Mini Review 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Roles of Andromedins in Prostate development and Cancer
*Corresponding author: Deepak Pandey, Department of Reproductive Biology, All India Institute of Medical Sciences, Ansari Nagar, New Delhi-110029.
Received: July 19, 2024; Published: July 24, 2024
DOI: 10.34297/AJBSR.2024.23.003070
Abstract
Prostatic tissue experiences and responds to a host of paracrine, autocrine and endocrine factors during its development, maintenance and pathological conditions like cancer. Structural and functional phenotypes of the prostatic epithelial compartment largely depend on the factors secreted by the stroma. Except for only a few, the cells from both the compartments of the prostate are androgen responsive. Many of such stromal factors which are produced and secreted under the influence of androgens are commonly named as ‘andromedins’. This minireview encompasses brief accounts on the functions of the andromedins and their signaling pathways involved in the glandular development and pathogenesis of the prostate.
Keywords: Andromedins, Prostate, Stromal-epithelial interaction, Glandular morphogenesis, Cancer
Abbreviations: AR: Androgen receptor; FGF: Fibroblast growth factor; KGF: Keratinocyte growth factor; EGF: Epithelial growth factor; HGF: Hepatocyte growth factor; IGF: Insulin-like growth factor
Introduction
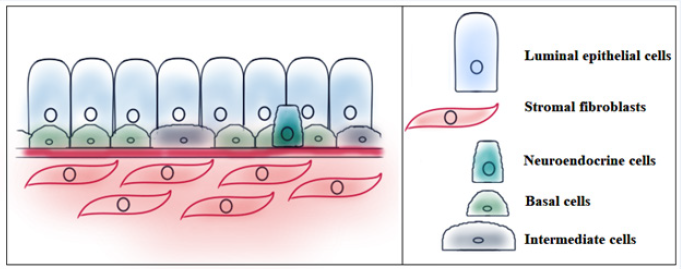
Prostate is a tubuloalveolar gland which acts as a male accessory sex organ. The growth, differentiation and morphogenesis of prostatic tissues largely depend on the sex-steroid hormones [1]. Closely apposed cell types (epithelial and stromal) interact with each other in the prostate to maintain homeostasis in the gland [2]. A schematic transverse section showing all the cell types of the glandular prostate has been presented in Figure 1.
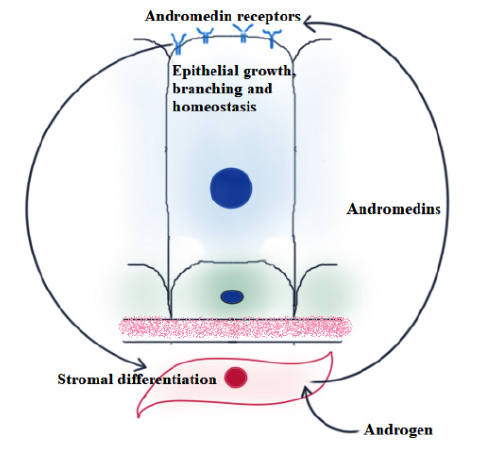
Studies have shown how the sex-steroids affect the individual cell types and their mutual interaction which, in turn, lead to alteration of tissue homeostasis [3,4]. Androgens, especially DHT act on both cell types via binding to AR. Upon binding, AR-DHT complex moves into the nucleus to regulate gene transcription [5]. Driven by androgen action, prostate stromal cells release certain growth-factors that induce epithelial cell proliferation, differentiation and luminal organization. The generic name used for such chemical factors is ‘andromedin’ [6,7]. Figure 2 describes the phenomenon schematically. Fasciana, et al. and Lu, et al. respectively identified FGF7 and FGF10 as the two foremost known members of the andromedin family [8,7]. In this review, we will focus on the contribution of some of the mostly investigated andromedins like KGF or FGF7, FGF10, EGF, HGF and IGF in prostatic epithelium during development and pathogenesis.
