Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Intestinal Parasites and Irritable Bowel Syndrome: A Double Jeopardy
*Corresponding author: Raúl Argüello-García, Department of Genetics and Molecular Biology, Center for Research and Advanced Studies of the National Polytechnic Institute, Mexico City, Mexico.
Received: July 09, 2024; Published: July 16, 2024
DOI: 10.34297/AJBSR.2024.23.003060
Abstract
Irritable Bowel Syndrome (IBS) is a gastrointestinal disorder of growing incidence causing abdominal pain, low grade inflammation, diarrhea, constipation, altered bowel habits often associated to anxiety and depression with multifactorial etiology, in which intestinal parasitic infections and concomitant changes in microbiota are included. The four most prevalent protists (Giardia, Cryptosporidium, Blastocystis and Entamoeba) have been associated to IBS cases while helminths are scarcely related. However Post-Infectious IBS (PI-IBS) is gaining attention and besides the mounting evidence of its association with these protists, particularly Giardia, experimental models in mice infected by helminths as Trichuris muris and Trichinella spiralis reproduce the most important features of clinical IBS and even psychological stress can trigger PI-IBS in rats. This review emphasizes the main steps in pathogeny of parasite-derived IBS and poses a landscape profiling therapeutic optimization strategies intervening the enteric system, the gut-brain axis and very likely the endocannabinoid system.
Keywords: Irritable bowel syndrome, Intestinal protozoa, Intestinal helminths, Pathogeny, Microbiota
Irritable Bowel Syndrome: Prevalence, Control and Etiology
After a first case description on 1820 and conceptual introduction by 1944, the Irritable Bowel Syndrome (IBS) has attracted attention in the last decades not only by its complex nature but by its sequelae on patient´s quality of life and significant healthcare costs. IBS is defined as a functional gastrointestinal disorder involving abdominal discomfort with altered bowel habits and defecation along to disturbed brain-gut axis functions. This entity is prevalent in rates ranging from 1.1-45.0% (mean: 11.2%) worldwide with higher rates at South America and lower at Southeast Asia, with women having slightly higher global prevalence (12%) than men (8.6%) [1]. Primary symptoms include abdominal pain with frequent diarrhea and constipation, tenesmus, bloating, recurrent gastroesophageal reflux and chronic fatigue syndrome. Psychiatric signs mainly consist of depression, anxiety and sleep disorders [2]. At the current time, IBS control relies on diagnosis and treatment while prophylactic measures are virtually not established yet. Detecting IBS has not been easily standardized but the Rome classification (I-IV, 1989 through 2017) of fecal depositions is the most accepted criterion to assist IBS diagnosis [3]. As per Rome IV criteria, patient must report abdominal pain coupled to changed bowel habits one or more days a week for at least 3 months and cases fall within 4 subtypes: diarrhea-predominant (IBS-D), constipation-predominant (IBS-C), mixed (IBS-M) and unclassified (IBS-U) (Figure 1).
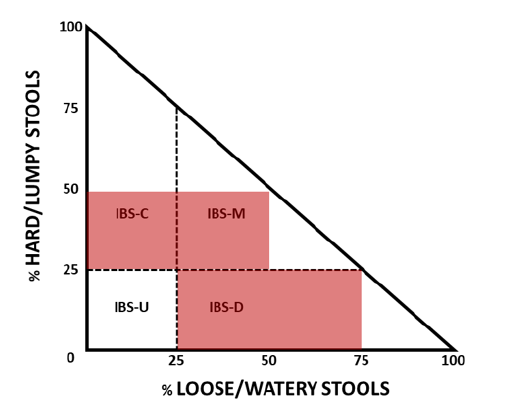
Figure 1: Two-dimensional diagram of the main IBS subtypes and PI-IBS. IBS-C: IBS with constipation; IBS-D: IBS with diarrhea; IBS-M: IBS with diarrhea/constipation; IBS-U, unclassified IBS; PI-IBS: post-infection IBS. The zone marked in light red corresponds to PI-IBS where IBS-D prevails. Adapted from Longstreth GF, et al., (2006): Functional Bowel Diseases, Gastroenterology 130: 1482.
Nevertheless, a patient could move amongst these subtypes indeed requiring a change in treatment strategy [4]. Therapy for IBS targets symptoms and commonly includes medication (e.g. dexamethasone, pinaverium bromide-dimeticone, loperamide, tricyclic antidepressants, serotonin transporter inhibitors), dietary measures (e.g. low diet in Fermentable Oligo-, Di-and Monosaccharides and Polyols [FODMAP]), probiotics, Fecal Microbial Transplantation (FMT), antibacterial overgrowth agents as Rifaximin as well as psychotherapy including hypnosis [5,6]. The precise etiology of IBS remains still unclear because genetic, environmental and psychosocial factors have been related to its appearance and symptom maintenance [7]. However, there is growing consensus on a relevant role for brain-gut axis and particularly neuro-immune disturbances. Recent studies suggest that allergy episode(s) that activate peripheral immune responses may underline the onset of abdominal pain in IBS patients [8]. Importantly, serotonin imbalances and allergy to food or microbial components are contributing factors to IBS induction. On a hand, serotonin (5-hydroxytryptamine, 5-HT) production is influenced by gut microbiome and besides regulating emotions, mood and even pain sensitivity through its receptor, it modulates gut peristalsis hence its altered levels lead to gut dysfunction and symptoms as visceral hypersensitivity, diarrhea and constipation [9]. On the other, the increased permeability at intestinal barrier associated with IBS (with some genetic association to IBS-D) may promote reactions to toxins, microbial excretory/secretory products or food allergens gaining access from lumen to mucosal intestinal compartments thus interacting with lymphocytes and mast cells [10,11]. In fact, tenapanor, an inhibitor of Sodium-Proton exchanger NHE3 that promotes permeability restoration is currently approved for use in IBS-C [12]. In line with these facts, serotonin produced in the gut by Enterochromaffin Cells (ECCs) is a well-known general regulator of membrane permeability [13]. Within studies needed in IBS diagnosis are patient history, abdominal and rectal examination along to laboratory tests to discard other conditions such as anemia, Inflammatory Bowel Disease (IBD), Celiac Disease (CD) and colorectal cancer [14].
However, a significant number of cases are preceded by episodes of acute infectious gastroenteritis; in fact, there is a six-fold increase of risk to develop IBS after a gastrointestinal infection [15]. Here, a bidirectional gut-brain-microbiome bidirectional axis takes place as viable mechanism. Intestinal microbiome includes several communities of prokaryotic (bacteria and archaea) and eukaryotic (fungi, parasites) microorganisms and viruses as well, which have dynamic interactions that under certain contexts may change not only each other´s pathogenicity but change bowel homeostasis. When intestinal parasites (protozoa, helminths) are concerned, IBS onset may occur after infection has been successfully treated; this subtype is known as Post-Infectious IBS (PI-IBS). The first report of PI-IBS corresponds to abdominal disturbances after acute amoebic disentery [16] and it has been estimated that this subtype accounts for 6-50% % of all IBS cases, mostly derived from traveller´s diarrhea or gastroenteritis outbreaks where IBS-D prevails and where parasitic infection even occurred several years ago [17,18]. As with other IBS subtypes, for PI-IBS pathophysiological factors and mechanisms have not been fully unveiled but it is acknowledged that parasites may elicit mechanisms affecting the brain-gut axis and promote persistent inflammation, malabsorption and even bacterial overgrowth at intestine [19,20]. Of note, parasitic protists of recognized medical and veterinary importance able to colonize small (Giardia, Cryptosporidium) and large (Entamoeba, Blastocystis) intestines may only cause infection but not disease in a subset of cases, the so-called asymptomatic carriers; likewise, helminthes being harmful at high burdens may be mutualists under other contexts [21,22]. In the next sections the parasitic factors and effects on gut homeostasis with a posible link to the onset of PI-IBS are addressed.
