mentioned in the Results section, the E/I ratio (1.113±0.061) observed in cancer patients is considered relatively low, indicating poorer health. Thus, impaired cardiovascular parasympathetic control may characterize cancer patients compared to healthy subjects, emphasizing the role of E/I ratio and resting HR as key indices in assessing autonomic function in cancer.
Ben-David's study, involving 798 patients, investigated HRV as an indicator of ANS dysfunction in cancer patients [176]. It revealed that cancer patients exhibit significantly reduced HRV metrics (SDNN and RMSSD) compared to non-cancer controls in cases of breast, gastrointestinal, genitourinary, or respiratory cancers. Moreover, the HRV metrics declined progressively with disease advancement across all cancer stages, independent of common co-morbidities such as cardiovascular diseases, diabetes, and obesity [176]. Non-cancer patients consistently demonstrated higher HRV metrics, including RMSSD (11.1 to 13.9 ms) and SDNN (22.8 to 27.7 ms), compared to those with stage I or II cancer. In contrast, patients with stage III and IV cancer exhibited even lower HRV metrics: HR reductions of -11.8 to -14.0 bpm, increases in RMSSD of +31.7 to +32.8 ms, and higher SDNN values of +45.2 to +45.8 ms. Specifically, individuals with stages I and II cancer had HRs of 69.9±5.5 and 82.4±5.9 bpm, RMSSDs of 40.0±5.5 and 26.4±5.5 ms, and SDNNs of 43.8 ± 5.8 and 30.1 ± 5.9 ms, respectively. In contrast, patients with advanced cancer (stages III and IV) exhibited elevated HRs of 81.3 ± 4.4 and 88.1 ± 3.5 bpm, and reduced RMSSD values of 15.9 ± 2.9 and 9.4 ± 2.7 ms, and SDNN values of 20.5 ± 2.8 and 14.7 ± 2.5 ms, respectively. On average, non-cancer patients had HRs of 71.2 ± 8.1 bpm, rMSSD of 45.6 ± 7.9 ms, and SDNN of 63.0 ± 11.1 ms. Ben-David’s study illustrates a reduced HRV pattern that persisted across cancer stages (I-IV) and locations, suggesting HRV could serve as a non-invasive marker for cancer detection and staging. The study underscores the need for further research to explore HRV variations across demographics.
Early detection through screening is crucial for improving cancer survival rates, yet current methods often involve burdensome preparation or discomfort, deterring adherence [177-183]. Previous studies have consistently reported lower HRV in various cancers, suggesting dysregulation of the SNS and PNS may influence cancer progression and prognosis [79,92,184-187]. Torres-Juarez, et al. highlight the ongoing debate and accumulating evidence suggesting that nerve activity modulation may disrupt cellular and tissue homeostasis, thereby promoting cancer progression [188]. Additionally, higher HRV has been associated with improved overall survival in cancer patients, contrasting findings that suggest its limited prognostic value in advanced stages [92,189-191]. Advanced stages of cancer are associated with exacerbated sympathetic drive, potentially promoting tumor growth and metastasis [192,193]. Overall, increasing HR and decreasing HRV metrics with increasing cancer stage, reflecting heightened autonomic disturbance and potentially impacting patient outcomes [92,187]. Further research is needed to clarify HRV's utility in cancer prognosis and its potential mechanisms in tumor progression.
Chemotherapy studies using both short-duration and 24-hour recordings have consistently reported exhibit altered ANS function, as evidenced by diminished HRV [194,195]. However, HRV measurements can be influenced by immediate stressors such as medical consultations, potentially skewing results [79]. Short-term ECG measurements during stressful situations may not reflect a patient’s typical HRV patterns, although high HRV in such contexts could indicate resilience. To mitigate these issues, the review recommends using 24-hour Holter ECG for more accurate HRV assessments in cancer patients. Regarding chemotherapy, specifically vincristine, doxorubicin, and certain combination therapies have been linked to reduced HRV, while findings regarding paclitaxel have been mixed, with some studies indicating reduced variability and others showing no significant change [195-199]. Contrary to some reports suggesting chemotherapy-induced attenuation of HRV, Adams, et al. found no significant changes in parasympathetic activity based on short-term HRV analysis during rest periods before tilt tests [200]. This aligns with the idea that acute changes induced by chemotherapy may be subtle and harder to detect with short-term recordings compared to 24-hour monitoring [199]. In addition to HRV, aberrant BP pressure variability and maladaptive orthostatic responses have been observed in patients treated with paclitaxel, taxanes, vinca alkaloids, and cisplatin [201-204]. These drugs appear to affect both HR and BP regulation, highlighting broader impacts on autonomic function. Overall, chemotherapy treatments appear to disrupt ANS balance, potentially shifting towards sympathetic dominance, which could lead to chronically higher metabolic rates and physiological stress, impacting overall patient health and well-being [205,206]. While HRV shows promise in diabetic neuropathy assessment and athlete fitness evaluation, its role as an independent prognostic tool in cancer, particularly in advanced stages, remains debated [94,146,207-210]. Further research is needed to clarify HRV's correlation with cancer prognosis, exploring whether enhanced coping abilities contribute to better outcomes.
HRV Analysis in Diseased Conditions - Alcohol Toxicity
The decline in autonomic nervous system (ANS) function with age and its susceptibility to existing comorbidities are well-documented [211]. Medications can also influence ANS and cardiovascular reflex responses, potentially confounding study outcomes. Using short-term HRV recordings in 55 AD adults aged 20-55, results by Muthuswamy, et al. showed significantly reduced HF, a marker of parasympathetic activity, and increased LF, indicating sympathetic dominance, in the AD group [60]. The LF/HF ratio, reflecting sympathetic-vagal balance, was also elevated in AD individuals. These findings suggest alcohol-induced cardiac autonomic dysfunction with diminished parasympathetic activity and heightened sympathetic activity. The study concludes that HRV in the frequency domain offers a non-invasive method to assess autonomic dysfunction in AD patients. In health, the intrinsic HR ranges from 100 to 120 beats per minute (bpm), while the resting HR typically ranges from 60 to 90 bpm, regulated by a balance between sympathetic (which accelerates HR) and parasympathetic dominance (which slows HR), with the vagus nerve playing a key role in HR control [12,212]. In AD, both sympathetic and parasympathetic nerve fibers suffer functional and structural damage, contributing to impaired cardiac autonomic function [213,214].