Prostate Development
As the process of prostate organogenesis is understood, it can be divided into four sequential stages: (1) Induction, (2) Epithelial budding and ductal growth, (3) Branching morphogenesis, (4) Ductal lumen formation [9,6]. Although the exact molecular mechanisms of induction are not well characterized, there are two main hypotheses: 1. Smooth muscle model and 2. Andromedin model. The ‘smooth muscle model’ proposes an inhibitory role of smooth muscles (differentiated by androgen signaling) on the prostatic epithelium budding which is otherwise regulated constitutively. On the other hand, ‘andromedin model’ suggests a direct stimulatory role of stromal andromedins to induce adjacent epithelial budding [10]. In rodents, before and during prostate development the mesenchymal (stromal) cells exhibit a lofty expression of Androgen Receptor (AR), whereas epithelial cells start expressing AR much later. This suggests the androgenic effects on epithelium are independent of epithelial AR but mediated via stromal AR [11]. In mouse models, embryonic Urogenital Epithelia (UGE) in combination culture with AR-positive Urogenital Mesenchyme (UGM) leads to development of normal epithelial structures, while UGE combined with AR-negative or non-functional AR containing mesenchyme leads to malformed/under-developed glands with stratified squamous epithelium [12,13]. An extensive study by Singh, et al. with the human fetal prostate as a model system showed that AR expression starts as early as 7th week across stromal cells, while epithelial cells show AR expression only during 11-13th weeks. At 22nd week of development, the expression happens to be across the gland [14]. This clearly indicates that early induction, budding and ductal growth of epithelial cells rely more on indirect androgenic effects via stromal paracrine factors and only at later stage direct androgenic signals are important for cytodifferentiation and completion of development.
In vitro prostate stromal cell culture displayed an increase in both Fgf7 and Fgf10 expression levels in the presence of androgens [7]. Kgf/Fgf7 null mutants lack a prostate phenotype, whereas Fgf10 null mutants show either complete lack of budding or formation of incomplete prostate buds [15,16]. Epithelium-specific deletion of Fgfr2, the receptor of Fgf7 and Fgf10 was found to obliterate the formation of anterior and ventral lobes [17]. Fgf7 and Fgf10 are expressed in mouse Urogenital Mesenchyme (UGM), the embryonic precursor of prostatic stroma [18]. Addition of exogenous Fgf7 and Fgf10 has shown to promote growth and branching of Urogenital Sinus Epithelium (UGE), cultured as an explant [19-21,16]. Fgfr2 is suggested to incite the PI3K/mTOR signaling pathway. Impairment or inhibition of either the receptor or the pathway is reported to suppress the growth and branching morphogenesis of prostate during development [22]. Conditional knockout of Fgfr2 leads to androgen-dependent tissue homeostasis without disrupting the androgen-dependent secretory behaviour of adult prostate. Epithelial cells devoid of Fgfr2 happen to have reduced expressions of Bone Morphogenetic Protein (BMP) 4, Transforming Growth Factor (TGF) β, HOXD13 [17].
Though crosstalk between Wnt (wingless) and Androgen (AR) signaling pathways has been widely studied in the context of prostate cancer, recently AR signaling is appreciated to induce Axin2, otherwise a direct downstream target of β-catenin/Wnt pathway. These cells have progenitor properties to regenerate prostatic epithelium [23]. Wnt signaling is found to be active in mouse UGM before budding and in both UGM and UGE during ductal growth [24,25]. Androgen Receptor (AR) coincidentally has similar kind of expression pattern, though during induction it binds with androgen in UGM and not in UGE cells [26,27]. β-catenin, principal mediator of Wnt signaling, if conditionally targeted, results in the formation of prostatic rudiments lacking expression of epithelial marker Nkx3.1 [24,28-30]. AR, though not directly, acts as an indispensible factor to mediate the expression of Axin2, supposed to support early development and regeneration of prostatic epithelium [23]. In 2008, Placencio, et al. discovered inter-dependence among the Androgen, Wnt and Tgfβ signaling pathways in the context of stromal-epithelial interaction in prostate. Androgen antagonist leads to an elevation of Wnts in Tgfβ-knockout stromal cells. High Wnt activity in epithelial cells maintains a high level of proliferation. On the contrary, Neutralization of Wnt signaling by Secreted Frizzled Related Protein (SFRP) 2 results in decreased epithelial cell proliferation [31]. Though the amount of work done in this direction is insufficient and inconclusive, it is important to execute a more detailed analysis of the Tgfβ-Wnt-androgen axis to reveal the actions of andromedins behind the development of the prostate gland.