Pathogenic Role of Intestinal Protists in IBS
As compared to other pathogens, protist-caused enteritis displays the higher risk for developing PI-IBS [23]. At intestinal level, several traits of disruption of gut homeostasis are observed in IBS patients and are potentially related to intestinal parasites and other microbiome communities of which bacteria are the most extensively characterized [24]. First, parasites can secrete or export several kinds of molecules, from toxins to metabolites, that have a direct effect on host intestinal epithelium; secondly, the intestinal barrier permeability is increased in IBS with concomitant decreases in expression of cell junctional proteins as ZO-1 and even fecal supernatants from IBS-D patients induce higher paracellular permeability in mice models [25,26]. Thirdly, a low grade of immune activation is observed in significant proportions of IBS patients that exhibit increased numbers (i.e. hyperplasia) of mast cells and T lymphocytes as well as increased levels of proinflammatory cytokines as IL1β, IL-6, IL-8 and TNFα, and decreased levels of antiinflammatory ones as IL-10 [27-29]. Fourth, parasitic infection promotes visceral hypersensitivity than includes abdominal pain, a hallmark symptom in IBS spectrum; and fifth, parasites may induce changes in bacterial intestinal communities that might trend to the onset of IBS (Figure 2).
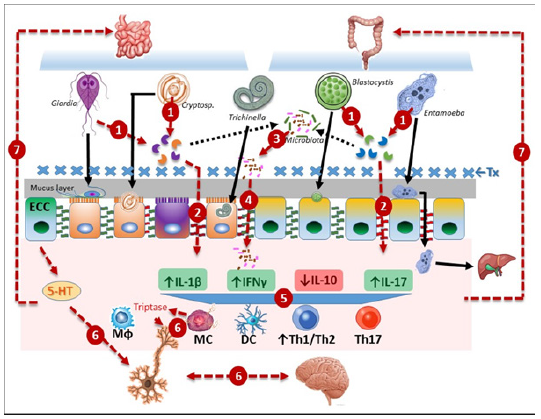
Figure 2: Steps involved in pathogeny of IBS/PI-IBS. The parasites more likely linked to IBS (genus indicated) upon interaction with intestinal epithelium secrete virulence factors exemplified by extracellular cysteine proteases [33] (1) that can disrupt intercellular junctions (tight, adherens) promoting increased intestinal permeability (2). Also, microbial dysbiosis (3) is produced by parasite-derived factors which allows bacterial translocation (4). Thereafter activation of the immune system occurs and pro-inflammatory cytokines that include, but are not limited to, IL-1β, IFNγ and IL-17 are up-regulated along to decreased levels of anti-inflammatory cytokines as IL-10 with concomitantly higher Th1/Th2 ratios and increased populations of Th17 cells, monocyte phagocytic cells (Mɸ) and Dendritic Cells (DC) and importantly Mast Cell (MC) hyperplasia and activation at the proximitiy of nerve terminals (5). Enterochromaffin Cells (ECC) have altered serotonin (5-HT) levels that combined to triptase release from MC and activity of the Protease-Activated Receptor 2 (PAR-2), produce neuronal activation and perturbations in the bidirectional brain-gut axis (6). This altered immuno-neural environment leads to intestinal dysfunction and hypersensitivity (7). In PI-IBS, antiparasitic Treatment (Tx) or immunological clearance of the pathogen is followed by the reactivation of a lowgrade inflammation in which remnant microbiota plays an important role, with further appearance of recurrent symptoms as abdominal pain, visceral hypersensitivity, altered gut habits and psychological imbalances.
Cryptosporidium sp
Cryptosporidium is a genus complex of apicomplexan alveolate protists causing cryptosporidiosis, this infection is initiated by the infective oocyst that releases sporozoites that upon infecting epithelial cells undergo several morphological changes including gametogony and thereafter it replicates within a parasitophorous vacuole at microvillous layer. Humans are mainly infected (90% cases) by zoonotic C. parvum and C. hominis species. Typical symptoms involve diarrhea, nausea, vomiting and malaise but in C. hominis infections, extraintestinal symptoms as joint pain, eye discomfort, fatigue and recurrent headaches may exist [30]. Further, a third of patients infected with C. hominis or C. parvum had persistent diarrhea and abdominal pain for 12 months [31]. In experimental murine models, C. parvum produces several IBS traits such as jejunal hypersensitivity, mast cell hyperplasia and intraepithelial lymphocye infiltration [32]. In these organisms none virulence factor has been characterized in depth nor explored in relation to IBS albeit the presence of the cryptopain family of cysteine proteases in C. parvum, particularly secreted cryptopains 4 and 5 [33], would shed important insights in future research. Otherwise, proteomics studies in the epithelial cell line HCT-8 infected with C. parvum showed a decreased expression of proteins involved in α-linoleic acid synthesis, a metabolite that alleviates IBS symptoms [34].
With regard to intestinal permeability, in neonatal calves infected with C. parvum an important increase in paracellular permeability has been reported [35] and this is associated with decreased presence of proteins from tight and adherens junctions [36]. Also, activation of the immune system by Cryptosporidium shares some features with IBS. For instance, increased levels of proinflammatory IFNγ, IL-12 and IL-18 have been observed in infected mice [37]; likewise, the same trend occurs with TLR4 and ICAM-1, two surface molecules increased in IBS patients but linked to limited inflammation [38]. In relation to jejunal hypersensitivity, it has been shown to be induced by C. parvum in rats along to abdominal distension that persisted up to 100 days after parasite clearance [39] and this could be associated to recruitment of activated mast cells [32]. Cryptosporidium infection concomitantly induces multiple changes in intestinal microbiota (dysbiosis). Among others, mice infected with Cryptosporidium spp. showed lower relative aundance of Firmicutes (Bacillota) and higher abundances of Bacteroidetes (Bacteroidota) [40]. Of interest, in IBS patients the disbalanced presence of enterotoxigenic Bacteroides fragilis promotes diarrhea and abdominal discomfort [24]. From these observations, the similarity in mechanisms of immune activation and microbiota changes are proposed to be a possible link between cryptosporidiosis and PI-IBS [41].
Giardia duodenalis
Giardia duodenalis (G. lamblia, G. intestinalis) is a microaerophilic flagellated protist causing giardiasis which is transmitted by the infective cyst stage while motile, pathogenic trophozoites attach and reproduce at the microvillus border of small intestine in humans and a wide variety of companion, livestock and wild host species. Common symptoms and signs are diarrhea, flatulence, abdominal cramps, bloating and intestinal malabsorption that led to weight loss. Symptomatic infections may be self-limiting within 2-3 months while chronic cases are long lasting (several years). This species is composed of eight genetic lineages (assemblages A-H) of which assemblages A and B infect humans. Lately or after infection, several sequelae may be observed and include muscle and/or eye alterations, skin allergy, arthritis, metabolic unbalances and impaired cognitive performance [42]. Based on experimental, clinical and epidemiological evidence, giardiasis is currently a recognized risk to develop PI-IBS. In experimental models, Giardia infection was shown to drive persistent tight junctional damage promoting bacterial penetration and mucosal inflammation that was present even after effective treatment [42,43]. Epidemiological data support this notion; for instance, in a follow-up study in a giardiasis outbreak where infected and treated individuals were included, 46.1% presented PI-IBS after 3 years and 39.4% developed PI-IBS after 6 years, while these rates were 14 and 11.6% in control group [44]; likewise in matched cohorts, one-year incidence of PI-IBS was 37.7/1000 in giardiasis group and 4.4/1000 in non-giardiasis group [45].
This protozoan secretes a variety of proteins considered as virulent factors (toxin-like, cysteine rich surface proteins including Variant Surface Proteins [VSPs] and High Cysteine Membrane Proteins [HCMPs], metabolic enzymes from arginolysis as Arginine Deiminase (ADI) and Ornithine Carbamoyl Transferase (OCT) as well as glycolytic enzymes as enolase, tenascins and worth noting cysteine proteases as Giardipain-1 [46].