The study conducted by Muthuswamy, et al. revealed significant differences in HRV parameters between AD individuals and non-AD controls [60]. Specifically, they found that HF values were 158.13±134.99 in the AD group and 197.16±42.29 in the non-AD, indicating a statistically significant decrease in HF among AD individuals. This suggests reduced parasympathetic tone in alcoholics. Regarding LF, which represents sympathetic activity, the study noted increased values of 398.27±345.15 in the AD group compared to 121.22±31.27 in the non-AD group. This difference was highly significant, indicating heightened sympathetic tone in AD individuals. Similarly, the LF/HF ratio showed a substantial increase in the AD group (3.61±1.15) compared to the non-AD group (0.62±0.15). The LF/HF ratio for the AD group in the current study was higher than that in Muthuswamy’s short-term HRV study (6.7±4.1 vs. 3.61±1.15), potentially due to differences in experimental conditions and subject variables. Multiple studies corroborated these findings of reduced HF or increased LF, and elevated LF/HF [63,215-217]. Kumar, et al. found similar results of reduced HF and increased LF/HF ratio, indicating diminished parasympathetic activity and potentially increased sympathetic tone [219]. Pop, et al. highlighted reduced HF and trends towards elevated LF and LF/HF ratio in association with increased alcohol intake, linking these changes to alcoholic cardiomyopathy and associated cardiac complications [62]. Contrastingly, using 24-hour ECG recording in women subjects, Jansky, et al. found no correlation between alcohol consumption and HRV indices in a cohort study, which differs from the findings of the present study and others suggesting alcohol's impact on autonomic balance [220]. Not surprisingly, contextual factors matter for HRV metrics, analysis, and interpretation, as well as the comparison of studies. Nonetheless, all these studies point to an imbalance that denotes sympathetic hyperactivity and impaired parasympathetic activity in the AD group, highlighting an autonomic imbalance favoring sympathetic over parasympathetic activity on the heart.
Overall, these studies and the current findings support the notion that alcohol disrupts ANS balance, characterized by reduced parasympathetic activity, increased sympathetic tone, and altered HRV parameters. Early detection of these changes through HRV analysis could aid in clinical interventions, including detoxification and rehabilitation, potentially mitigating the progression of alcohol-related cardiac dysfunction and reducing the risk of sudden cardiac events. Thus, using short-term (5 min) HRV recordings to aid in diagnosis and prognosis, HRV analysis in clinical practice serves as a valuable non-invasive tool for routinely assessing autonomic neuropathy and cardiac risk in individuals with AD, cancer and T2DM, as well as other chronic diseases.
Conclusion
The study of HRV across various health conditions highlights its significance in diagnosing and managing autonomic dysfunction, particularly in conditions such as T2DM and cancer. HRV parameters provide insights into the dynamic balance between sympathetic and parasympathetic activity, offering valuable prognostic information in cardiovascular diseases and potential cancer screening. Medeia Inc.’s ultimate goal is to expand its clinical patient databases to integrate HRV as a diagnostic and prognostic biomarker of cardiovascular health in both normal and diseased individuals. Future research should focus on elucidating HRV patterns among different demographics and integrating HRV with other physiological metrics for comprehensive diagnostic and therapeutic strategies. Enhancing HRV measurement methodologies and accessibility can improve early detection and personalized treatment, ultimately enhancing patient outcomes across diverse health conditions.
References
- Zygmunt A and Stanczyk J (2010) Methods of evaluation of autonomic nervous system function. Archives of Medical Science 6(1): 11-18.
- Gibbins I (2013) Functional organization of autonomic neural pathways. Organogenesis 9(3): 169-175.
- Janig W and McLachlan EM (1992) Specialized functional pathways are the building blocks of the autonomic nervous system. Journal of the Autonomic Nervous System 41(1-2): 3-13.
- Novak P (2019) Autonomic disorders. The American Journal of Medicine 132: 420-436.
- Chen X, Yang R, Kuang D, Zhang L and Lv R et al (2017). Heart rate variability in patients with major depression disorder during a clinical autonomic test. Psychiatry Research 256: 207-211.
- Montaquila JM, Trachik BJ and Bedwell JS (2015). Heart rate variability and vagal tone in schizophrenia: A review. Journal of Psychiatric Research 69: 57-66.
- Furlan R, Ardizzone S, Palazzolo L, Rimoldi A and Perego F et al (2006) Sympathetic overactivity in active ulcerative colitis: Effects of clonidine. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 290(1): R224-R232.
- Thorp AA and Schlaich MP (2015). Relevance of sympathetic nervous system activation in obesity and metabolic syndrome. Journal of Diabetes Research 341583.
- Malliani A and Montano N (2004) Sympathetic overactivity in ischaemic heart disease. Clinical Science (London, England: 1979) 106: 567-568.
- Julius S and Nesbitt S (1996). Sympathetic overactivity in hypertension: A moving target. American Journal of Hypertension 9(11 Pt 2): 113S-120S.
- Niedermaier ON, Smith ML, Beightol LA, Zukowska Grojec Z and Goldstein DS, et al (1993). Influence of cigarette smoking on human autonomic function. Circulation 88(2): 562-571.
- Task Force Report (1996) Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation 93(5): 1043-1065.
- Benarroch EE and Chang FL (1993). Central autonomic disorders. Journal of Clinical Neurophysiology 10(1): 39-50.
- Racosta JM and Kimpinski K (2016). Autonomic dysfunction, immune regulation, and multiple sclerosis. Clinical Autonomic Research 26(1): 23-31.
- Robertson D (1999). The epidemic of orthostatic tachycardia and orthostatic intolerance. The American Journal of the Medical Sciences 317(2): 75-77.
- Sheldon RS, Grubb BP, Olshansky B, Win Kuang Shen, Hugh Calkins, et al (2015). 2015 Heart Rhythm Society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 12(6): e41-e63.
- Benarroch EE (2012). Postural tachycardia syndrome: A heterogeneous and multifactorial disorder. Mayo Clinic Proceedings 87(12): 1214-1225.
- Wieling W, Jardine DL and de Lange FJ, et al (2016) Cardiac output and vasodilation in the vasovagal response: An analysis of the classic papers. Heart Rhythm 13(3): 798-805.
- Van Lieshout JJ, Wieling W, Karemaker JM and Secher NH (2003). Syncope, cerebral perfusion, and oxygenation. Journal of Applied Physiology (1985) 94(3): 833-848.
- Runser LA, Gauer RL and Houser A (2017). Syncope: Evaluation and differential diagnosis. American Family Physician 95(5): 303-312.
- The Task Force for the diagnosis and management of syncope of the European Society of Cardiology (ESC). (2018) 2018 ESC Guidelines for the diagnosis and management of syncope. European Heart Journal 39: 1883-1948.
- Liu X, Treister R, Lang M and Oaklander AL (2018). IVIg for apparently autoimmune small-fiber polyneuropathy: First analysis of efficacy and safety. Therapeutic Advances in Neurological Disorders 11: 1756285617744484.
- Freeman R (2005) Autonomic peripheral neuropathy. The Lancet 365(9466): 1259-1270.
