Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Prevalence And Risk Factors Related to Chlamydia Trachomatis and Mycoplasma Infections in Men Seeking Fatherhood at The Bafoussam Regional Hospital
*Corresponding author: Vanessa Wandja Kamgang, Assistant Lecturer at the Department of Biology, Higher Teacher Training College, University of Bamenda, Bambili, Cameroon.
Received: May 31, 2024; Published: July 10, 2024
DOI: 10.34297/AJBSR.2024.23.003054
Abstract
Background: The aetiologies and causes of male infertility include urogenital infections with the most prevalent species being Chlamydia trachomatis and mycoplasma.
Objectives: The present study aimed to determine the prevalence, and risk factors related to infections due to Chlamydia trachomatis and mycoplasma in men seeking fatherhood at the Bafoussam Regional Hospital of (BRH).
Methods: 43 patients were screened in a cross-sectional study from March to May 2021 at the RHB. Specimens were examined for the presence of trachomatis and mycoplasma species by the ELISA method and bacterial culture respectively.
Results:39% (n=35/43) of participants presented abnormal semen profiles. The prevalence of C. trachomatis infection was 6.98% (n=3/43) while that of mycoplasma infections was 25.58% (n=11/43) among which Ureaplasma urealyticum (n=10/43, 23.38%) and the coinfection M. hominis and U. urealyticum (n=1/43, 2.33%). Among men presenting sperm morphology<15%, 14.28% (n=5/43) were infected with U. urealyticum. In addition, a statistically significant relationship between mycoplasmas and semen abnormalities (p=0.02) was revealed. U. urealyticum was revealed sensitive to Clarithromycin, Josamycin and Roxithromycin at 90% each and resistant to ciprofloxacin and Clindamycin at 81.81% each. Overall, the multiplicity of sexual partners and unprotected sexual intercourse constituted risk factors for the occurrence of mycoplasmas infection.
Conclusions: These results show the involvement of mycoplasma and Chlamydia trachomatis infections in the aetiology of male infertility and emphasize the need for their early screening in men.
Keywords: Chlamydia trachomatis, Mycoplasma, Prevalence, Risk factors, Male infertility, Bafoussam regional hospital
Abbreviations: RHB: Bafoussam Regional Hospital; C. trachomatis: Chlamydia trachomatis; U. urealyticum: Ureaplasma urealyticum.
Introduction
Male infertility is a worldwide condition with Middle East, Central Eastern Europe, Latin America and Sub-Saharan Africa countries being the most affected [1,2]. According to the World Health Organization (WHO) [3,1], men are incriminated in about 20-30% of couple infertility. A few studies realized in African countries such as Sudan reported that the prevalence of male infertility was approximately 35.5% in couples whereas in Tanzania it was 6.8% [2]. The aetiologies and causes of male infertility are of different origins namely organic diseases, genetic factors, environmental risk factors, and their interactions [4]. It is estimated that 46% of Sub-Saharan African men suffer from infertility related to Sexually Transmissible Infections (STIs) [5]. The most recurrent sexually transmitted diseases are those caused by Chlamydia trachomatis and genital mycoplasmas [6]. In Sub-Saharan Africa, Chlamydia trachomatis is the most recurrent pathogen of STIs [7-9]. However, STIs have also been reported as frequently associated with genital mycoplasmas [10,11]. Chlamydia and Mycoplasma male urogenital infections are often asymptomatic and usually cause non-gonococcal urethritis. These microorganisms can cause complications and long-term scars in the upper genital tract thus affecting the reproductive health of both sexes [12]. In men, infections caused by these microorganisms affect sperm parameters. It is estimated that approximately 30-50% of male infertility in the world is related to poor sperm parameters [13]. Alterations caused by these microorganisms in men’s urogenital tract include reduction in sperm concentration [14,15], decreased motility, [15] and/or morphology modifications [16]. These pathogens also enhance the generation of excessive oxidative stress by sperm cells which will lead to deleterious consequences on spermatic parameters (volume, Ph, sperm concentration, motility, and morphology). In Cameroon, very few studies have been undertaken on male infertility and STIs. In a recent study undertaken in Yaoundé Tchoula, et al. [17] reported a prevalence of approximately 53.8% of secondary infertility in men. Whereas Momo, et al. [13] reported that in Dschang, 83.9% of men who were admitted to health centres were diagnosed with sperm damage. Further, a study undertaken at the urology service of Yaounde Central Hospital revealed that sperm damage in infertility accounted for 41.7% in infertile men [1]. Till date, limited data on male infertility related to STIs in Bafoussam, one of the biggest municipalities of Cameroon, is available. The objective of the present study was to determine the prevalence, and risk factors related to C. trachomatis, and mycoplasma infections in men in Bafoussam, Cameroon.