Consistent with a link between giardiasis and PI-IBS, duodenal biopsies from chronically infected patients showed increased paracellular permeability, i.e. decreased transepithelial resistance, increased rates of enterocyte apoptosis, decreased expression of tight junction proteins as claudin-1 and occludin-4, and recruitment of inflammatory cells at the intraepithelial compartment [47]. These alterations have been reproduced during in vitro interactions of intestinal IEC-6 cells with G. duodenalis trophozoites and even in experimental giardiasis using Mongolian gerbils infected with trophozoites transfected with plasmids containing the entire sequence of Giardipain-1 [48,49]. Other virulence factor, the arginolytic enzyme Arginine Deiminase (ADI) contained in Giardia´s secretome was shown able to actívate mast cells in vitro which produced IL-6 and TNFα; this effect was reproduced by the ADI product, citrulline [50]. Nonetheless these and other virulence factors as well as other molecules are not only present in the trophozoite´s secretome but are actively exported within exosomes or Extracellular Vesicles (EVs) which may penetrate intercellular epithelial junctions or directly fuse to enterocyte membrane to release its content in host cell cytoplasm. In vitro assays indicate that giardial EVs induce transcription and secretion of proinflammatory cytokines (IL-1β, IL-6 and TNFα) in murine macrophages [51]. Interestingly, treatment with giardial EVs in mice with dextran sulfate-induced cholitis (an IBD model) attenuate inflammation through down-regulation of IL-1β, TNFα and IFNγ [52], in agreement with the low grade of inflammation observed in IBS.
In addition, there are insights about an altered brain-gut axis in giardiasis connecting it to IBS-D because patients may have impaired serotonin release combined to increased cholecystokinin levels at duodenum and plasma, this latter being a predisposing factor for changes in bowel habits as delayed emptying and postprandial contractions in colon [53,54]. Furthermore, Giardia infections may induce dysbiosis in intestinal microbiota biofilms promoting bacterial translocation across the intestinal epithelial barrier with concomitant, proinflammatory stimulation of TLR4 signaling and IL-1β overproduction. Worth of mention, these alterations in microbiota were caused by parasite´s cysteine proteases. Otherwise in gerbils infected with trophozoites transfected to overexpress Giardipain-1, these parasites provoked changes in fecal bacterial communities albeit the inflammatory response that persisted after parasite clearance was thought to be associated to the parasite but not to the dysbiotic microbiota [49]. Likewise in mice infected with G. duodenalis, parasite colonization was related to increases in aerobic Proteobacteria and decreases in anaerobic Firmicutes and Melainabacteria as a direct result from parasite metabolism and indirectly from stimulation of inflammatory responses [55]. In summary, this protozoan fulfills the criteria to be associated to IBS in cases where it is erradicated by treatment, but main traits of IBS later emerge [23,44,45].
Blastocystis sp
The stramenopile, unicellular organism Blastocystis sp. is other key intestinal parasite of emerging epidemiological importance and still a matter of controversy regarding its pathogenicity and link to IBS yet these issues are gaining consensus in recent years. This heterokont infects the large intestine of humans and a wide repertoire of vertebrates through fecal-oral transmission where vacuolar and/or granular forms predominate in stool and ameboid stages are present in symptomatic individuals. Most infections are self-limiting and asymptomatic, the most frequent symptoms are diarrhea, abdominal pain, bloating, vomiting, nausea, anorexia and sometimes urticaria, itching and skin redness [56]. Currently there are up to 38 Subtypes (STs) identified, with ample genetic variability, of which ST1-ST9 can infect humans and other hosts whilst ST1-ST4 cause >90% of human infections with prevalence of ST3 in both symptomatic and asymptomatic cases [57]; indeed a relation between virulence and Blastocystis subtypes has not consensus but it is proposed to be rather a multifactorial result from the infecting subtype, intestinal microbiota and host immune response.
The association of blastocystosis and IBS prompts from their reported occurrence in a same individual, raising the notion that the parasite has a role in the onset and development of IBS [58,59]. Based on symptomatology, it is conceivable that the intestinal barrier is disrupted during blastocystosis, leading to epithelial damage, infiltration of immunitary cells and activation of innate responses [60]. In this sense, experimental data in Blastocystis-infected mice provide supporting evidence; for instance, this protist provoked an altered epithelial architecture, infiltration of both inflammatory cells at submucosa and parasites at all intestinal layers along to goblet cell hyperplasia, higher levels of IL-12 and TNFα and decreased ones of IL-4 and IL-10 [61]. Accordingly, intestinal explants from Blastocystis ST7-infected mice displayed higher levels of IL-1β and IL-6 through the MAPK signaling pathway [62]. In addition, patients with Blastocystis infection may exhibit an important decline in anti-inflammatory bacteria as Faecalibacterium prausnitzii [63]. On the contrary, Blastocystis ST4-infected mice increased the production of anti-inflammatory factors such as short-chain fatty acids and IL-10 [64]. Clinical data add insights in the latter sense. Thus, Blastocystosis has been linked with decreased levels of the neutrophil-derived inflammatory marker, fecal calprotectin, and normal bacterial alpha diversity in asymptomatic (“healthy”) individuals [63,65]; further, Blastocystis ST3 infection has been associated with a eubosis state with predominance of beneficial species as Ruminococcus (Firmicutes) and Prevotella (Bacteroidetes) [66].
Here, it is worth mentioning that a few virulent factors from Blastocystis sp. have been identified and characterized, particularly a secreted cysteine protease named Blastopain-1 that is activated by a Legumain-type protease. Blastopain-1 can disrupt the epithelial barrier in model epithelial cell lines [67] and it is known that cysteine proteases may be exported by this parasite as part of EVs´ cargo [68]. Moreover, EVs from Blastocystis ST1-3 have been shown to differentially induce the production of pro-and anti-inflammatory cytokines by human monocytes [68]. Clearly, it is necessary to assess the active role of this and other(s) from the 29 cysteine proteases predicted to be secreted by this organism [33] along to its serine protease(s) able to cleave secretory IgA [69] in experimental blastocystosis and further in symptomatic Blastocystis sp. infections. In the same sense, there is a lack of studies concerning brain-gut axis alterations caused by this parasitic heterokont, but a link is likely because it was observed that prevalence and pathogenicity of Blastocystis sp. are higher in people with stressed lifestyles in developing countries [70].
The ability of Blastocystis infection to alter intestinal microbiota is a hallmarked link with IBS onset and development. It has been observed that Blastocystis colonization promotes a significant decrease in probiotic Bifidobacterium sp. in men with IBS-C [63]. In the case of IBS patients infected with Blastocystis ST3, they displayed increased levels of serum IL-6 and TNFα [71]. Intriguingly in IBS-D patients the fecal prokaryotic microbiota is unchanged regardless of the presence or absence of both Blastocysitis sp. However, IBS was ultimately related to a rise in Firmicutes and a decrease in Bacteroidetes [72]. In relation to immune responses, IBS patients with concomitant Blastocystis infection had lower levels of CD3+, CD4+ and CD4+/CD8+ cell ratios [73]; further, ST1 or ST3-infected IBS patients overproduced pro-inflammatory cytokines (IL-8, IL-12 and TNFα) when compared to patients with only IBS [74]. In studies analyzing genetic background, Blastocystis carriers harbouring polymorphisms at alleles of pro-inflammatory IL-8+ 396 (GG) and anti-inflammatory IL-10-592 (C) cytokines displayed a risk to develop IBS [75]. In spite that these reports suggest likely relationships of at least IBS-C with proinflammatory microbiota and that recent meta-analyses of clinical reports show a positive relatio of Blastocystis sp. infection and risk to develop IBS (Odds Ratio 1.78, 95% confidence interval 1.29-2.44) [76], the ocurrence of PI-IBS after blastocystosis treatment still requires case-control, prospective clinical studies.