- de Greef BTA, Hoeijmakers JGJ, Gorissen Brouwers CML, Geerts M, Faber CG, et al (2018). Associated conditions in small fiber neuropathy - A large cohort study and review of the literature. European Journal of Neurology 25(2): 348-355.
- Oaklander AL (2016). Immunotherapy prospects for painful small-fiber sensory neuropathies and ganglionopathies Neurotherapeutics 13(1): 108-117.
- Peters MJH, Bakkers M, Merkies ISJ, Hoeijmakers JGJ, van Raak EPM, et al (2013). Incidence and prevalence of small-fiber neuropathy: A survey in the Netherlands. Neurology 81(15): 1356-1360.
- Birnbaum J and Bingham CO (2014). Non-length-dependent and length-dependent small-fiber neuropathies associated with tumor necrosis factor (TNF)-inhibitor therapy in patients with rheumatoid arthritis: Expanding the spectrum of neurological disease associated with TNF-inhibitors. Seminars in Arthritis and Rheumatism 43(5): 638-647.
- Vinik AI, Nevoret ML, Casellini C and Parson H (2013) Diabetic neuropathy. Endocrinology and Metabolism Clinics of North America 42(4): 747-787.
- Novak V, Freimer ML, Kissel JT, Periquet IM, Nash SM, et al (2001). Autonomic impairment in painful neuropathy. Neurology 56(7) 861-868.
- Freeman R (2014) Diabetic autonomic neuropathy. In A. V. Chaudhry & P. J. Dyck (Eds.), Handbook of Clinical Neurology 126: 63-79.
- Krishnasamy S and Abell TL (2018). Diabetic gastroparesis: Principles and current trends in management. Diabetes Therapy. Advance online publication 9(Suppl 1): 1-42.
- Vinik AI, Maser R E, Mitchell B D and Freeman R (2003). Diabetic autonomic neuropathy. Diabetes Care 26(5): 1553-1579.
- Ewing DJ, Martyn CN, Young RJ and Clarke BF (1985). The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care 8(5): 491-498.
- Hon EH and Lee ST (1963). Electronic evaluation of the fetal heart rate. VIII. Patterns preceding fetal death, further observations. American Journal of Obstetrics and Gynecology 87: 814-826.
- Wolf MM, Varigos GA, Hunt D and Sloman JG (1978). Sinus arrhythmia in acute myocardial infarction. Medical Journal of Australia 2(2): 52-53.
- Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, et al (1981). Power spectrum analysis of heart rate fluctuation: A quantitative probe of beat-to-beat cardiovascular control. Science 213(4504): 220-222.
- Casolo G, Balli E, Taddei T, Amuhasi J and Gori C (1989). Decreased spontaneous heart rate variability in congestive heart failure. The American Journal of Cardiology 64(18): 1162-1167.
- Cripps TR, Malik M, Farrell TG and Camm AJ (1991) Prognostic value of reduced heart rate variability after myocardial infarction: Clinical evaluation of a new analysis method. British Heart Journal 65(1): 14-19.
- Ponikowski P, Anker SD, Chua TP, Szelemej R, Piepoli M, et al (1997) Depressed heart rate variability as an independent predictor of death in chronic congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. The American Journal of Cardiology 79(12): 1645-1650.
- La Rovere MT, Bigger JT, Marcus FI, Mortara A and Schwartz PJ (1998) Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (autonomic tone and reflexes after myocardial infarction) investigators. The Lancet 351(9101): 478-484.
- Mäkikallio TH, Huikuri HV, Mäkikallio A, Sourander LB, Mitrani RD, et al (2001) Prediction of sudden cardiac death by fractal analysis of heart rate variability in elderly subjects. Journal of the American College of Cardiology 37(5): 1395-1402.
- Kleiger RE, Miller JP, Bigger JT, and Moss AJ (1987) Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. American Journal of Cardiology 59(4): 256-262.
- McCraty R and Shaffer F (2015) Heart rate variability: New perspectives on physiological mechanisms, assessment of self-regulatory capacity, and health risk. Global Advances in Health and Medicine 4(1): 46-61.
- Beckers F, Verheyden B and Aubert AE (2006). Aging and nonlinear heart rate control in a healthy population. American Journal of Physiology. Heart and Circulatory Physiology 290(6): H2560-H2570.
- Janig W (2008) Integrative action of the autonomic nervous system: Neurobiology of homeostasis. Cambridge, UK: Cambridge University Press.
- Gevirtz RN, Lehrer PM and Schwartz MS (2016) Cardiorespiratory biofeedback. In M. S. Schwartz & F. Andrasik (Eds.), Biofeedback: A Practitioner’s Guide. 196-213.
- Ferdousi S and Gyeltshen P (2021). Type 2 Diabetes Mellitus: Cardiovascular Autonomic Neuropathy and Heart Rate Variability. In Type 2 Diabetes - From Pathophysiology to Cyber Systems. IntechOpen.
- Olshansky B, Sabbah HN, Hauptman PJ and Colucci WS (2008). Parasympathetic nervous system and heart failure: Pathophysiology and potential implications for therapy. Circulation 118(8): 863-871.
- Tortora GJ and Derrickson BH (2017) Principles of Anatomy and Physiology (15th ed.). New York NY: John Wiley & Sons Inc.
- Sigurdsson MI, Waldron NH, Bortsov AV, Smith SB and Maixner W (2018) Genomics of cardiovascular measures of autonomic tone. Journal of Cardiovascular Pharmacology 71(3): 180-191.
- Verrier RL and Tan A (2009). Heart rate, autonomic markers, and cardiac mortality. Heart Rhythm 6(11 Suppl): S68-S75.
- Fox K, Borer JS, Camm AJ, Danchin N, Ferrari R, et al (2007) Heart Rate Working Group Resting heart rate in cardiovascular disease. Journal of the American College of Cardiology 50(9):823-830.
- Agarwal SK, Norby FL, Whitsel EA, Soliman EZ, Chen LY, et al (2017). Cardiac autonomic dysfunction and incidence of atrial fibrillation: Results from 20 years follow-up. Journal of the American College of Cardiology 69(3): 291-299.
- Salman IM (2016). Major autonomic neuroregulatory pathways underlying short- and long-term control of cardiovascular function. Current Hypertension Reports 18(3): 18.
- Victor RG (2015) Carotid baroreflex activation therapy for resistant hypertension. Nature Reviews Cardiology 12(8): 451-463.
- Mancia G and Grassi G (2014). The autonomic nervous system and hypertension. Circulation Research 114(11): 1804-1814.
- Coffman TM (2011) Under pressure: The search for the essential mechanisms of hypertension. Nature Medicine 17(11): 1402-1409.
- Xin W, Lin Z and Mi S (2014) Orthostatic hypotension and mortality risk: A meta-analysis of cohort studies. Heart 100(5): 406-413.