Materials and Methods
Study Design and Participants
This was a cross-sectional and analytic study conducted over three months from March 1 to May 31, 2021, at the Bafoussam Regional Hospital (RHB) in the West Region of Cameroon. Men seeking for childbirth attending the RHB gynaecology service and who gave their informed consent were included in the study. All participants underwent a sperm count analysis to investigate male infertility and were interviewed to provide information related to their level of knowledge, attitude, and practices (educational needs), as well as other risk factors of infections.
Data Collection and Analyses
Sperm Count Parameters: Participants were asked to abstain from sexual intercourse between 4 to 7 days before the sperm samples collection. All samples were collected in a sterile container at the RHB anatomopathological laboratory. Sperm count analysis was done using the Papanicolaou protocol to determine the following parameters: Ph, sperm motility, concentration, and vitality following the model of WHO 2010 [18].
Detection of Mycoplasma Hominis and Ureaplasma Urealyticum, and Their Susceptibility Profile: Urethral samples were collected using a sterile cotton swab for the detection of Mycoplasma hominis and Ureaplasma urealyticum. The mycoplasma Biosynex kit (REF: 1030007) was used for the study. The galleries prepared using the protocol and incubated at 37°C for 24-48H and read afterward. Performance and selection testing of the sensitivity was based on the Clinical and Laboratory Standard Institute (CLSI) recommendations.
Detection of Antibodies Igm and Igg of Anti-Chlamydia Trachomatis: Three millilitres of blood samples collected in an additive-free tube were centrifuged at 1500 rpm for 5 mins. The serum obtained was stored in a microtube at 2-8°C for at most 2 days and analysed by the ELISA indirect technique as indicated by the ERBA reagent kit (REF: 1530907, India). The wells were prepared and read using an ELISA lecturer for 15 minutes at the dual-wavelength 450 and 630 nm. Interpretation of result was done using the kit instructions.
Evaluation of the Risk Factors Relative to Chlamydia Trachomatis and Mycoplasma Infections: A pretested survey was administered to different participants to collect information on risk factors relative to these STDs.
Statistical Analysis
The minimum sample size calculated using the Lorentz formula gave a sample of 59 participants. The data analysis was done using Epi Info 7.2 and Excel 2010. Results were expressed as frequencies and percentages/ratios. An odd ratio with 95% CI was used to measure the strength of the association and Chi-square was used to assess the link between two categorical variables. Statistical significance was assumed at p<0,005.
Results and Discussion
Results
A total of forty-three (43) men participated in the study and the results obtained were distributed as follows.
Socio-Demographic Characteristics, Clinical and Gynaecological History of Participants: Men aged between 31 and 41 years were the most represented, with 18 men (41.86%) among the 43 participants with a mean age of approximately 36±62 years (Table 1). Considering marital status, level of education, and profession, married men (n= 26/43) represented 60.47% of participants, those who attended higher education (n=25/43) represented 58.14% and private-sector workers (n=15/43) with 34.88% were the most represented. Among the participants, 19/43 participants (27.90%) had an infectious history, 24/43 men (55.81%) had no children and 44.18% encountered reproductive difficulties for one year.