Prostate Cancer
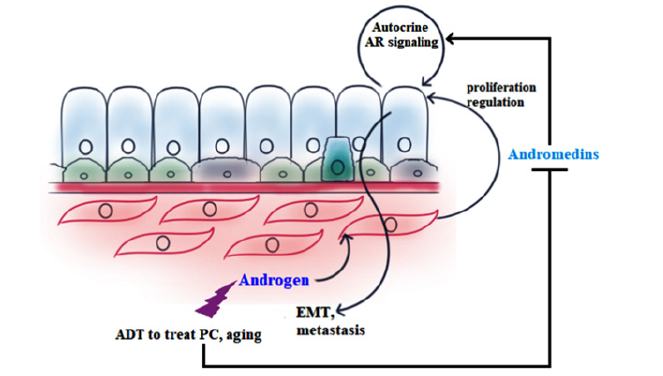
Prostate cancer is regarded as the most diagnosed non-skin malignancy in US. The age-adjusted incidence rate of prostate cancer is predicted to be much higher in currently developing countries than those which are already developed [32,33]. An interesting study by Wang, et al. showed that epithelial cells, if implanted in AR-positive UGM, develop into tumor upon androgen treatment in 36% mice [34]. When transformed Benign Prostatic Hyperplasia (BPH)-1 cells are grown in mice in combination with AR-positive stroma (WT UGM), cancer initiation and progression happen, but when combined with AR negative stroma (Tfm UGM), only small, irregular, non-cancerous glands develop [35]. Spontaneous development of Prostatic Intraepithelial Neoplasia (PIN) in PTEN+/- can be repressed to a certain extent in offspring bred with stromal AR-knockout (dARKO) mice [36]. Androgen Deprivation Therapy (ADT) in androgen-dependent prostate cancers has been reported to reduce FGF2, Interleukin (IL)6, Insulin-like Growth Factor (IGF)1 and TGFβ, all of which are able to increase cancer cell proliferation and tumor progression [37,38]. In the context of prostate cancer, androgen induced (obtained by microarray analysis) paracrine factors of stromal origin that unleash the proliferative ability in epithelial cells are Connective Tissue Growth Factor (CTGF), FGF 2,5,7, Hepatocyte Growth Factor (HGF), IGF 1,2, IL6, platelet derived growth factor (PDGF), TGFβ 1,2,3, Vascular Endothelial Growth Factor (VEGF), and Wnt. Decreased availability of proliferative growth factors upon ADT is suspected to cause Epithelial-to-Mesenchymal Transition (EMT) in search of more habitable microenvironment (metastasis) [39]. Figure 3 sketches a brief outline of this process. Extracellular Matrix (ECM) is composed of a huge pool of secreted peptides, glycoproteins, and proteoglycans which collectively provide the cells with the ground for attachment and a favorable milieu for growth and morphogenesis, entrapping growth factors and chemo attractants and functioning as a reservoir of the same. Androgen actively regulates the ECM secretion from the Cancer-Associated Fibroblasts (CAF)s. Androgen is shown to regulate the total volume of ECM and to alter the Matrix Metalloproteinase (MMP) activity in tumour stroma [40,39]. Collagen I, a principal component of ECM is attributed to increased tissue stiffness in solid tumour [41]. On addition of collagen I, RWPE-1 normal prostate epithelial cells in in vitro 3D overlay culture tend to form more invasive dysmorphic structures and less acini compared to the control growing in plain matrigel [42].
From the studies in rodents, it is now evident that AR in prostate stroma is essential to secrete and deposit collagen. Stromal AR knockout mice show a drastic decline in collagen level in ECM though the serum androgen level remains unchanged [13]. Impelled by Dihydrotestosterone (DHT), prostate stromal cells upregulate adhesive ECM proteins COL1A1, COL3A1, COL4A6 and FBN1, whereas the ECM degrading enzymes (MMPs) are downregulated. Hence, it is suggested loss of stromal AR gives rise to such an ECM environment which is less adhesive for cancer epithelial cells and more favorable for metastatic spread [43]. In 2015 Zou, et al., proposed the model of malignant transformation of the prostate epithelial cells due to the activation of autonomous AR signaling in epithelial cells instead of the homeostatic andromedin-based paracrine signaling. Also, epithelial stem cells are more prone to accumulate mutations because of the decline of the overall testosterone level with age [44,45].