- Barlow PA, Otahal P, Schultz MG, Shing CM and Sharman JE (2014) Low exercise blood pressure and risk of cardiovascular events and all-cause mortality: Systematic review and meta-analysis. Atherosclerosis 237(1): 13-22.
- Indumathi Muthuswamy (2024) Exploring heart rate variability in alcohol-dependent subjects using frequency-domain analysis. National Journal of Physiology Pharmacy and Pharmacology 14(07): 1424-1431.
- GBD 2020 Alcohol Collaborators (2022) Population-level risks of alcohol consumption by amount, geography, age, sex, and year: A systemic analysis for the global burden of disease 2020. The Lancet 400: 185-235.
- Pop GN, Christodorescu R, Velimirovici DE, Sosdean R, Corbu M, et al (2021). Assessment of the impact of alcohol consumption patterns on heart rate variability by machine learning in healthy young adults Medicina 57(9): 956.
- Vaschillo EG, Bates ME, Vaschillo B, Lehrer P, Udo T, et al (2008). Heart rate variability response to alcohol, placebo, and emotional picture cue challenges: Effects of 0.1 Hz stimulation. Psychophysiology 45(5): 847-858.
- Fisher VL and Tahrani AA (2017) Cardiac autonomic neuropathy in patients with diabetes mellitus: Current perspectives. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy 10: 419.
- Spallone V, Ziegler D, Freeman R, Bernardi L, Frontoni S, (2011) Cardiovascular autonomic neuropathy in diabetes: Clinical impact, assessment, diagnosis, and management. Diabetes/Metabolism Research and Reviews 27(7): 639-653.
- American Diabetes Association (2005) Diagnosis and classification of diabetes mellitus. Diabetes Care, 28(Suppl 1): S37-S42.
- Kaiser AB, Zhang N and Van Der Pluijm W (2018) Global prevalence of type 2 diabetes over the next ten years (2018-2028) Diabetes 67(Supplement_1): 202-LB.
- World Health Organization (2019) Classification of diabetes mellitus.
- Pinhas Hamiel O and Zeitler P (2007). Acute and chronic complications of type 2 diabetes mellitus in children and adolescents. The Lancet 369(9575): 1823-1831.
- Chawla A, Chawla R and Jaggi S. (2016). Microvascular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian Journal of Endocrinology and Metabolism 20(4): 546.
- Vinik AI and Ziegler D (2007). Diabetic cardiovascular autonomic neuropathy. Circulation 115(3): 387-397.
- Spallone V (2019). Update on the impact, diagnosis and management of cardiovascular autonomic neuropathy in diabetes: What is defined, what is new, and what is unmet. Diabetes & Metabolism Journal 43(1): 3-30.
- Dimitropoulos G, Tahrani AA and Stevens MJ (2014). Cardiac autonomic neuropathy in patients with diabetes mellitus. World Journal of Diabetes 5(1): 17.
- Clemente de Souza Pereira Rolim L, de Sá JR, Chacra AR and Dib SA (2008). Diabetic cardiovascular autonomic neuropathy: Risk factors, clinical impact and early diagnosis. Arquivos Brasileiros de Cardiologia, 90(4): 23-31.
- Malik M (1996). Heart rate variability: Standards of measurement, physiological interpretation, and clinical use: Task force of the European Society of Cardiology and the North American Society for Pacing and Electrophysiology. Annals of Noninvasive Electrocardiology 1(2): 151-181.
- Cha SA, Park YM, Yun JS, Lee SH, Ahn YB et al (2018). Time- and frequency-domain measures of heart rate variability predict cardiovascular outcome in patients with type 2 diabetes. Diabetes Research and Clinical Practice 143: 159-169.
- Bellavere F, Balzani I, DeMasi G, Carraro M, Carenza P, et al (1991) Power spectral analysis of heart rate variations improves assessment of diabetic cardiac autonomic neuropathy. Diabetes 41: 633-640.
- Twombly R (2005). Cancer surpasses heart disease as leading cause of death for all but the very elderly. Journal of the National Cancer Institute 97(5): 330-333.
- Kloter E, Barrueto K, Klein SD, Scholkmann F and Wolf U (2018). Heart rate variability as a prognostic factor for cancer survival—A systematic review. Frontiers in Physiology 9: 623.
- Escutia Reyes D, de Jesús Garduño García J, Emilio López Chávez G, Gómez Villanueva Á, Pliego Carrillo AC, et al (2021). Differences in heart rate variability and body composition in breast cancer survivors and women without cancer. Scientific Reports 11(1): 14460.
- Guo Y, Palmer JL, Strasser F, Yusuf SW and Bruera E (2013). Heart rate variability as a measure of autonomic dysfunction in men with advanced cancer. European Journal of Cancer Care 22(5): 612-616.
- Bijoor SN, NK S and Banerjee S (2015) Cardiac autonomic modulation in cancer patients as assessed by time domain measures of heart rate variability. International Journal of Health Sciences & Research 5(2): 194-198.
- Hansen SW (1994). Autonomic neuropathy after treatment with ciplastin, vinblastine and bleomycin for germ cell cancer. British Medical Journal 300(6723): 511-512.
- Bruera E, Chadwick S, Fox R, Hanson J and MacDonald N (1986) Study of cardiovascular autonomic insufficiency in advanced cancer patients. Cancer Treatment Reports 70(12); 1383-1387.
- Walsh D and Nelson K (2002). Autonomic nervous system dysfunction in advanced cancer. Supportive Care in Cancer 10(7): 523-528.
- Gould G, Ashworth M and Lewis G (1986) Are cardiovascular reflexes more commonly impaired in patients with bronchial carcinoma? Thorax 41(5): 372-375.
- Strasser F, Palmer JL, Schover LR, Yusuf SW, Pisters K, et al (2006) The impact of hypogonadism and autonomic dysfunction on fatigue, emotional function, and sexual desire in male patients with advanced cancer: A pilot study Cancer 107(12): 2949-2957.
- Roca E, Bruera E, Politi PM, Barugel M, Cedaro L, et al (1985). Vinca alkaloid-induced cardiovascular autonomic neuropathy. Cancer Treatment Reports 69(2): 149-151.
- Verstappen CCP, Heimans JJ, Hoekman K and Postma TJ (2003). Neurotoxic complications of chemotherapy in patients with cancer: Clinical signs and optimal management. Drugs 63(15): 1549-1563.
- Stone CA, Kenny RA, Nolan B and Lawlor PG (2012). Autonomic dysfunction in patients with advanced cancer: Prevalence, clinical correlates and challenges in assessment. BMC Palliative Care 11: 3.