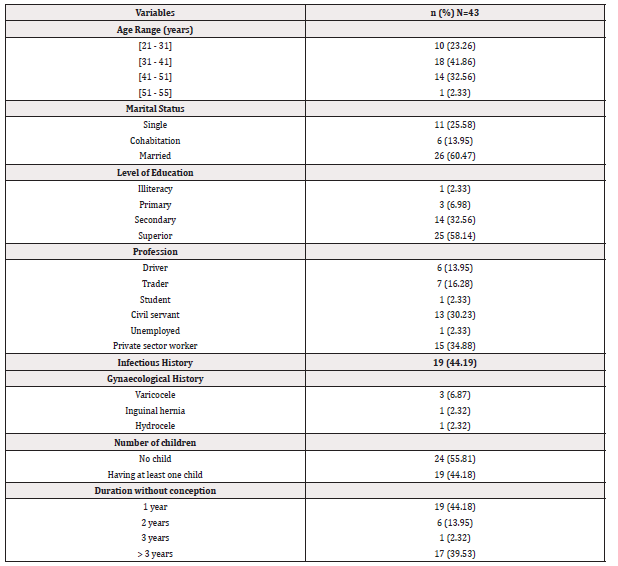
Table 1: Socio-demographic characteristics, clinical and gynaecological history of participants at the RHB.
Note*: n= Numbers of participants.
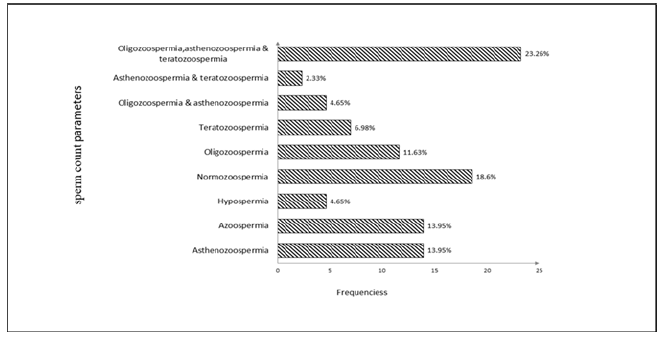
Semen Quality of Participants: Among the total population studied, the prevalence of men affected with semen abnormalities represented 81.39% (n=35/43; Figure 1). The abnormalities frequently observed were oligo-astheno-teratozoospermia (10/43; 23.26%), followed by isolated cases of azoospermia and asthenozoospermia (6/43; 13.95% each). Only eight men (18.60%) presented normal semen after analysis.
Serological Profile of Chlamydia Trachomatis Infection and Frequency of Genital Mycoplasma: Overall, only three participants were infected by Chlamydia trachomatis; consequently, the prevalence was approximately 6.98%. They showed seropositivity to both antibodies IgM and IgG of anti-Chlamydia trachomatis. Six participants (13.95%) presented a positive IgG serology indicating a scar of trachomatis infection (Table 2).
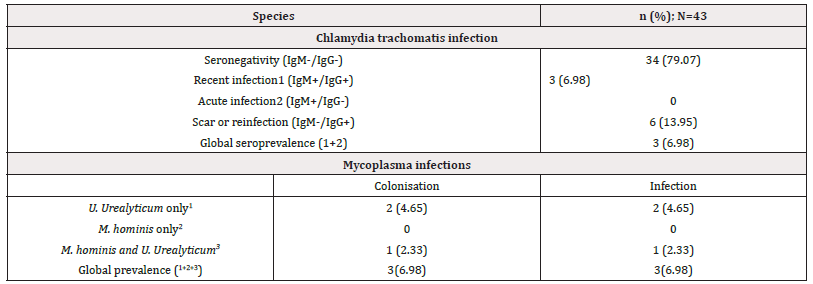
Table 2: Prevalence of Chlamydia trachomatis and mycoplasmas infections among men seeking for childbirth at the BRH.
Note*: N= total number of participants.
Out of the 43 individuals tested, 11 (25.58%) were positive for mycoplasmas species and three specimens (6.98%) presented colonization (bacterial growth<104) (Table 2). The overall prevalence of mycoplasma was 25.58%, with 23.26% (10) representing the prevalence of U. urealyticum but no one had M. hominis. Furthermore, the prevalence of coinfection with M. hominis and U. urealyticum was 2.33% with one infected patient.