Entamoeba histolytica
The Entamoeba histolytica/dispar complex inhabits the colonic mucosa of humans and alternates between two main stages: the infective cyst and the vegetative, pathogenic trophozoite. The disease caused, amebiasis, may have intestinal manifestations in ≈10% cases with symptoms as acute abdominal pain, dysentery, amoebic colitis and watery or mucus-containing feces. In ≈1% cases the parasite may cross the epithelial layers and migrate through portal vein to liver, causing amebic liver abscess which is fatal if untreated. For colonization and invasiveness, these protists express several types of molecules aimed to interact with and degrade host targets. These include several cell surface glycoproteins as the Gal-GalNac lectin, a lipopeptidoglycan, and adhesins as ADH112; glycosidases that degrade MUC2; β-amylases for energy source during invasion and remarkably cysteine proteases like CP112 that form a complex with ADH112 and degrade intercellular junction proteins (tight and adherens junctions as well as desmosomes) and CP-A5 (expressed only by E. histolytica) that (a) degrades mucus and even microbial films, (b) induce collagen degradation through matrix metalloproteinase activation, and (c) promotes tissue inflammation through binding to αvβ3 integrin receptors on goblet cells. Another factor secreted by trophozoites is the inflammatory, secreted prostaglandin E2 that also disrupts tight junctions as do some serine proteases [33,77]. E. histolytica is identified as the virulent species and E. dispar as the non-virulent one. Accordingly, humoral and cellular immune responses are activated by E. histolytica, initiating with neutrophil infiltration at colonic (intra)epithelium, activation of mast cells, macrophages, natural killer cells and T lymphocytes which led to secretion of pro-inflammatory cytokines [78]. However, in recent times a role for microbiota in the control of inflammation has been proposed [79].
As far as the role of intestinal microbiota in the modulation of E. histolytica virulence is concerned, certainly Entamoeba feeds on comensal bacteria and it triggers not only its invasiveness but provoke significant changes in the population structure of human intestinal microbiota, i.e. dysbiosis, with distinct trends. In this sense, Indian patients with amebic colitis had decreases in populations as Bacteroides, Clostridium coccoides/leptum, Lactobacillus, Campylobacter and Eubacterium with concomitant increases in Bifidobacterium species [80]. However amebic colonization in children has been found associated to enrichment of the pro-inflammatory bacterium Prevotella copri in diarrheal samples [81]; similarly in other study E. histolytica-positive samples from African patients exhibited greater frequencies of Firmicutes and lower of Bacteroidetes as compared to healthy controls [82]. Despite the obvious inflammatory potential and the aggressive parasite behaviour, an association of symptomatic amebiasis with IBS remains unraveled despite pioneer reports proposing IBS to be likely associated to amebic dysentery in British soldiers [16], because subsequent studies carried out in Indian individuals and further at Nicaragua did not demonstrate an actual relationship [83]. However, more recent case-control studies in an Indian tribal cohort showed Giardia as the most prevalent parasite in IBS patients (33/120, 27.5%) followed by E. histolytica (16/120, 13%) as compared to infected individuals without IBS (Giardia 8/80, 10% and E. histolytica 2/80, 2.5%) [84]. Moreover, in recent retrospective studies at Spain where 31 out of 74 individuals were diagnosed with parasite-caused traveller´s diarrhea and developed PI-IBS within six months after travel return and parasite clearance, 20 cases were associated to Giardia, 6 to Blastocystis sp., 3 to Dientamoeba fragilis while 2 cases were associated to previous E. histolytica infection [85]. Thus, the latter studies provide a suggestive basis to associate E. histolytica infection with both IBS and PI-IBS, albeit future studies around these notions are mandatory.
Pathogenesis of IBS by Intestinal Helminths
Overview
Inflammation is a hallmark of IBS where cellular (i.e. predominant Th1 type) host immune responses predominate, but are not exclusive to, infections by eukaryotic single-celled pathogens as those previously described. Nevertheless, these parasites could promote and/or directly modulate host pro-inflammatory responses in order to provide a state of limited inflammation as it often occurs in IBS [38]. In the case of metazoan, intestinal worm parasites (helminths) from which extracellular, soil-transmitted nematodes (e.g. Ascaris, Trichuris, Ancylostoma, Strongyloides) and intracellular Trichinella along to cestodes (e.g. Taenia, Hymenolepis) are outstanding genera, these organisms colonize the intestines of humans, livestock and wild hosts. As opposite to protists, helminths elicit a predominant Th2-type response that renders an anti-inflammatory environment in immunocompetent hosts. At this reductonist scenario, it could be assumed that a relationship of helminthic infections and IBS would be unlikely to occur. Concurrently the information in this sense is scarce although interesting insights are emerging from recent experimental research. At this respect, several helminths as Ascaris lumbricoides, Taenia spp. and Hymenolepis nana were associated in some cases (2.5-6%, 0-1% in controls) to IBS development in case-control studies, similarly to E. histolytica [84]. In the case of infections by other helminths as Trichuris trichura, despite its high incidence and by virtue that it elicits similar symptoms to IBS, this issue hampers its detection and relationship with IBS [86]. Nonetheless the species T. muris has been used to develop a PI-IBS model using Th1-biased susceptible AKR mice treated with oxantel pamoate, where it was found that the continued presence of the parasite was not necessary for the maintenance of eosinophil-including inflammation and gut dysfunction, and these alterations were normalized with dexamethasone after helminth elimination [87].
Trichinella-PI-IBS Models
The nematode Trichinella spiralis is possibly the helminth most closely related to IBS as judged by some reports [88]. Its life cycle involves the ingestion of meat contaminated by larvae that upon activation at stomach migrate to small intestine, burrow into the mucosal layer, mature into adults which reproduce and females release hundreds of newborn larvae that may migrate through the bloodstream towards striated muscle, where they encyst and remain latent for months or years. After several weeks (5-8 in humans and 2-4 in rodents) adults are expelled but the intestinal inflammatory response remains and is then diminished by a protective Th2 response. Based on this, from the last two decades Trichinella-infected rodents serve as experimental models of PI-IBS due to the transient infection followed by gut dysfunction and low inflammation. In the standard Trichinella-PI-IBS model, NIH mice are gavaged with 300-500 larvae; at 2 weeks p.i. severe inflammation is detected and by 8 weeks (70 days) p.i. inflammation ceases, indicating the onset of PI-IBS [89]. This model has provided valuable cues about the patophysiology of PI-IBS that mimic clinical findings. These include immune dysfunction, where PI-IBS mice exhibit higher jejunal/ileal IFNγ and β7 levels, IL-10 was decreased and IL-1β remained unchanged [90]; also, dendritic cells at lamina propia were activated and displayed a Th2-type response [91] which may contribute to the maintenance of small intestine hypercontractility [92].
Another coincident feature is visceral hypersensitivity, in which luminal antigen exposure maintains gut dysmotility and hyperalgesia that is reversed by dexamethasone [89]; here, CD11c+ lamina propia mononuclear phagocytes transferred from PI-IBS to normal mice caused visceral hypersensitivity and mucosal barrier dysfunction [93] which may be normalized by using protease activated receptor 2 (PAR-2) antagonists [94]. Regarding changes in microbiota, particularly bacterial overgrowth at small intestine (SIBO), a third of PI-IBS mice showed this alteration associated to decreased numbers of interstitial cells of Cajal and increased intraepithelial lymphocytes at ileum [95]. Likewise, deficiencies in absorptive/secretory functions as that related to bile acid trafficking are reproduced in this model although a relationship with expression of sodium-dependent bile acid transporters was not demonstrated [96]. Furthermore, some protecting factors such as Heat Shock Protein 70 (HSP70) have been identified; in these studies, heat–treated PI-IBS mice reversed intestinal dysfunction and inflammatory responses by promoting Nitric Oxide (NO) production and inhibiting NF-kB activation [97]. In relation to therapeutical strategies, the mice PI-IBS model has provided evidence on the beneficial effect of Rifaximin, an antibiotic that binds bacterial RNA polymerase and is currently approved for IBS treatment, which suppressed IL-12 and IL-17 expression and promoted expression of tight junction proteins as occludin at colon and ileum with minimal effect on abundance of Lactobacillus and Bifidobacterium populations [98]; likewise probiotic Lactobacillus paracasei (or its spent medium) normalized the altered muscle activity and energy metabolism as compared to untreated PI-IBS mice [99]; in addition, treatment by FMT or Bifidobacterium longum supplementation restore barrier function and increase pain thresholds as compared to controls [100].