- Kim D, Choi YS, Kim JA, Kim S and Kim DG (2007) Heart rate variability for prediction of life span in hospice cancer patients. In 10th Congress of the European Association for Palliative Care: Budapest Hungary 25(8): 1140-1145.
- Fadul N, Strasser F, Palmer JL, Yusuf SW, Guo Y, et al (2010). The association between autonomic dysfunction and survival in male patients with advanced cancer: A preliminary report. Journal of Pain and Symptom Management 39(2): 283-290.
- Chiang JK, Koo M, Kuo TBJ and Fu CH (2010) Association between cardiovascular autonomic functions and time to death in patients with terminal hepatocellular carcinoma. Journal of Pain and Symptom Management 39(4): 673-679.
- De Couck M and Gidron Y (2013). Norms of vagal nerve activity, indexed by heart rate variability, in cancer patients. Cancer Epidemiology 37(5): 737-741.
- Zhang J (2007) Effect of age and sex on heart rate variability in healthy subjects. Journal of Manipulative and Physiological Therapeutics 30(5): 374-379.
- Saleem S, Hussain MM, Majeed SMI and Khan MA (2012). Gender differences of heart rate variability in healthy volunteers. JPMA. The Journal of the Pakistan Medical Association 62(5): 422-425.
- Benichou T, Pereira B, Mermillod M, Tauveron I, Pfabigan D, et al (2018). Heart rate variability in type 2 diabetes mellitus: A systematic review and meta-analysis PLOS ONE 13(4): e0195166.
- Young AT, Danev S and Lakey JRT (2024) Construction and validation of a new BrainView qEEG discriminant database. Acta Scientific Neurology 7(6): 742.
- Novak P (2011) Quantitative autonomic testing. Journal of Visualized Experiments (53): e2502.
- Low PA, Denq JC, Opfer Gehrking TL, Dyck PJ, O Brien PC, et al (1997) Effect of age and gender on sudomotor and cardiovagal function and blood pressure response to tilt in normal subjects. Muscle & Nerve, 20: 1561-1568.
- Ajdaraga E and Gusev M (2017). Analysis of sampling frequency and resolution in ECG signals. In Proceedings of the 2017 25th Telecommunication Forum (TELFOR), Belgrade Serbia 21-22.
- Shaffer F and Ginsberg JP (2017). An overview of heart rate variability metrics and norms. Frontiers in Public Health, 5: 258.
- Shaffer F, McCraty R and Zerr CL (2014) A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Frontiers in Psychology 5: 1040.
- Guidelines American TN Guidelines (1996). Guidelines heart rate variability. European Heart Journal 17: 354-381.
- Tarvainen MP, Lipponen J, Niskanen JP, Ranta Aho P (2017). Kubios HRV Version 3 - User’s Guide. Kuopio: University of Eastern Finland.
- Ernst G (2017) Hidden signals - The history and methods of heart rate variability. Frontiers in Public Health16(5): 265.
- Parashar R, Amir M, Pakhare A, Rathi P and Chaudhary L (2016). Age related changes in autonomic functions. Journal of Clinical and Diagnostic Research 10(3): CC11-CC15.
- Jha RK, Acharya A and Nepal O (2018). Autonomic influence on heart rate for deep breathing and Valsalva maneuver in healthy subjects. JNMA J Nepal Med Assoc 56(211): 670-673.
- Umetani K, Singer DH, McCraty R and Atkinson M (1998). Twenty-four hour time domain heart rate variability and heart rate: Relations to age and gender over nine decades. Journal of the American College of Cardiology 31(3): 593-601.
- Miranda LS, Stein PK, Imamura F, Sattelmair J, Lemaitre RN et al (2012). Trans-Fatty Acid consumption and heart rate variability in 2 separate cohorts of older and younger adults. Circulation Arrhythmia and Electrophysiology 5(4): 728-738.
- Reardon M and Malik M (1996). Changes in heart rate variability with age. Pacing and Clinical Electrophysiology 19(11 Pt 2): 1863-1866.
- Mendonca GV, Heffernan KS, Rossow L, Guerra M, Pereira FD, et al (2010). Sex differences in linear and nonlinear heart rate variability during early recovery from supramaximal exercise. Applied Physiology Nutrition, and Metabolism 35(4): 439-446.
- Dietrich DF, Schindler CH, Schwartz J, Barthélémy JC, Tschopp JM, et al (2006) Heart rate variability in an nsageing population and its association with lifestyle and cardiovascular risk factors: Results of the SAPALDIA study Europace 8(7): 521-529.
- Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, et al (1986). Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog Circulation Research 59(2): 178-193.
- Chu Duc H, Nguyen Phan K and Nguyen Viet D (2013). A review of heart rate variability and its applications. APCBEE Procedia 7: 80-85.
- Valenza G, Citi L, Saul JP and Barbieri R (2018). Measures of sympathetic and parasympathetic autonomic outflow from heartbeat dynamics. Journal of Applied Physiology 125(1): 19-39.
- Billman GE (2013). The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Frontiers in Physiology 4: 26.
- Pagani M, Lombardi F, Guzzetti S, Sandrone G, Rimoldi O, Malfatto G, (1984) Power spectral density of heart rate variability as an index of sympatho-vagal interaction in normal and hypertensive subjects. Journal of Hypertension 2: S383-S385.
- Kappus RM, Ranadive SM, Yan H, Lane Cordova AD, Cook MD, et al (2015). Sex differences in autonomic function following maximal exercise. Biology of Sex Differences 6: 28.
- Ashok C, Anandhi D, Jayakumar B and Jawahar V (2022). Heart rate variability among gym-goers and age-matched sedentary individuals. Bulletin of Faculty of Physical Therapy 27: 53.
- Selye H (1950) Stress and the general adaptation syndrome. British Medical Journal 1: 1383-1392.
- Baron R and Ewing DJ (1999) Heart rate variability. In G. Deuschl & A. Eisen (Eds.), Recommendations for the practice of clinical neurophysiology: Guidelines of the International Federation of Clinical Physiology 283-286.
- Risk M, Bril V, Broadbridge C and Cohen A (2001). Heart rate variability measurement in diabetic neuropathy: Review of methods. Diabetes Technology & Therapeutics 3(1): 63-76.
- Sundkvist G, Almer L and Lilja B (1979). Respiratory influence on heart rate in diabetes mellitus. British Medical Journal 1: 924-925.
- Hilz MJ and Dütsch M (2006). Quantitative studies of autonomic function. Muscle & Nerve 33(1): 6-20.
- Smith SA (1982) Reduced sinus arrhythmia in diabetic autonomic neuropathy: Diagnostic value of an age-related normal range. British Medical Journal 285: 1599-1601.
- Manandhar SA and Pramanik T (2019). Immediate effect of slow deep breathing exercise on blood pressure and reaction time. Mymensingh Medical Journal 28(4): 925-929.