Correlation Between Semen Quality and Infections Due to Chlamydia Trachomatis and Genital Mycoplasma ( Homins and U. Urealyticum): Overall, mycoplasma infections were more recurrent in men with teratozoospermia (n=3/43; 27.27%) and infections with U. urealyticum (n=3/43; 30%); whereas C. trachomatis infections were recurrent in men with oligo-astheno-teratozoospermia (n=1/43; 33.33%) (Table 3). In addition, the presence of mycoplasma species seemed to have an impact on semen quality as analysis gave a p-value of 0.02.
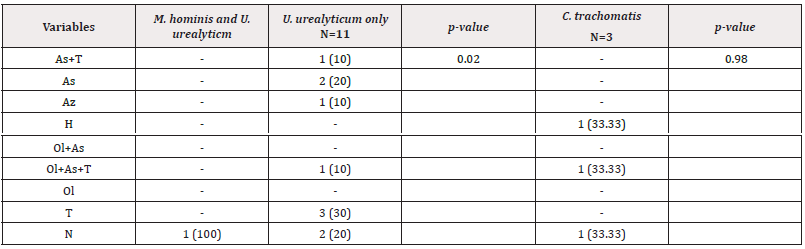
Table 3: Distribution of infections in relation to semen quality among men seeking for childbirth at the BRH.
Note*: Teratozoospermia=T; Oligozoospermia=Ol; Asthenozoospermia=As; Hypospermia=H; Azoospermia=Az; Asthenozoospermia and teratozoospermia=As+T; Oligozoospermia and asthenozoospermia=Ol+As; Oligozoospermia, Asthenozoospermia and teratozoospermia=Ol+As+T; Normozoospermia=N.
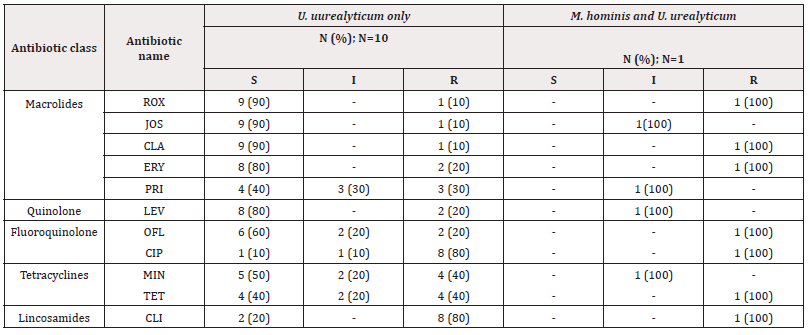
Antimicrobial Susceptibility Among Isolates of Genital Mycoplasmas Species: The U. urealyticum isolates from the 10 infected participants were highly sensitive to macrolides especially Clarithromycin (90%), Josamycin (90%), and Roxithromycin (90%). In contrast, mycoplasmas were highly resistant to lincosamides such as Clindamycin (81.81%) and to fluoroquinolone such as Ciprofloxacin (81.81%; Table 4).
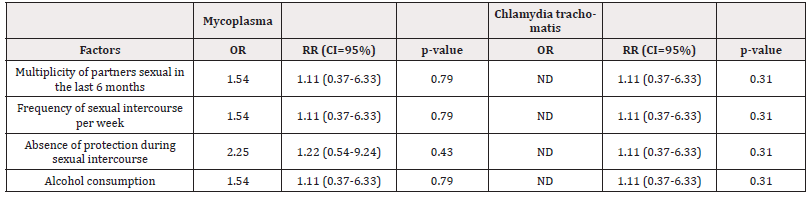
Risk Factors: Analysis revealed there was an association between mycoplasma infections and parameters such as multiplicity of sexual partners (OR=1.54; CI=0.37-6.33), frequency of sexual intercourse with a suspicious partner (OR=1.54; CI=0.37-6.33) (Table 5), excessive alcohol consumption and the absence of protection during sexual intercourse (OR=2.25; CI=0.54-9.24); the latter inducing twice the risk of contracting infections. The risk factors of trachomatis infections were not elucidated during this study.