Conclusion
The prostate is one of the most extensively used model systems to study glandular development and the roles of endocrine mediators. A series of experiments with Androgen Receptor Knockout (ARKO) mice indicated the dependance of prostate epithelial morphogenesis on the stromal counterpart. Studies in Prostate Epithelial Cell Specific (pes-ARKO) mice showed the importance of AR in the maintenance of the relative abundance of luminal and basal cell subpopulations in prostatic epithelium [44,36]. Stromal Fibroblast Specific ARKO (FSP-ARKO) leads to increased apoptosis, decline in epithelial proliferation and less collagen matrix [36]. Both fibroblast and smooth muscle specific ARKO (dARKO) were reported to affect epithelial cell viability, branch formation and anterior lobe size [46]. A complete knockout of AR in all the prostate cell types leads to no prostate development at all [47]. All these results, put together, clearly reveal how important the heterotypic cell-cell interaction is especially in the context of prostate development. Epithelial development seems to depend more on stromal contributions. Although there are already reports, as discussed above, on how stromal mediators induced by androgens assist and modulate epithelial morphogenesis, many of them remain to be firmly established as ‘andromedins by definition’. Keeping the recent developments in mind, it looks promising to investigate ‘androgens→ stromal mediators→ epithelial morphology’ axis with greater details in the context of both normal and malignant development, preferably by employing the state-of-the-art tissue culture techniques and the new age ‘omics’ approaches.
Highlights
i. Under the influence of androgens, the stroma of the prostate gland secretes growth promoting factors (andromedins) which cause proliferation of the epithelial compartment.
ii. Targeting andromedins instead of androgen itself, hopefully, may open a new door to prostate cancer therapeutics.
iii. This minireview encompasses brief accounts on the functions of the andromedins and their signalling pathways involved in the glandular development and pathogenesis of the prostate.
Introduction
Compliance with Ethical Standards
The manuscript does not contain any human or animal studies performed by any of the authors.
Funding
This research was funded by an Extramural ad hoc research Grant (RFC no-RBMCH/Adhoc/14/2020-21) from the Indian Council of Medical Research, New Delhi, and an Intramural Research Grant (A-789) from the All-India Institute of Medical Sciences, New Delhi.
Acknowledgements
None.
Conflict of Interest
None.
References
- Hayward SW, Cunha GR (2000) The Prostate: development and physiology. Radiol Clin North Am 38(1): 1-14.
- Hayward SW, Rosen MA, Cunha GR (1997) Stromal–epithelial interactions in the normal and neoplastic prostate. Br J Urol 79(2): 18-26.
- Neuzillet Y, Raynaud J, Radulescu C, Fiet J, Giton F, et al. (2017) Sexual steroids in serum and prostatic tissue of human non-cancerous prostate (STERPROSER trial). Prostate 77(15): 1512-1519.
- Silva MHAD and Souza DBD (2019) Current evidence for the involvement of sex steroid receptors and sex hormones in benign prostatic hyperplasia. Res Rep Urol 11: 1-8.
- Basu S, Tindall DJ (2010) Androgen Action in Prostate Cancer. Hormones and Cancer 1(5): 223-228.
- Tenniswood M (1986) Role of epithelial-stromal interactions in the control of gene expression in the prostate: an hypothesis. Prostate 9(4): 375-385.
- Lu W, Luo Y, Kan M, McKeehan WL (1999) Fibroblast growth factor-10. A second candidate stromal to epithelial cell andromedin in prostate. J Biol Chem 274(18): 12827-12834.
- FascianaC, Made AC, Faber PW, Trapman J (1996) Androgen Regulation of the Rat Keratinocyte Growth Factor (KGF/FGF7) Promoter. BiochemBiophys Res Commun 220(3): 858-863.