- Garg P, Mendiratta A, Banga A, Bucharles A, Victoria P, et al (2024) Effect of breathing exercises on blood pressure and heart rate: A systematic review and meta-analysis. International Journal of Cardiology and Cardiovascular Risk Prevention 20: 200232.
- Bernardi L, Porta C, Spicuzza L, Bellwon J, Spadacini G, et al (2002). Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation 105(2): 143-145.
- Nunan D, Sandercock GRH and Brodie DA (2010). A quantitative systematic review of normal values for short-term heart rate variability in healthy adults. Pacing and Clinical Electrophysiology 33(11): 1407-1417.
- Laborde S, Mosley E and Thayer JF (2017) Heart rate variability and cardiac vagal tone in psychophysiological research - Recommendations for experiment planning, data analysis, and data reporting. Frontiers in Psychology 8: 213.
- Saul JP, Albrecht P, Berger RD and Cohen RJ (1988). Analysis of long-term heart rate variability: Methods, 1/f scaling and implications. Computers in Cardiology 14: 419-422.
- Kuusela T (2013) Methodological aspects of heart rate variability analysis. In M. V. Kamath, M. A. Watanabe, & A. R. M. Upton (Eds.), Heart Rate Variability (HRV) Signal Analysis 9-42.
- Jeyhani V, Mahdiani S, Peltokangas M and Vehkaja A (2015). Comparison of HRV parameters derived from photoplethysmography and electrocardiography signals. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society 5952-5955.
- Peltola MA (2012). Role of editing of R-R intervals in the analysis of heart rate variability. Frontiers in Physiology 3: 148.
- Shaffer F and Combatalade DC (2013) Don’t add or miss a beat: a guide to cleaner heart rate variability recordings. Biofeedback 41: 121-30.
- Merri M, Farden DC, Mottley JG and Titlebaum EL (1990). Sampling frequency of the electrocardiogram for spectral analysis of heart rate variability. IEEE Transactions on Biomedical Engineering, 37: 99-106.
- Meehan Z, Muesenfechter N, Gravett N, Watson T, Smith A, et al. (Forthcoming). Breathing effort may not reduce heart rate variability when respiration rate is controlled [Abstract]. Applied Psychophysiology and Biofeedback.
- Meehan Z, Muesenfechter N, Gravett N, Watson T, Smith A, et al. (Forthcoming). A 1:2 inhalation-to-exhalation ratio does not increase heart rate variability during 6-bpm breathing [Abstract]. Applied Psychophysiology and Biofeedback.
- Hirsch JA and Bishop B (1981). Respiratory sinus arrhythmia in humans: How breathing pattern modulates heart rate. American Journal of Physiology 241(4): H620-H629.
- Serhiyenko VA and Serhiyenko AA (2018). Cardiac autonomic neuropathy: Risk factors, diagnosis and treatment. World Journal of Diabetes 9(1): 1.
- Bonnemeier H, Richardt G, Potratz J, Wiegand UK, Brandes A, et al (2003). Circadian profile of cardiac autonomic nervous modulation in healthy subjects: Differing effects of aging and gender on heart rate variability. Journal of Cardiovascular Electrophysiology 14(7): 791-799.
- Almeida-Santos MA, Barreto-Filho JA, Oliveira JL, Reis FP, da Cunha Oliveira CC, et al (2016). Aging, heart rate variability and patterns of autonomic regulation of the heart. Archives of Gerontology and Geriatrics 63: 1-8.
- Koenig J and Thayer JF (2016). Sex differences in healthy human heart rate variability: A meta-analysis. Neuroscience & Biobehavioral Reviews 64: 288-310.
- Zhang D, Shen X and Qi X (2016). Resting heart rate and all-cause and cardiovascular mortality in the general population: A meta-analysis. Canadian Medical Association Journal 188(3): E53-E63.
- De Meersman RE (1993). Heart rate variability and aerobic fitness. American Heart Journal 125(3): 726-731.
- Aubert AE, Seps B and Beckers F (2003). Heart rate variability in athletes. Sports Medicine 33(12): 889-919.
- Bigger JT, Fleiss JL, Steinman RC, Rolnitzky LM, Schneider WJ, et al (1995). RR variability in healthy, middle-aged persons compared with patients with chronic coronary heart disease or recent acute myocardial infarction. Circulation 91(7): 1936-1943.
- Agelink M, Boz C, Ullrich H and Andrich J (2002). Relationship between major depression and heart rate variability: Clinical consequences and implications for antidepressive treatment. Psychiatry Research 113(2): 139-149.
- Ziegler D, Dannehl K, Muhlen H, Spuler M and Gries FA (1992). Prevalence of cardiovascular autonomic dysfunction assessed by spectral analysis, vector analysis, and standard tests of heart rate variation and blood pressure responses at various stages of diabetic neuropathy. Diabetic Medicine 9(9): 806-814.
- Vinik AI, Erbas T and Casellini CM (2013). Diabetic cardiac autonomic neuropathy, inflammation and cardiovascular disease. Journal of Diabetes Investigation 4(1): 4-18.
- Agashe S and Petak S (2018). Cardiac autonomic neuropathy in diabetes mellitus. Methodist DeBakey Cardiovascular Journal 14(4): 251.
- Trivedi GY, Saboo B, Singh RB, Maheshwari A, Sharma K, et al (2019). Can decreased heart rate variability be a marker of autonomic dysfunction, metabolic syndrome and diabetes? Journal of Diabetology 10(2): 48.
- Rothberg LJ, Lees T, Clifton Bligh R and Lal S (2016). Association between heart rate variability measures and blood glucose levels: Implications for noninvasive glucose monitoring for diabetes. Diabetes Technology & Therapeutics 18: 366-376.
- Gerritsen J, Dekker JM, TenVoorde BJ, Kostense PJ, Heine RJ, et al. (2001). Impaired autonomic function is associated with increased mortality, especially in subjects with diabetes, hypertension, or a history of cardiovascular disease: The Hoorn study. Diabetes Care 24(10): 1793-1798.
- Hufnagel C, Chambres P, Bertrand PR and Dutheil F (2017). The need for objective measures of stress in autism. Frontiers in Psychology 8: 64.
- Boudet G, Walther G, Courteix D, Obert P, Lesourd B, et al (2017). Paradoxical dissociation between heart rate and heart rate variability following different modalities of exercise in individuals with metabolic syndrome: The RESOLVE study. European Journal of Preventive Cardiology 24(3): 281-296.
- Solanki JD, Basida SD, Mehta HB, Panjwani SJ and Gadhavi BP (2016). Comparative study of cardiac autonomic status by heart rate variability between under-treatment normotensive and hypertensive known type 2 diabetics. Indian Heart Journal. Advance online publication 69(1): 52-56.