Discussion
The current study aimed at determining the prevalence of infections due to Chlamydia trachomatis and mycoplasmas in men consulting for fatherhood desire at the Bafoussam regional hospital. The limitations of our study were firstly the low number of our population (only 43 men) and secondly the absence of a control group composed of fertile men. Thus, we have limited our comparison between infected and uninfected men. However important information was gathered from this study. Findings of the present study showed that there were high prevalences of semen abnormalities 81.3% and infections due to Chlamydia trachomatis 6.9% and Ureaplasma urealyticum 23.2%. The multiplicity of sexual partners, unprotected sexual intercourses, and insufficient levels of knowledge, attitudes, and practices relative to these infections constituted risk factors for the occurrence of these infections. The mean age of patients was 36 years with extremes of 21 and 55. Similar data were reported by Moussa and colleagues in Niamey, with 69.66% of patients aged less than 40 years [19].
The high prevalence of semen abnormalities obtained is close to the 83.9% reported by Momo et al. at the Dschang District Hospital [13]. These abnormalities could be associated with the patient's habits, gynaecological or infectious diseases impacting semen quality. The prevalence of Chlamydia trachomatis infection obtained was higher than the global prevalence of African regions published in 2016 (2.7%) [20] but lower than that reported (10,71%) by Nwankwo, et al. [21] in Kano, Nigeria. The result obtained from the current study could be explained by the sexual habits of the individuals, the late manifestations, and the delay in the diagnosis of infections. Also, insufficient knowledge, attitudes, and practices relative to sexually transmitted infections (STIs) and their consequences could explain the high prevalence obtained. It is widely known that C. trachomatis infections are asymptomatic in merely 50% of men. Hence, they are usually lately diagnosed [22], and most men usually go for traditional medicine as their first intention in case of sexual problems before consulting a medical doctor. This could nevertheless be a reason for the observed elevated prevalence. This prevalence of Ureaplasma urealyticum infections is higher than that reported in Yaoundé, Cameroon (10.8%) [23] and Tunisia (5.8%) [24]. The result obtained could be justified by frequent unprotected sexual intercourse (44.1%) and inappropriate knowledge related to mycoplasma infections.
Concerning the relationship between Chlamydia trachomatis, mycoplasmas infections, and semen abnormalities, C. trachomatis infected and non-infected patients presented no difference in their semen profiles. Previous studies have reported that the semen parameters differed between fertile and infertile men, with fertile men having higher semen volume (p<0.002), sperm concentration (p<0.0001) [25] different from our results. The relation could not be well elucidated due to the low prevalence reported during the study making it difficult to compare. On the other hand, out of 35 participants presenting abnormal semen, U. urealyticum was isolated from 8 men. Participants who presented sperms morphology inferior to 15 % and progressive mobility inferior to 30% were highly infected with U. urealyticum isolates. Furthermore, a p-value of 0.02 was obtained after matching semen abnormalities and mycoplasma infections’ prevalence. The present result revealed the possible role of these infections in the modification of semen parameters. These results differ from the findings of Radhouane et al. in Tunisia who reported no significant difference in the frequency of mycoplasma species in both normal and abnormal semen [26]. Strong sensitivities to Clarithromycin 90%, Josamycin 81.8%, and Roxithromycin 81.8%, and strong resistances to Ciprofloxacin 81.8%, and Clindamycin 81.8% by mycoplasma isolates were observed. The result observed is similar to that reported in Cameroon by Njunda, et al. [27] where high sensitivities to pristinamycin (72.4%), Josamycin (79.3%) and Clarithromycin (65.5%) and resistances to Ciprofloxacin (93.1%) and Clindamycin (100%) was reported. These sensitivities could be explained by the fact that these antibiotics belong to macrolides, and they act at the level of protein synthesis by binding to the 50S subunit of bacterial ribosomes. It is well known that M. hominis and U. urealyticum present different sensibilities to antibiotics with a natural resistance of M. hominis to macrolides with 14C (Erythromycin and Clarithromycin) and 15C (Azithromycin); whereas U. urealyticum is naturally resistant to lincosamides (lincomycin) as stipulated by Waites, et al. 2012 [28]. The resistance of U. urealyticum species to clindamycin and ciprofloxacin could be explained by the inappropriate use of antibiotics, through self-medication, and the large-scale use of these antibiotics in the management of STIs. Sensitizing couples on early screening of these infections could largely reduce the incidence of these infections. The multiplicity of sexual partners and the absence of protection during sexual intercourse constituted risk factors for the occurrence of mycoplasma infections. More so, the frequency of unprotected sexual intercourse with an infected person exposes more to the infection. Even though risk factors of C. trachomatis could not be elucidated in the present study, other studies reported that age of less than 30 years in men [29], the multiplicity of sexual partners (more than one partner in the last 6 months), celibacy and unprotected sexual intercourse [29] constituted risk factors of C. trachomatis infections.