A similar model has been also established in rats infected with 1,500 larvae then at 100 days p.i. the PI-IBS is induced by Acute Cold Restraint Stress (ACRS) [101]. In this model the post-infection stress has effectively induced intestinal dysmotility and visceral hypersensitivity, providing a valuable insight on the role of psychological stress that in humans is believed to be an inducer factor for IBS or even PI-IBS [102]. The same model was applied in mast cell-deficient rats (Ws/Ws) and, as compared to normal rats (+/+), they did not show disturbed neurotransmitter balance (acetylcholine/substance P) and IL-1 β/IL-10 ratios as +/+ rats did, which also displayed hyperplasia and activation of mast cells, thus demonstrating an important role of these latter in the development of PI-IBS [103]. In a general landscape, these models have not only contributed to gain important mechanistic insights underlying the onset and development of IBS/PI-IBS but are themselves a proof of concept regarding the relationship of at least these helminths with PI-IBS.
Remarks and Future Directions
Intestinal parasitic infections are embedded within the complex spectrum of etiology and pathophysiology of IBS and PI-IBS and outstand as risk factor over viral, bacterial and fungal pathogens on the basis of clinical studies and epidemiological meta-analyses [1,17,20,29,76]. The four protists included herein are the most frequent intestinal unicellular pathogens [33] while the helminths that may model PI-IBS (Trichinella, Trichuris) have a second-line frequency after geohelminths. Certainly, some environmental and lifestyle factors such as climate changes, intensive human traveling and increased globalization may worsen the epidemiological impact of these pathogens. For instance, in some regions with endemic parasitosis by geohelminths, the incidence of Blastocystis sp. is increasing not only due to changes in dietary habits, inflammatory intestinal diseases and even asymptomatic infections that can modify microbiota structure in people but also after mass drug administration campaigns applied since 1990s that are devoted to deworming children [65,66]. From experimental and clinical evidence, the onset of IBS is preceded by acute gastroenteritis that occurs in a subset of protist- and helminth-infected individuals whenever asymptomatic infections have a high prevalence.
As assessed here and in previous reviews [14,23,33], parasite-derived IBS involves the following main processes: increase in intestinal barrier permeability, low grade of immune pro-inflammatory response, induction of visceral hypersensitivity and microbiota dysbiosis. By one side, these steps in pathogenesis are preceded by the action of the so-called virulence factors, located at parasite cell surface or secreted into the soluble secretome or within EVs, which deserve not only to be emphasized as the earliest event in IBS pathophysiology but to evaluate their individual or collective contribution to IBS onset (Figure 2). For instance, in the protozoan consensually associated to IBS, i.e. Giardia duodenalis, the secreted cysteine protease named as Giardipain-1, when expressed by plasmid-mediated transfection in trophozoites, these parasites caused chronic inflammatory infiltrates that were more severe after 6 months p.i. –much after parasite clearance- at duodenum/jejunum of infected Mongolian gerbils, concomitantly to changes in microbiota which were, however, not representative of pro-inflammatory communities, even after FMT treatment [104]. Otherwise, cysteine proteases from G. duodenalis were, at least in part, responsible to induce bacterial dysbiosis and translocation that were linked to up-regulation of proinflammatory molecules such as TLR4 and IL-1β in humanized germ-free mice [105]. Conversely, the ability to modulate inflammation by some pathogenic protists or its derivatives might be further exploited to profile new pre- or probiotics, e.g. Giardia EVs for colitis [55] and Blastocystis sp. for inflammatory diseases [65] respectively.
In addition to functional and mechanistic studies on gut dysfunction, the role of the brain-gut axis in parasite-derived IBS deserves further clinical research. Besides of the importance of serotonin [ 9,13,53,54] and the important proximity of activated mast cells to colonic nerve terminations [27] with implications in neurotransmitter dysbalances found in stress-triggering rodent models [103], other factors clearly play a role. In fact, IBS does not only imply alterations at gut because the brain also develops anatomical differences such as abnormalities in grey and white matter, fronto-limbic and sensorimotor networks and affective and pain processing by habenula among others. Indeed, IBS is believed to be a somatization of psychosocial morbidities as 40-60% IBS cases have comorbid depression or anxiety disorders. Recent meta-analyses show that IBS-C is related with the highest incidence of depression (38%) and anxiety (40%) followed by IBS-D, IBS-M, and IBS-U [106]. As far as IBS-D and IBS-M are the most frequent varieties, and that protozoal infections like giardiasis, blastocystosis and amebiasis may imply diarrhea-constipation alternancy, it is tempting to assess what IBS variety is associated to PI-IBS along to the presence of psychological disorders. These studies could have implications for the need on recent treatment approaches such as hypnotherapy for PI-IBS symptomatology, based on possible epigenetic cellular mechanisms driving long-term stress memories at the enteric nervous system [107].
Another system in the body with influence on bowel function is the Endocannabinoid System (ECS) that comprises two cannabinoid receptors (CB1 and CB2) and its cognate ligands anandamine and 2-Arachidonoglycerol (2-AG); noteworthy activation of CB1/CB2 decreases intestinal motility, secretions, inflammation and hypersensitivity. Phytocannabinoids from Cannabis sativa (THC or tetrahidrocannabinol and CBD or cannabidiol) preferentially activate CB1 and CB2 respectively but THC has psychotropic effects as CB1 are also located at central nervous system while CB2 is present at peryphery including the gut and immune cells. Therefore, cannabinoids have been used in IBS and SNPs at gene members of ECS have been proposed to be linked to IBS [108]. Dronabinol, a commercially available CB2 agonist showed improvements in colonic motility and fasting left in IBS-D and IBD-M patients; indeed, CBD analogs have been proposed for IBS-D therapy and CBD2 antagonists to relieve IBS-C [109]. Moreover, Phyto cannabinoids from C. sativa have promising antiparasitic activities [110]. Notwithstanding these facts, the use of (phyto)cannabinoids remains controversial hence the use of THC and even CBD in medical research is waiting for legalization in most countries worldwide.
Conclusion
Parasitic intestinal infections are a relevant contributing factor for IBS onset and PI-IBS development where clinical studies leading to meta-analyses and experimental models provide a deeper understanding of the etiology and pathophysiology related to this inflammatory disorder. The association of IBS with the four intestinal protists and helminths used to model PI-IBS assessed herein is gaining consensus, particularly for G. duodenalis, albeit evidence for the others is not compelling to date. Indeed, the control of IBS involving diagnosis and detection of intestinal parasites in more prospective and retrospective clinical studies including the routine application of the Rome criteria as well as additional alternatives for treatment including new drugs, phytochemicals and pre/probiotic blends combined to psychological support on demand still warrants further research.
Acknowledgements
The author is grateful to QFB Raquel Romo-Ramirez for her valuable support and comments during the preparation of this manuscript.
Conflict of Interest
None.
References
- Oka P, Parr H, Barberio B, Black CJ, Savarino EV, et al. (2020) Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: a systematic review and meta-analysis. Lancet Gastroenterol Hepatolo 5(10): 908-917.
- Sperber AD, Dekel R (2010) Irritable Bowel Syndrome and Co-morbid Gastrointestinal and Extra-gastrointestinal Functional Syndromes. J Neuro gastroenterol Motil 16(2): 113-119.
- Lacy BE, Pimentel M, Brenner DM, Chey WD, Keefer LA, et al. (2021) ACG clinical guideline: Management of irritable bowel syndrome. Am J Gastroenterol 116(1): 17-44.
- El Salhy M (2912) Irritable bowel syndrome: diagnosis and pathogenesis. World J Gastroenterol 18(37): 5151-5163.
- Palsson OS, Peery A, Seitzberg D, Amundsen ID, McConnell B, et al. (2020) Human Milk Oligosaccharides Support Normal Bowel Function and Improve Symptoms of Irritable Bowel Syndrome: A Multicenter, Open-Label Trial. Clin Transl Gastroenterol 11(12): e00276.
- Chey WD, Kurlander J, Eswaran S (2015) Irritable bowel syndrome: a clinical review. JAMA 313(9): 949-958.
- Tanaka Y, Kanazawa M, Fukudo S, Drossman DA (2011) Biopsychosocial model of irritable bowel syndrome. J Neurogastroenterol Motil 17(2): 131-139.
- Rothenberg ME (2021) An Allergic Basis for Abdominal Pain. N Eng J Med 384(22): 2156-2158.
- Mittal R, Debs LH, Patel AP, Nguyen D, Patel K, et al. (2017) Neurotransmitters: The critical modulatord regulating gut–brain axis. J Cell Physiol 232(9): 2359-2372.