- Lotric MB, Stefanovska A, Stajer D and Urbancic Rovan V (2000) Spectral components of heart rate variability determined by wavelet analysis. Physiological Measurement 21(4): 441-457.
- Roy B and Ghatak S (2013). Nonlinear methods to assess changes in heart rate variability in type 2 diabetic patients. Arquivos Brasileiros de Cardiologia 101(4): 317-327.
- Goit RK, Khadka R, Sharma SK, Limbu N and Paudel BH (2012). Cardiovascular autonomic function and vibration perception threshold in type 2 diabetes mellitus. Journal of Diabetes and its Complications 26(4): 339-342.
- Coopmans C, van der Heijden AA, Rutten GEHM, Koster A, Savelberg HHCM, et al (2020). Both prediabetes and type 2 diabetes are associated with lower heart rate variability: The Maastricht study. Diabetes Care 43(5): 1126-1133.
- Hadad R, Akobe SF, Weber P, Madsen CV, Larsen BS, et al (2022). Parasympathetic tonus in type 2 diabetes and pre-diabetes and its clinical implications. Scientific Reports 12: 18020.
- Geijselaers SLC, Sep SJS, Stehouwer CDA, Biessels GJ, Biessels GJ, et al (2017). The role of hyperglycemia, insulin resistance, and blood pressure in diabetes-associated differences in cognitive performance—The Maastricht study. Diabetes Care 40(11): 1537-1547.
- Andersson J (2005) The inflammatory reflex—Introduction. Journal of Internal Medicine 257(2): 122-125.
- Kannel WB, Kannel C, Paffenbarger RS and Cupples LA (1987). Heart rate and cardiovascular mortality: The Framingham study. American Heart Journal 113(6): 1489-1494.
- Pavithran P, Madanmohan T and Nandeesha H (2008). Sex differences in short-term heart rate variability in patients with newly diagnosed essential hypertension. Journal of Clinical Hypertension 10(12): 904-910.
- Singh JP, Larson MG, O Donnell CJ, Wilson PF, Tsuji H, et al (2000). Association of hyperglycemia with reduced heart rate variability (The Framingham Heart Study). The American Journal of Cardiology 86(3): 309-312.
- Breder IS and Sposito AC (2019). Cardiovascular autonomic neuropathy in type 2 diabetic patients. Revista da Associação Médica Brasileira 65(1): 56-60.
- Studer V, Rocchi C, Motta C, Lauretti B, Perugini J, et al (2017). Heart rate variability is differentially altered in multiple sclerosis: Implications for acute, worsening and progressive disability. Multiple Sclerosis Journal—Experimental, Translational and Clinical 3: 2055217317701317.
- Fagundes CP, Murray DM, Hwang BS, Gouin JP, Thayer JF, et al (2011). Sympathetic and parasympathetic activity in cancer-related fatigue: More evidence for a physiological substrate in cancer survivors. Psychoneuroendocrinology 36(8): 1137-1147.
- Hayano J, Sakakibara Y and Yamada A (1991). Accuracy of assessment of cardiac vagal tone by heart rate variability in normal subjects. American Journal of Cardiology 67(2): 199-204.
- Subbalakshmi NK, Adhikari PMR and Sathyanarayana Rao KN (2012). Deterioration of cardiac autonomic function over a period of one year in relation to cardiovascular and somatic neuropathy complications in type 2 diabetes mellitus. Diabetes Research and Clinical Practice 97(2): 313-321.
- Kenneth C and Bilchik Ronald D (2006). Heart rate variability. Journal of Cardiovascular Electrophysiology 17: 691-694.
- Bijoor SN, NK S and Banerjee S (2015). Cardiac autonomic modulation in cancer patients as assessed by time domain measures of heart rate variability. International Journal of Health Sciences & Research 5(2): 194-198.
- Ben David K, Wittels HL, Wishon MJ, Lee SJ, McDonald SM, et al (2024). Tracking cancer: Exploring heart rate variability patterns by cancer location and progression. Cancers (Basel) 16(5): 962.
- Barzi A, Lenz HJ, Quinn DI and Sadeghi S (2017). Comparative effectiveness of screening strategies for colorectal cancer. Cancer 123: 1516-1527.
- Loud JT and Murphy J (2017). Cancer screening and early detection in the 21st century. Seminars in Oncology Nursing 33: 121-128.
- Philipson TJ, Durie T, Cong Z and Fendrick AM (2023). The aggregate value of cancer screenings in the United States: Full potential value and value considering adherence. BMC Health Services Research 23: 829.
- Alexandraki I and Mooradian AD (2010). Barriers related to mammography use for breast cancer screening among minority women. Journal of the National Medical Association 102: 206-218.
- Bernstein E, Bade BC, Akgün KM, Rose MG and Cain HC (2022). Barriers and facilitators to lung cancer screening and follow-up. Seminars in Oncology 49: 213-219.
- Muthukrishnan M, Arnold LD and James AS (2019). Patients’ self-reported barriers to colon cancer screening in Federally Qualified Health Center settings. Preventive Medicine Reports 15: 100896.
- Nagelhout E, Comarell K, Samadder NJ and Wu YP (2017). Barriers to colorectal cancer screening in a racially diverse population served by a safety-net clinic. Journal of Community Health 42(4): 791-796.
- Hu S, Lou J, Zhang Y and Chen P (2018). Low heart rate variability relates to the progression of gastric cancer. World Journal of Surgical Oncology 16(1): 49.
- McGovern J, Leadbitter S, Miller G, Hounat A, Kamande I, et al (2023). The relationship between heart rate variability and TNM stage, co-morbidity, systemic inflammation and survival in patients with primary operable colorectal cancer. Scientific Reports 13(1): 8157.
- Wu S, Chen M, Wang J, Shi B and Zhou Y (2021). Association of short-term heart rate variability with breast tumor stage. Frontiers in Physiology 12: 678428.
- Frye JN, Sutterfield SL, Caldwell JT, Behnke BJ, Copp SW, et al (2018). Vascular and autonomic changes in adult cancer patients receiving anticancer chemotherapy. Journal of Applied Physiology 125(1): 198-204.
- Torres Juárez KV, Queiroga FL and Romero Romero LP (2022). The Nervous System as a Regulator of Cancer Hallmarks: Insights into Therapeutic Implications. Cancers 14(18): 4372.
- Zhou X, Ma Z, Zhang L, Zhou S, Wang J, et al (2016). Heart rate variability in the prediction of survival in patients with cancer: A systematic review and meta-analysis. Journal of Psychosomatic Research 89: 20-25.
- Kim DH, Kim JA, Choi YS, Kim SH, Lee JY et al (2010). Heart rate variability and length of survival in hospice cancer patients. Journal of Korean Medical Science 25(8): 1140-1145.