- Thomson AA (2008) Mesenchymal mechanisms in prostate organogenesis. Differentiation 76(6): 587-598.
- Toivanen R, Shen MM (2017) Prostate organogenesis: tissue induction, hormonal regulation and cell type specification. Development 144(8): 1382-1398.
- Takeda H, Chang C (1991) Immunohistochemical and in situ hybridization analysis of androgen receptor expression during the development of the mouse prostate gland. J Endocrinol 129(1): 83-89.
- Cunha GR, Lung B (1978) The possible influence of temporal factors in androgenic responsiveness of urogenital tissue recombinants from wild-type and androgen-insensitive (Tfm) mice. J Exp Zool 205(2): 181-193.
- Yu S, Yeh C, Niu Y, Chang H, Tsai Y, et al. (2015) Altered Prostate Epithelial Development in Mice Lacking the Androgen Receptor in Stromal Fibroblasts. Prostate 72(4): 437-449.
- Singh M, Jha R, Melamed J, Shapiro E, Hayward SW, et al. (2014) Stromal Androgen Receptor in Prostate Development and Cancer. Am J Pathol 184(10): 2598-2607.
- Guo L, Degenstein L, Fuchs E (1996) Keratinocyte growth factor is required for hair development but not for wound healing. Genes Dev 10(2): 165-175.
- Donjacour AA, Thomson AA, Cunha GR (2003) FGF-10 plays an essential role in the growth of the fetal prostate. Dev Biol 261(1): 39-54.
- Lin Y, Liu G, Zhang Y, Hu YP, Yu K, et al. (2007) Fibroblast growth factor receptor 2 tyrosine kinase is required for prostatic morphogenesis and the acquisition of strict androgen dependency for adult tissue homeostasis. Development 134(4): 723-734.
- FinchPW, Cunha GR, Rubin JS, Wong J, Ron D (1995) Pattern of keratinocyte growth factor and keratinocyte growth factor receptor expression during mouse fetal development suggests a role in mediating morphogenetic mesenchymal-epithelial interactions. Dev Dyn 203(2): 223-240.
- Thomson AA, CunhaGR (1999) Prostatic growth and development are regulated by FGF10. Development 126(16): 3693-3701.
- SugimuraY, Foster BA, HomYK, Lipschutz JH, Rubin JS, et al. (1996) Keratinocyte growth factor (KGF) can replace testosterone in the ductal branching morphogenesis of the rat ventral prostate. IntJ Dev Biol 40(5): 941-951.
- Huang L, Pu Y, Alam S, Birch L, Prins GS (2005) The role of Fgf10 signaling in branching morphogenesis and gene expression of the rat prostate gland: lobe-specific suppression by neonatal estrogens. Dev Biol 278(2): 396-414.
- Ghosh S, Lau H, Simons BW, Powell JD, Meyers DJ, et al. (2011) PI3K/mTOR signaling regulates prostatic branching morphogenesis. Dev Biol 360(2): 329-342.
- He Y, Hooker E, Yu E, Wu H, Cunha GR, et al. (2018) An Indispensable Role of Androgen Receptor in Wnt Responsive Cells During Prostate Development, Maturation, and Regeneration. Stem Cells 36(6): 891-902.
- Simons BW, Hurley PJ, Huangc Z, Rossc AE, Millerc R, et al. (2012) WntSignaling Though Beta-catenin is Required for Prostate Lineage Specification. Dev Biol 371(2): 246-255.
- Francis JC, Swain A (2018) Prostate Organogenesis. Cold Spring Harb Perspect Med 8(7): a030353.
- Cunha GR, Chung LW, Shannon JM, Taguchi O, Fujii H (1983) Hormone-induced morphogenesis and growth: role of mesenchymal-epithelial interactions. Recent Prog Horm Res 39: 559-598.
- Cunha GR, Donjacour AA, Cooke PS, Mee S, Bigsby RM, et al. (1987) The endocrinology and developmental biology of the prostate. Endocr Rev 8(3): 338-362.
- Francis JC, Thomsen MK, Taketo MM, Swain A (2013) beta-catenin is required for prostate developmentand cooperates with Pten loss to drive invasive carcinoma. PLoS Genet 9(1): e1003180.