- Shulman RJ, Jarrett ME, Cain KC, Broussard EK, Heitkemper MM (2014) Associations among gut permeability, inflammatory markers, and symptoms in patients with irritable bowel syndrome. J Gastroenterol 49(11): 1467-1476.
- Vazquez Roque MI, Camilleri M, Smyrk T, Joseph A Murray, Jessica O Neill, et al. (2012) Association of HLA-DQ gene with bowel transit, barrier function, and inflammation in irritable bowel syndrome with diarrhea. Am J Physiol Gastrointestinal Liver Physiol 303(11): G1262-G1269.
- Lacy BE, Rosenbaum D, Edelstein S, Kozuka K, Williams LA, et al. (2024) Intestinal Permeability, Irritable Bowel Syndrome with Constipation, and the Role of Sodium-Hydrogen Exchanger Isoform 3 (NHE3). Clin Exp Gastroenterol 17: 173-183.
- Szőke H, Kovács Z, Bókkon I, Vagedes J, Szabó AE, et al. (2020) Gut dysbiosis and serotonin: intestinal 5-HT as a ubiquitous membrane permeability regulator in host tissues, organs, and the brain. Rev in Neurosci 31(4): 415-425.
- Vakili O, Adibi Sedeh P, Pourfarzam M (2024) Metabolic biomarkers in irritable bowel syndrome diagnosis. Clin Chim Acta 560: 119753.
- Enck P, Aziz Q, Barbara G, Farmer AD, Fukudo S, et al. (2016). Irritable bowel syndrome. Nat Rev Dis Primers 2: 16014.
- Chaudhary NA, Truelove SC (1962) The irritable colon syndrome. A study of the clinical features, predisposing causes, and prognosis in 130 cases. Q J Med 31: 307-322.
- Thabane M, Kottachchi DT, Marshall JK (2007) Systematic review and meta-analysis: the incidence and prognosis of post-infectious irritable bowel syndrome. Aliment Pharmacol Therapy 26(4): 535-544.
- Saha L (2014) Irritable bowel syndrome: Pathogenesis, diagnosis, treatment, and evidence-based medicine. World J Gastroenterol 20: 6759-6773.
- Spiller RC (2007) Role of infection in irritable bowel syndrome. J Gastroenterol 42(Suppl 17): 41-47.
- Ford AC, Spiegel BM, Talley NJ, Moayyedi P (2009) Small intestinal bacterial overgrowth in irritable bowel syndrome: systematic review and meta-analysis. ClinGastroenterol Hepatol 7(12): 1279-1286.
- Parfrey LW, Walters WA, Knight R (2011) Microbial eukaryotes in the human microbiome: ecology, evolution, and future directions. Frontiers Microbiolo 2: 153.
- Wammes LJ, Mpairwe H, Elliott AM, Yazdanbakhsh M (2014) Helminth therapy or elimination: epidemiological, immunological, and clinical considerations. Lancet Infect Dis 14(11): 1150-1162.
- Berumen A, Edwinson AL, Grover M (2021) Post-infection irritable bowel syndrome. Gastroenterology Clinics of North America 50(2): 445-461.
- Pittayanon R, Lau JT, Yuan Y, Leontiadis GI, Tse F, et al. (2019) Gut Microbiota in Patients With Irritable Bowel Syndrome-A Systematic Review. Gastroenterol 157(1): 97-108.
- Dunlop SP, Hebden J, Campbell E, Naesdal J, Olbe L, et al. (2006) Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol 101(6): 1288-1294.
- Gecse K, Roka R, Ferrier L, Leveque M, Eutamene H, et al. (2008) Increased faecal serine protease activity in diarrhoeic IBS patients: A colonic lumenal factor impairing colonic permeability and sensitivity. Gut 57(5): 591-599.
- Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, et al. (2004) Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterol 126(3): 693-702.
- Bennet SM, Polster A, Tornblom H, Isaksson S, Capronnier S, et al. (2016) Global Cytokine Profiles and Association With Clinical Characteristics in Patients With Irritable Bowel Syndrome. Am J Gastroenterol 111(8): 1165-1176.
- Bashashat M, Rezaei N, Shafieyoun A, McKernan DP, Chang L, et al. (2014) Cytokine imbalance in irritable bowel syndrome: A systematic review and meta-analysis. Neurogastroenterol Motil 26(7): 1036-1048.
- Hunter PR, Hughes S, Woodhouse S, Raj N, Syed Q, et al. (2004) Health sequelae of human cryptosporidiosis in immunocompetent patients. Clin Infect Dis 39(4): 504-510.
- Carter BL, Stiff RE, Elwin K, Hutchings HA, Mason BW, et al. (2019) Health sequelae of human cryptosporidiosis-a 12-month prospective follow-up study. Eur J Clin Microbiol Infect Dis 38(9): 1709-1717.
- Khaldi S, Gargala G, Le Goff L, Parey S, Francois A, et al. (2009) Cryptosporidium parvum isolate-dependent postinfectious jejunal hypersensitivity and mast cell accumulation in an immunocompetent rat model. Infect Immun 77: 5163-5169.
- Argüello García R, Carrero JC, Ortega Pierres MG (2023) Extracellular Cysteine Proteases of Key Intestinal Protozoan Pathogens-Factors Linked to Virulence and Pathogenicity. Int J Mol Sci 24(16): 12850.
- Stenlund H, Nilholm C, Chorell E, Roth B, D'Amato M, et al. (2021) Metabolic profiling of plasma in patients with irritable bowel syndrome after a 4-week starch- and sucrosereduced diet. Metabolites 11(7): 440.
- Klein P, Kleinová T, Volek Z, Simůnek J (2008) Effect of Cryptosporidium parvum infection on the absorptive capacity and paracellular permeability of the small intestine in neonatal calves. Vet Parasitol 152(1): 53-59.
- Kumar A, Chatterjee I, Anbazhagan AN, Jayawardena D, Priyamvada S, et al. (2018) Cryptosporidium parvum disrupts intestinal epithelial barrier function via altering expression of key tight junction and adherens junction proteins. Cell Microbiol 20(6): e12830.
- Jalanka J, Lam C, Bennett A, Hartikainen A, Crispie F, et al. (2021) Colonic gene expression and fecal microbiota in diarrhea-predominant irritable bowel syndrome: Increased toll-like receptor 4 but minimal inflammation and no response to mesalazine. Journal of Neurogastroenterolo Motil 27: 279-291.
- Mitselou A, Grammeniatis V, Varouktsi A, Papadatos SS, Klaroudas A, et al. (2021) Immunohistochemical study of adhesion molecules in irritable bowel syndrome: A comparison to inflammatory bowel diseases. Adv Biomed Res 10: 21.
- Marion R, Baishanbo A, Gargala G, François A, Ducrotté P, et al. (2006) Transient neonatal Cryptosporidium parvum infection triggers long-term jejunal hypersensitivity to distension in immunocompetent rats. Infect Immun 74(7): 4387-4389.
- Wang L, Cao L, Chang Y, Fu Y, Wang Y, et al. (2023) Microbiome-metabolomics analysis of the impacts of Cryptosporidium muris infection in BALB/C mice. Microbiol Spectr 11(1): e0217522.
- Alsaady IM (2024) Cryptosporidium and irritable bowel syndrome. Trop Parasitol 14(1): 8-15.
- Halliez MC, Buret AG (2013) Extra-intestinal and long-term consequences of Giardia duodenalis infections. World J Gastroenterol 19 (47): 8974-8985.
- Chen TL, Chen S, Wu HW, Lee TC, Lu YZ, et al. (2013). Persistent gut barrier damage and commensal bacterial influx following eradication of Giardia infection in mice. Gut Pathog 5(1): 26.
- Hanevik K, Wensaas K A, Rortveit G, Eide G E, Morch K, et al. (2014) Irritable bowel syndrome and chronic fatigue 6 years after giardia infection: a controlled prospective cohort study. Clin Infect Dis 59 (10): 1394-1400.
- Nakao JH, Collier SA Gargano JW (2017) Giardiasis and Subsequent Irritable Bowel Syndrome: A Longitudinal Cohort Study Using Health Insurance Data. J Infect Dis 215(5): 798-805.