Conclusion
The present study shows that at the BRH, West region of Cameroon, urogenital infections are indeed present in men seeking fatherhood. They presented different semen abnormalities that could be at the origin of their infertility. Generally, multiple sex partners and the absence of protection during sexual intercourse appeared as the major risk factors. Since urogenital infections are known to cause various sperm abnormalities; and infertility, it is therefore essential that these infections be screened early to anticipate the severity of their effects.
Acknowledgement
The authors are grateful to the University of Dschang Cameroon, for facilities and we acknowledge the support provided by the Bafoussam Regional Hospital.
Conflict of Interest
The authors declare that they have no competing interests.
References
- Ashok Agarwal, Aditi Mulgund, Alaa Hamada, Michelle Renee Chyatte (2015) A unique view on male infertility around the globe. Reprod Biol Endocrinol 13: 37.
- Elhussein OG, Ahmed MA, Suliman SO, Yahya leena I, Adam I (2019) Epidemiology of infertility and characteristics of infertile couples requesting assisted reproduction in a low-resource setting in Africa, Sudan. Fertil Res Pract 5: 7.
- Kumar N, Singh AK (2015) Trends of male factor infertility, an important cause of infertility: A review of literature. J Hum Reprod Sci 8(4): 191-196.
- Han LJ, He XF, Ye XH (2020) Methylenetetrahydrofolate reductase C677T and A1298C polymorphisms and male infertility risk: An updated meta-analysis. Medicine (Baltimore) 99(51): e23662.
- Gerais AS, Rushwan H (1992) Infertility in Africa. Popul Sci Cairo Egypt 12: 25-46.
- Ljubin Sternak S, Meštrović T (2014) Chlamydia trachomatis and Genital Mycoplasmas: Pathogens with an Impact on Human Reproductive Health. J Pathog 183167.
- Santé HA(2010) Diagnostic biologique de l’infection à Chlamydia trachomatis–Avis sur les actes.
- Barbeyrac B, Bébéar C (2005) Chlamydial pathogenesis: diagnostic and therapeutic consequences. Arch Pediatr 1:12 Suppl 1): S26-31.
- John Libbey Eurotext (2022) Annals of Clinical Biology - Limits and prospects of serological diagnosis in the era of in vitro gene amplification: genital chlamydia trachomatis infections and Chlamydia pneumoniae and Mycoplasmapneumoniae respiratory infections.
- Nwadioha S, Egesie JO, Emejuo H, Iheanacho E (2010) A study of female genital swabs in a Nigerian Tertiary Hospital. Asian Pac J Trop Med 3(7): 577-579.
- Karou SD, Djigma F, Sagna T, Nadembega C, Zeba M, et al. (2012) Antimicrobial resistance of abnormal vaginal discharges microorganisms in Ouagadougou, Burkina Faso. Asian Pac J Trop Biomed 2(4): 294-297.
- Nora Miro, Demetra Socolov, Mihai Mareş, Gabriela Anton, Valentin Nastasa, et al. (2013) Bacteriological agents which play a role in the development of infertility in: Acta Microbiol Immunol Hung 60(1): 41-53.
- Césaire Momo Tetsatsi A, Alumeti Munyali D, Romeo Bonsou Fozin G, Ngadjui E, Wankeu Nya M, et al. (2020) Semen quality among men attending urology services in the Dschang Health District, west Cameroon: A retrospective study on 379 Int J Reprod Biomed IJRM 18(2): 121-128.