- Mehta V, Schmitz CT, Keil KP, Joshi PS, Abler LL, et al. (2013) Betacatenin (CTNNB1) induces Bmp expression in urogenital sinus epithelium and participates inprostatic bud initiation and patterning. Dev Biol 376(2): 125-135.
- Julio MK, Shibata M, Desai N, Melissa M, Halili MV, et al. (2013) Canonical Wnt signaling regulates Nkx3.1 expression andluminal epithelial differentiation during prostate organogenesis. Dev Dyn 242(10): 1160-1171.
- Placencio VR, Sharif-Afshar A, Li X, Huang H, Consolate U, et al. (2008) Stromal TGF-ß signaling mediates prostatic response to androgenablation by paracrine Wnt activity. Cancer Res 68(12): 4709-4718.
- Brawley OW (2012) Trends in prostate cancer in the United States. J Natl Cancer Inst Monogr 2012(45): 152-156.
- TeohJCY, Hirai HW, Ho JMW, Chan FCH, Tsoi KKF, et al. (2019) Global incidence of prostate cancer in developing and developed countries with changing age structures. PLoS One 14(10): e0221775.
- Wang Y, Sudilovsky D, Zhang B, Haughney PC, et al. (2001) Human prostaticepithelial model of hormonal carcinogenesis. Cancer Res 61(16): 6064-6072.
- Ricke EA, Williams K, Lee YF, Couto S, Wang Y, et al. (2012) Androgen hormone action in prostatic carcinogenesis:stromal androgen receptors mediate prostate cancer progression,malignant transformation and metastasis. Carcinogenesis 33(7): 1391-1398.
- Lee SO, Tian J, Huang CK, Ma Z, Lai KP, et al. (2012) Suppressor role of androgen receptor in proliferation of prostatebasal epithelial and progenitor cells. J Endocrinol 213(2): 173-182.
- Nieto CM, Rider LC, Cramer SD (2014) Influence of stromal-epithelialinteractions on androgen action. Endocr Relat Cancer 21(4) : T147-160.
- KatzenwadelA, Wolf P (2015) Androgen deprivation of prostate cancer: Leading to a therapeutic dead end. Cancer Lett 367(1): 12-17.
- LeachDA, Buchanan G (2017) Stromal Androgen Receptor in Prostate CancerDevelopment and Progression. Cancers (Basel) 9(1): 10.
- Liao X, Thrasher JB, Pelling J, Holzbeierlein J, et al. (2003) Androgen Stimulates Matrix Metalloproteinase-2 Expression in Human Prostate Cancer. Endocrinology 144(5): 1656-1663.
- Burns CoxN, Avery NC, Gingell JC, Bailey AJ (2001) Changes in collagen metabolism in prostate cancer: a host response that may alter progression. J Urol 166(5): 1698-1701.
- Tyson DR, Inokuchi J, Tsunoda T, Lau A, Ornstein DK (2007) Culture requirements of prostatic epithelial cell lines for acinar morphogenesis and lumen formation in vitro: role of extracellular calcium. Prostate 67(15): 1601-1613.
- Leach DA, Need EF, Toivanen R, Trotta AP, PalenthorpeHM, (2015) Stromal androgen receptor regulates the composition of themicroenvironment to influence prostate cancer outcome. Oncotarget 6(18): 16135-16150.
- Zhou Y, Bolton EC, Jones JO (2015) Androgens and androgenreceptorsignaling in prostate tumorigenesis. J Mol Endocrinol 54(1): R15-29.
- ZenzmaierC, Untergasser G, Berger P (2008) Aging of the prostate epithelial stem/progenitor cell. Exp Gerontol 43(11): 981-985.
- Lai KP, Yamashita S, Huang CK, Yeh S, Chang C (2012) Loss of stromal androgen receptor leads tosuppressed prostate tumourigenesis via modulation of pro-inflammatory cytokines/chemokines.EMBO Mol Med 4(8): 791-807.
- De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, et al. (2004) A Sertoli cell-selective knockout of the androgen receptor causesspermatogenic arrest in meiosis. Proc Natl Acad Sci U S A 101(5): 1327-1332.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.