- Argüello-García R, Ortega-Pierres MG (2021) Giardia duodenalis Virulence - "To Be, or Not To Be". Curr Trop Med Rep 8(4): 246-256.
- Troeger H, Epple HJ, Schneider T, Wahnschaffe U, Ullrich R, et al. (2007) Effect of chronic Giardia lamblia infection on epithelial transport and barrier function in human duodenum. Gut 56(3): 328-35.
- Ortega Pierres G, Argüello García R, Laredo Cisneros MS, Fonseca Linán R, Gómez Mondragón M, et al. (2018) Giardipain-1, a protease secreted by Giardia duodenalis trophozoites, causes junctional, barrier and apoptotic damage in epithelial cell monolayers. Int J Parasitol 48(8): 621-639.
- Quezada Lázaro R, Vázquez Cobix Y, Fonseca Liñán R, Nava P, Hernández Cueto DD, et al. (2022) The Cysteine Protease Giardipain-1 from Giardia duodenalis Contributes to a Disruption of Intestinal Homeostasis. Int J Mol Sci 23(21): 13649.
- Muñoz Cruz S, Gomez García A, Matadamas Martínez F, Alvarado Torres JA, Meza Cervantez P, et al. (2018) Giardia lamblia: identification of molecules that contribute to direct mast cell activation. Parasitol Res 117(8): 2555-2567.
- Zhao P, Cao L, Wang X, Li J, Dong J, et al. (2021) Giardia duodenalis extracellular vesicles regulate the proinflammatory immune response in mouse macrophages in vitro via the MAPK, AKT and NF-kappaB pathways. Parasit Vectors 14(1): 358.
- Kim HJ, Lee YJ, Back SO, Cho SH, Lee HI, et al. (2022) Treatment with Extracellular Vesicles from Giardia lamblia Alleviates Dextran Sulfate Sodium-Induced Colitis in C57BL/6 Mice. Korean J Parasitol 60(5): 309-315.
- Dizdar V, Spiller R, Singh G, Hanevik K, Gilja OH, et al. (2010) Relative importance of abnormalities of CCK and 5-HT (serotonin) in Giardia-induced post-infectious irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol The 31(8): 883–891.
- Chey WY, Jin HO, Lee MH, Sun SW, Lee K (2001) Colonic motility abnormality in patients with irritable bowel syndrome exhibiting abdominal pain and diarrhea. Am J Gastroenterol 96(5): 1499-1506.
- Barash NR, Nosala C, Pham JK, McInally SG, Gourguechon S, et al. (2017) Giardia colonizes and encysts in high-density foci in the murine small intestine. mSphere 2(3): e00343-16.
- Stensvold CR, Lewis HC, Hammerum AM, Porsbo LJ, Nielsen SS, et al. (2009) Blastocystis: Unravelling potential risk factors and clinical significance of a common but neglected parasite. EpidemioInfect 137(11): 1655-1663.
- Roberts T, Stark D, Harkness J, Ellis J (2014) Update on the Pathogenic Potential and Treatment Options for Blastocystis sp. Gut Pathog 6: 17.
- Yakoob J, Jafri W, Beg MA, Zaigham Abbas, Shagufta Naz, et al. (2010) Blastocystis hominis and Dientamoeba fragilis in patients fulfilling irritable bowel syndrome criteria. Parasitol Res 107(3): 679-684.s
- Ragavan ND, Kumar S, Chye TT, Sanjiv Mahadeva, Ho ShiawHooi (2015) Blastocystis sp. in Irritable Bowel Syndrome (IBS) - Detection in stool aspirates during colonoscopy. PLoS One 10(9): e0121173.
- Vitetta L, Saltzman ET, Nikov T, Isabelle Ibrahim, Sean Hall (2016) Modulating the gut microenvironment in the treatment of intestinal parasites. J Clin Med 5(11): 102.
- Abdel Hafeez EH, Ahmad AK, Abdelgelil NH, Abdellatif MZM, Kamal AM, et al. (2016) Immunopathological assessments of human blastocystis spp. in experimentally infected immunocompetent and immunosuppresed mice. Parasitol Res 115(5): 2061-2071.
- Lim MX, Png CW, Tay CYB, Teo JDW, Jiao H, et al. (2014) Differential regulation of proinflammatory cytokine expression by mitogen-activated protein kinases in macrophages in response to intestinal parasite infection. Infect Immun 82(11): 4789-4801.
- Nourrisson C, Scanzi J, Pereira B, NkoudMongo C, Wawrzynia I, et al. (2014) Blastocystis is associated with decrease of fecal microbiota protective bacteria: Comparative analysis between patients with irritable bowel syndrome and control subjects. PloS One 9(11): e111868.
- Deng L, Tan KSW (2022) Interactions between blastocystis subtype ST4 and gut microbiota in vitro. Parasitol Vectors 15(1): 80.
- Nieves Ramırez ME, Partida Rodrıguez O, Laforest Lapointe I, Reynolds LA, Brown EM, et al. (2018) Asymptomatic intestinal colonization with protist blastocystis is strongly associated with distinct microbiome ecological patterns. mSystems 3(3): e00007-e00018.
- Iebba V, Totino V, Gagliardi A, Santangelo F, Cacciotti F, et al. (2016) Eubiosis and dysbiosis: the two sides of the microbiota. New Microbiol 39(1): 1-12.
- Nourrisson C, Wawrzyniak I, Cian A, Livrelli V, Viscogliosi E, et al. (2016) On Blastocystis Secreted Cysteine Proteases: A Legumain-Activated Cathepsin B Increases Paracellular Permeability of Intestinal Caco-2 Cell Monolayers. Parasitol 143(13): 1713-1722.
- Norouzi M, Pirestani M, Arefian E, Dalimi A, Sadraei J, et al. (2022) Exosomes Secreted by Blastocystis Subtypes Affect the Expression of Proinflammatory and Anti-Inflammatory Cytokines (TNFα, IL-6, IL-10, IL-4). Front Med (Lausanne) 9: 940332.
- Puthia MK, Vaithilingam A, Lu J, Tan KSW (2005) Degradation of human secretory immunoglobulin A by blastocystis. Parasitol Res 97(5): 386-389.
- Chandramathi S, Suresh K, Sivanandam S, Umah Rani Kuppusamy (2014) Stress exacerbates infectivity and pathogenicity of Blastocystis hominis: in vitro and in vivo evidence. PLoS One 14(9): e94567.
- Azizian M, Basati G, Abangah G, Mahmoudi MR, Mirzaei A (2016) Contribution of blastocystis hominis subtypes and associated inflammatory factors in development of irritable bowel syndrome. Parasitol Res 115(5): 2003–2009.
- Nagel R, Traub RJ, Allcock RJ, Kwan MM, Bielefeldt Ohmann H (2016) Comparison of faecal microbiota in Blastocystis-positive and Blastocystis-negative irritable bowel syndrome patients. Microbiome 4(1): 47.
- Wang KX, Li CP, Wang J, Cui YB (2002) Epidemiological survey of Blastocystis hominis in Huainan City, Anhui Province, China. World J Gastroenterol 8(5): 928-932.
- Yakoob J, Abbas Z, Usman MW, Sultana A, Islam M, et al. (2014) Cytokine changes in colonic mucosa associated with Blastocystis spp. subtypes 1 and 3 in diarrhoea-predominant irritable bowel syndrome. Parasitology 141(7): 957-969.
- Olivo Diaz A, Romero Valdovinos M, Gudino Ramirez A, Reyes Gordillo J, Jimenez Gonzalez DE, et al. (2012) Findings related to IL-8 and IL-10 gene polymorphisms in a Mexican patient population with irritable bowel syndrome infected with Blastocystis. Parasitol Res 111(1): 487-491.
- Abedi SH, Fazlzadeh A, Mollalo A, Sartip B, Mahjour S, Bahadory S, et al. (2022) The neglected role of Blastocystis sp. and Giardia lamblia in development of irritable bowel syndrome: A systematic review and meta-analysis. Microb Pathog 162: 105215.
- Nancy Guillén (2023) Pathogenicity and virulence of Entamoeba histolytica, the agent of amoebiasis. Virulence 14(1): 2158656.