- Wang Y, Liang CL, Wu JQ, Xu C, Qin SX, et al. (2006) Do Ureaplasma urealyticum infections in the genital tract affect semen quality? Asian J Androl 8(5): 562-568.
- Naessens A, Foulon W, Debrucker P, Devroey P, Lauwers S (1986) Recovery of microorganisms in semen and relationship to semen evaluation. Fertil Steril 45(1): 101-105.
- Xu C, Sun GF, Zhu YF, Wang YF (1997) The correlation of ureaplasma urealyticum infection with infertility. Andrologia 29(4): 219-226.
- Corinne TM, Anatole PC, Jeanne NY (2020) Comparison of Serum Inhibin B and Follicle-Stimulating Hormone (FSH) Level between Normal and Infertile Men in Yaoundé. Int J Reprod Med: 1-9.
- Lu JC, Huang YF, Lü NQ (2010) WHO Laboratory Manual for the Examination and Processing of Human Semen: its applicability to andrology laboratories in China. Zhonghua Nan Ke Xue Natl J Androl 16(10): 867-871.
- Moussa D, Soumana A, Amadou SM, Soli I, Tahirou I, et al. (2016) Profil hormonal chez l’homme en cas d’infertilité au laboratoire de radio immunologie de l’institut des radioisotopes de Niamey. Afr J Urol 22(4): 305-309.
- Rowley J, Vander Hoorn S, Korenromp E, Low N, Unemo M, et al (2019) Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ 97(8): 548-562P.
- Nwankwo E, Magaji N (2014) Prevalence of Chlamydia trachomatis infection among patients attending infertility and sexually transmitted diseases clinic (STD) in Kano, Northwestern Nigeria. Afr Health Sci 14(3): 672-678.
- Malhotra M, Sood S, Mukherjee A, Muralidhar S, Bala M, et al. (2013) Genital Chlamydia trachomatis: An update. Indian J Med Res 138(3): 303-316.
- Ahouga Voufo R, Maïdadi MF, Mbah EC, Esemu LF, Fouodji HP, et al (2020) STUDY on the gender prevalence and sensitivity of urogenital mycoplasmas to antibiotics in YAOUNDE, CAMEROON. Sci Afr 8: e00372.
- Sellami H, Znazen A, Sellami A, Mnif H, Louati N, et al (2014) Molecular Detection of Chlamydia trachomatis and Other Sexually Transmitted Bacteria in Semen of Male Partners of Infertile Couples in Tunisia: The Effect on Semen Parameters and Spermatozoa Apoptosis Markers. Kumar A, editor. PLoS ONE 9(7): e98903.
- Iwamoto T, Nozawa S, Mieno MN, Yamakawa K, Baba K, et al (2013) Semen quality of 1559 young men from four cities in Japan: a crosssectional population-based study. BMJ Open 3(4): e002222.
- Gdoura R, Kchaou W, Chaari C, Znazen A, Keskes L, et al (2007) Ureaplasma urealyticum, Ureaplasma parvum,Mycoplasma hominis and Mycoplasma genitalium infections and semen quality of infertile men. BMC Infect Dis 7(1): 129.
- Longdoh NA, Gregory H EE, Djeumako WA, Nguedia AJ C, Francois Xavier M K, et al. (2018). The Occurrence and Antimicrobial Susceptibility Patterns of Mycoplasma hominis and Ureaplasma urealyticum in Pregnant Women in Three District Hospitals in Douala, Cameroon. J Adv Med Med Res 27(11): 1-11.
- Waites KB, Duffy LB, Bébéar CM, Matlow A, Talkington DF, et al (2012). Standardized Methods and Quality Control Limits for Agar and Broth Microdilution Susceptibility Testing of Mycoplasma pneumoniae, Mycoplasma hominis, and Ureaplasma urealyticum. J Clin Microbiol 50(11): 3542-3547.
- Tadongfack TD, Nitcheu ILS, Ngoune R, Nkouayep VR, Selabi ACN, et al (2021). Seroprevalence of Chlamydia trachomatis IgA, IgM and IgG Antibodies and Associated Risk Factors among Sexually Active Individuals at Saint Vincent de Paul Hospital in Dschang, West Cameroon. Int STD Res Rev: 13-21.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.