- Mouton C, Ronson A, Razavi D, Delhaye F, Kupper N, et al (2012) The relationship between heart rate variability and time-course of carcinoembryonic antigen in colorectal cancer. Autonomic Neuroscience: Basic & Clinical 166(1-2): 96-99.
- Magnon C (2015). Role of the autonomic nervous system in tumorigenesis and metastasis. Molecular & Cellular Oncology 2(2): e975643.
- Horvathova L and Mravec B (2016). Effect of the autonomic nervous system on cancer progression depends on the type of tumor: Solid are more affected than ascitic tumors. Endocrine Regulations 50(4): 215-224.
- Hirvonen HE, Salmi TT, Heinonen E, Antila KJ and Valimaki IA (1989). Vincristine treatment of acute lymphoblastic leukemia induces transient autonomic cardioneuropathy. Cancer 64(4): 801-805.
- Hrushesky WJ, Fader DJ, Berestka JS, Sommer M, Hayes J et al (1991) Diminishment of respiratory sinus arrhythmia foreshadows doxorubicin-induced cardiomyopathy. Circulation 84(2): 697-707.
- Hansen SW (1990) Autonomic neuropathy after treatment with cisplatin, vinblastine, and bleomycin for germ cell cancer. British Medical Journal 300(6723): 511-512.
- Turner ML, Boland OM, Parker AC and Ewing DJ (1993) Subclinical autonomic dysfunction in patients with Hodgkin's disease and non-Hodgkin's lymphoma. British Journal of Haematology 84(4): 623-626.
- Ekholm E, Rantanen V, Syvänen K, Jalonen J, Antila K, (2002). Docetaxel does not impair cardiac autonomic function in breast cancer patients previously treated with anthracyclines. Anticancer Drugs 13(4): 425-429.
- Ekholm E, Salminen E, Huikuri H, Jalonen J, Antila K, et al (2000). Impairment of heart rate variability during paclitaxel therapy. Cancer 88(9): 2149-2153.
- Adams SC, Schondorf R, Benoit J and Kilgour RD (2015) Impact of cancer and chemotherapy on autonomic nervous system function and cardiovascular reactivity in young adults with cancer: A case-controlled feasibility study. BMC Cancer 15: 414.
- Ekholm E, Rantanen V, Antila K and Salminen E (1997) Paclitaxel changes sympathetic control of blood pressure. European Journal of Cancer 33(9): 1419-1424.
- Jerian SM, Sarosy GA, Link CJ, Fingert HJ, Reed E et al (1993) Incapacitating autonomic neuropathy precipitated by taxol. Gynecologic Oncology 51(2): 277-280.
- Ekholm E, Rantanen V, Bergman M, Vesalainen R, Antila K, et al (1999) Docetaxel and autonomic cardiovascular control in anthracycline treated breast cancer patients. Anticancer Research 20(3B): 2045-2048.
- Quasthoff S and Hartung HP (2002). Chemotherapy-induced peripheral neuropathy. Journal of Neurology 249(1): 9-17.
- Rosenwinkel ET, Bloomfield DM, Arwady MA and Goldsmith RL (2001) Exercise and autonomic function in health and cardiovascular disease. Cardiology Clinics 19(3): 369-387.
- Thayer JF and Lane RD (2007) The role of vagal function in the risk for cardiovascular disease and mortality. Biological Psychology 74(2): 224-242.
- De Couck M, Mravec B and Gidron Y (2012) You may need the vagus nerve to understand pathophysiology and to treat diseases. Clinical Science 122: 323-328.
- Chen JL, Yeh DP, Lee JP, Chen CY, Huang CY, et al (2011) Parasympathetic nervous activity mirrors recovery status in weightlifting performance after training. Journal of Strength and Conditioning Research 25(6): 1546-1552.
- Teisala T, Mutikainen S, Tolvanen A, Rottensteiner M, Leskinen T, et al (2014) Associations of physical activity, fitness, and body composition with heart rate variability-based indicators of stress and recovery on workdays: A cross-sectional study. Journal of Occupational Medicine and Toxicology 9(1): 16.
- Kim K, Chae J and Lee S (2015) The role of heart rate variability in advanced non-small-cell lung cancer patients. Journal of Palliative Care 31(2): 103-108.
- Low PA (2008) Clinical autonomic disorders (3rd ed.) Lippincott Raven.
- Berntson GG, Norman GJ, Hawkley LC and Cacioppo JT (2008) Cardiac autonomic balance versus cardiac regulatory capacity. Psychophysiology 45: 643-652.
- Sadock BJ, Sadock VA and Ruiz P (2017) Kaplan and Sadock's Comprehensive Textbook of Psychiatry (18th ed.). Lippincott Williams and Wilkins.
- Penzlin AI, Siepmann T, Illigens BM, Weidner K and Siepmann M (2015) Heart rate variability biofeedback in patients with alcohol dependence: A randomized controlled study. Neuropsychiatric Disease and Treatment 11: 2619-2627.
- Rajan I, Murthy PJ, Ramakrishnan AG, Gangadhar BN and Janakiramaiah N (1998) Heart rate variability as an index of cue reactivity in alcoholics. Biological Psychiatry 43(7): 544-546.
- Sehested J, Heringlake M and Schmidt V (1998) Neurohumoral cardiovascular responses to alcohol and their modulation by peroral fluid. American Journal of Cardiology 81(6): 761-765.
- Romanowicz M, Schmidt JE, Bostwick JM, Mrazek DA and Karpyak VM (2011). Changes in heart rate variability associated with acute alcohol consumption: Current knowledge and implications for practice and research. Alcoholism: Clinical and Experimental Research 35(6): 1092-1105.
- Sufke S, Fielder S, Djonlagic H and Kibbel T (2009) Continuous analysis of heart rate variability for examination of cardiac autonomic nervous system after alcohol intoxication. Medizinische Klinik (Munich) 104(7): 511-519.
- Kumar DM, Kumar SC, Sudarshan B, Yadhuraj SR and Janakiramaiah N (2014) HRV analysis for the study of neurological and cardiac effects of alcohol on youth of India. International Journal of Computer Science Engineering and Information Technology Research 4: 117-124.
- Jansky I, Ericson M, Blom M, Georgia’s A, Magnusson JO, et al (2005) Wine drinking is associated with increased heart rate variability in women with coronary heart disease. Heart 91(3): 314-318.

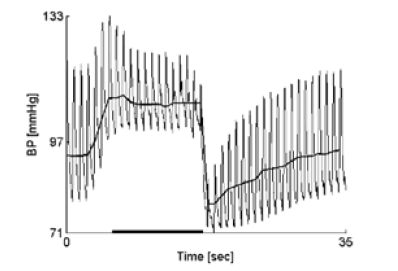


 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.