- Ghosh S, Padalia J, Moonah S (2019) Tissue destruction caused by Entamoeba histolytica parasite: cell death, inflammation, invasion, and the gut microbiome. Curr Clin Microbiol Rep 6(1): 51-57.
- Leon Coria A, Kumar M, Workentine M, France Moreau, Michael Surette, et al. (2021) Muc2 mucin and nonmucin microbiota confer distinct innate host defense in disease susceptibility and colonic injury. Cell Molecul Gastroenterol Hepatol 11(1): 77-98.
- Verma AK, Verma R, Ahuja V, Jaishree Paul (2012) Real-time analysis of gut flora in Entamoeba histolytica infected patients of Northern India. BMC Microbiol 12: 183.
- Gilchrist CA, Petri SE, Schneider BN, Daniel J Reichman, Nona Jiang, et al. (2016) Role of the gut microbiota of children in diarrhea due to the protozoan parasite Entamoeba histolytica. J Infect Dis 213(10): 1579e1585.
- Morton ER, Lynch J, Froment A, Sophie Lafosse, Evelyne Heyer, et al. (2015) Variation in rural African gut microbiota is strongly correlated with colonization by Entamoeba and subsistence. PLoS Genet 11(11): e1005658.
- Morgan DR, Benshoff M, Cáceres M, Becker Dreps S, Cortés L, et al. (2012) Irritable bowel syndrome and gastrointestinal parasite infection in a developing nation environment. Gastroenterol Res Pract 2012: 343812.
- Das BB, Panda AK, Patra MP, Nayak K (2022) Study on the Role of Gastrointestinal Parasite in Irritable Bowel Syndrome Patients in a Tribal Region of India. Cureus 14(6): e26091.
- España Cueto S, Oliveira Souto I, Salvador F, Goterris L, Treviño B, et al. (2023) Post-infectious irritable bowel syndrome following a diagnosis of traveller's diarrhoea: a comprehensive characterization of clinical and laboratory parameters. J Travel Med 30(6): taad030.
- Diniz Santos DR, Jambeiro J, Mascarenhas RR, Silva LR (2006) Massive Trichuris trichiura infection as a cause of chronic bloody diarrhea in a child. J Tropical Pediatrics 52(1): 66-68.
- Motomura Y, Khan WI, El-Sharkawy RT, Verma-Gandhu M, Grencis RK, et al. (2010) Mechanisms underlying gut dysfunction in a murine model of chronic parasitic infection. Am J Physiol Gastrointest Liver Physiol 299(6): G1354-G1360.
- Soyturk M, Akpinar H, Gurler O, Pozio E, Sari I, et al. (2007) Irritable bowel syndrome in persons who acquired trichinellosis. Am J Gastroenterol 102(5): 1064-1069.
- Bercík P, Wang L, Verdú EF, Mao YK, Blennerhassett P, et al. (2004) Visceral hyperalgesia and intestinal dysmotility in a mouse model of postinfective gut dysfunction. Gastroenterology 127(1): 179-187.
- Yang B, Zhou X, Lan C (2015) Changes of cytokine levels in a mouse model of post-infectious irritable bowel syndrome. BMC Gastroenterology 15: 43.
- Long Y, Wang W, Wang H, Hao L, Qian W, et al. (2012) Characteristics of intestinal lamina propria dendritic cells in a mouse model of postinfectious irritable bowel syndrome. J Gastroenterol Hepatol 27(5): 935-944.
- Akiho H, Deng Y, Blennerhassett P, Kanbayashi H, Collins SM (2005) Mechanisms underlying the maintenance of muscle hypercontractility in a model of postinfective gut dysfunction. Gastroenterology 129(1): 131-141.
- Ren YJ, Zhang L, Bai T, Yu HL, Li Y, et al. (2017) Transfer of CD11c+ lamina propria mononuclear phagocytes from post-infectious irritable bowel syndrome causes mucosal barrier dysfunction and visceral hypersensitivity in recipient mice. Int J Mol Med 39(6): 1555-1563.
- Du L, Long Y, Kim JJ, Chen B, Zhu Y, et al. (2019) Protease Activated Receptor-2 Induces Immune Activation and Visceral Hypersensitivity in Post-infectious Irritable Bowel Syndrome Mice. Dig Dis Sci 64(3): 729-739.
- Chen B, Zhu S, Du L, He H, Kim JJ, et al. (2017) Reduced interstitial cells of Cajal and increased intraepithelial lymphocytes are associated with development of small intestinal bacterial overgrowth in post-infectious IBS mouse model. Scand J Gastroenterol 52(10): 1065-1071.
- Kalia N, Hardcastle J, Keating C, Grasa L, Keating C, et al. (2008) Intestinal secretory and absorptive function in Trichinella spiralis mouse model of postinfective gut dysfunction: role of bile acids. Gut 57(1): 41-49.
- Zhou X, Dong L, Yang B, He Z, Chen Y, et al. (2015) Preinduction of heat shock protein 70 protects mice against post-infection irritable bowel syndrome via NF-κB and NOS/NO signaling pathways. Amino Acids 47(12): 2635-2645.
- Jin Y, Ren X, Li G, Li Y, Zhang L, et al. (2018) Beneficial effects of Rifaximin in post-infectious irritable bowel syndrome mouse model beyond gut microbiota. J Gastroenterol Hepatol 33(2): 443-452.
- Martin FP, Verdu EF, Wang Y, Dumas ME, Yap IK, et al. (2006) Transgenomic metabolic interactions in a mouse disease model: interactions of Trichinella spiralis infection with dietary Lactobacillus paracasei supplementation. J Proteome Res 5(9): 2185-2193.
- Bai T, Zhang L, Wang H, Wei Qian , Jun Song, et al. (2018) Fecal microbiota transplantation is effective in relieving visceral hypersensitivity in a postinfectious model. BioMed Research International 2018: 3860743.
- Leng YX, Wei YY, Zhou SP, Duan LP (2009) Establishment of irritable bowel syndrome rat model by combination of intestinal infection with Trichinella spiralis and acute stress. Zhonghua Yi Xue Za Zhi 89(42): 2992-2996.
- Barreau F, Ferrier L, Fioramonti J, Bueno L (2007) "New insights in the etiology and pathophysiology of irritable bowel syndrome: contribution of neonatal stress models". Pediatric Research 62 (3): 240-245.
- Leng YX, Wei YY, Chen H, Zhou SP, Yang YL, et al. (2010) Alteration of cholinergic and peptidergic neurotransmitters in rat ileum induced by acute stress following transient intestinal infection is mast cell dependent. Chin Med J 123(2): 227-233.
- Quezada-Lázaro R, Vázquez-Cobix Y, Fonseca-Liñán R, Nava P, Hernández-Cueto DD, et al. (2022) The Cysteine Protease Giardipain-1 from Giardia duodenalis Contributes to a Disruption of Intestinal Homeostasis. Int J Mol Sci 23(21): 13649.
- Beatty JK, Akierman SV, Motta JP, Muise S, Workentine ML, et al. (2017) Giardia duodenalis induces pathogenic dysbiosis of human intestinal microbiota biofilms. Int J Parasitol 47(6): 311-326.
- Hu Z, Li M, Yao L, Wang Y, Wang E, et al. (2021) The level and prevalence of depression and anxiety among patients with different subtypes of irritable bowel syndrome: a network meta-analysis. BMC Gastroenterol 21(1): 23.
- Császár-Nagy N, Bókkon I (2022) Hypnotherapy and IBS: Implicit, long-term stress memory in the ENS? Heliyon 9(1): e12751.
- DiPatrizio NV (2016) Endocannabinoids in the gut. Cannabis Cannabinoid Res 1(1): 67–77.
- Wong BS, Camilleri M, Busciglio I, Paula Carlson, Lawrence A Szarka, et al. (2011) Pharmacogenetic trial of a cannabinoid agonist shows reduced fasting colonic motility in patients with nonconstipated irritable bowel syndrome. Gastroenterology 141(5): 1638-1647.
- Argüello-García R (2023) Cannabis sativa: a source of antiparasitic compounds? Biochemical Journal of Scientific & Technical Research 50 (3): 41701-41707.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.