Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Serum Leptin Levels in Predicting Polycystic Ovary Syndrome (PCOS)
*Corresponding author: Delna NS, EMS Memorial Co-operative Hospital and Research Centre-College of Paramedical Sciences, Panambi, Perinthalmanna, Malappuram, Kerala, India.
Received: July 24, 2024; Published: July 30, 2024
DOI: 10.34297/AJBSR.2024.23.003076
Abstract
Polycystic Ovary Syndrome (PCOS) is a complex endocrine disorder with significant impacts on women’s health. This review focuses on elaborating the diagnostic potential of leptin in PCOS. A comprehensive review of the literature from the past decade was conducted using databases such as Google Scholar, PubMed, and Scopus. The search strategy was aimed at gathering relevant studies on the relationship between leptin and PCOS, including genetic aspects. All the reviewed studies unanimously confirm the diagnostic potential of leptin in PCOS, emphasizing its relevance in metabolic disorders associated with PCOS. The evidence gathered highlights elevated leptin levels, particularly in obesity, as a consistent characteristic of PCOS. The positive correlation between leptin and fat cell quantity underscores its potential role in PCOS pathogenesis. Leptin extends its impact beyond weight regulation, influencing ovum maturation and activating ovarian enzymes involved in steroid production. Genetic studies on Leptin Receptor Gene (LEPR) polymorphisms indicate associations between specific genetic variants and PCOS susceptibility. Combining leptin with markers like Anti-mullerian Hormone (AMH) shows high diagnostic accuracy, offering potential utility in clinical assessments. The multifaceted impact of leptin on both reproductive and metabolic aspects is evident. The findings support the integration of leptin with other markers for enhanced diagnostic accuracy, providing a promising avenue for future clinical applications in PCOS assessments.
Keywords: Polycystic ovary syndrome (PCOS), Leptin, Diagnostic markers, Obesity, Genetic aspects, Metabolic disorders, Ovarian enzymes, Leptin receptor gene (LEPR) polymorphisms, SNPs, Anti-Müllerian hormone (AMH)
Abbreviations: PCOS: Polycystic Ovary Syndrome; FSH: Follicle-stimulating hormone; LH: Luteinizing Hormone; LEP: Leptin; DHEAS: Dehydroepiandrosterone Sulfate; AMH: Anti-Müllerian Hormone; BMI: Body Mass Index; Sob-R: Soluble Leptin Receptor.
Introduction
Polycystic Ovary Syndrome (PCOS) is a prevalent endocrine disorder impacting women of reproductive age, spanning from adolescence through post-menopause [1]. This intricate condition affects the endocrine and metabolic systems, characterized by features such as anovulation, infertility, obesity, insulin resistance, and the presence of polycystic ovaries. Multiple risk factors, encompassing lifestyle choices, exposure to environmental pollutants, genetic predisposition, gut dysbiosis, neuroendocrine shifts, and obesity, contribute to the vulnerability of females to PCOS, these factors may collectively contribute to the development of metabolic syndrome by inducing conditions such as hyperinsulinemia, oxidative stress, hyperandrogenism, impaired folliculogenesis, and irregular menstrual cycles [2]. The exact underlying causes of PCOS remain unknown. Understanding the complex relationship between environmental influences and genetic predisposition is vital for unraveling the development of PCOS and, as a result, for developing more effective diagnostic and treatment strategies. Leptin, a multifunctional adipokine, plays a vital role in energy homeostasis and has been implicated in various physiological processes, including reproduction and metabolism [3]. PCOS poses a heightened risk for the development of diabetes, cardiovascular diseases, and metabolic syndrome in affected individuals [4]. In the pursuit of a more accurate assessment of insulin resistance, new markers are needed. Several proteins have emerged as potential indicators, including adipocytokines (adiponectin, visfatin, vaspin, and apelin), copeptin, irisin, PAI-1, and zonulin, which are strongly associated with PCOS physiopathology and insulin resistance [5]. Additionally, proteins like resistin, leptin, RBP4, kisspeptin, and ghrelin have been proposed as potential markers, although their roles remain controversial [5].
This review aims to expound the complex relationship between leptin and the pathophysiology of polycystic ovary syndrome (PCOS), with a specific emphasis on understanding how serum leptin serves as a potential indicator. By examining the connection between leptin, the leptin receptor gene, obesity, and the diverse metabolic and hormonal irregularities inherent in PCOS, the objective is to contribute to the current body of knowledge by exploring the nuanced role of leptin in the development and progression of PCOS, ultimately considering its potential utility as an early detection marker in the clinical context. We conducted a thorough review of the literature published in the past decade to gather information on the relationship between leptin and PCOS. Databases searched include Google Scholar, PubMed, and Scopus.
Leptin (LEP)
Obesity is a prevailing characteristic observed in polycystic ovary syndrome (PCOS) patients. Leptin plays a crucial role in the pathogenesis of PCOS in the context of obesity and oxidative stress [6]. Leptin classified within the “tumor necrosis factor” family as a cell factor, is an amino peptide consisting of 167 amino acids. Produced by adipose cells in fat tissues, LEP circulates either freely or in combination with a soluble isomer. Furthermore, LEP levels exhibit a positive correlation with the quantity of fat cells. In humans, LEP primarily functions to regulate appetite and facilitate heat generation for weight control. Elevated leptin levels have been validated to stimulate the appestat, potentially leading to reduced heat generation [7,6]. Beyond its role in weight regulation, LEP exerts modulatory effects on the maturation of female egg cells and activates ovarian enzymes involved in steroid production, including Follicle-Stimulating Hormone (FSH) and Luteinizing Hormone (LH). Additionally, LEP has been proposed as an indicator of peripheral signals, providing insights into the adequacy of reproductive functions’ nutritional support [8] (Figure 1).
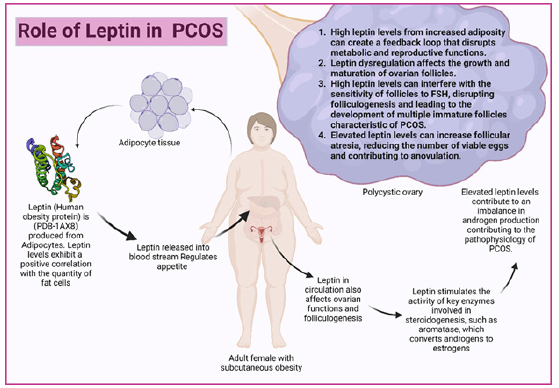
Figure 1: Leptin contributes to the development and progression of PCOS through multiple pathways, including hormonal imbalances (hyperandrogenism and disrupted gonadotropin secretion), impaired folliculogenesis, increased follicular atresia, obesity-related leptin resistance, chronic inflammation, oxidative stress, and genetic predispositions. These factors collectively lead to the characteristic symptoms of PCOS, such as anovulation, infertility, and metabolic disturbances.
Role of Leptin in PCOS as a Predictive Marker: Leptin is derived from adipocytes and is encoded by the ‘ob’ gene, it has a crucial role in relaying metabolic signals to the brain and modulating the hypothalamic-pituitary-ovarian axis. Elevated leptin levels are strongly linked to obesity, often coexisting with Polycystic Ovarian Syndrome (PCOS) [9]. PCOS involves hyperandrogenism, inappropriate leutinising hormone secretion, insulin resistance, and hyperinsulinemia. The relationship between leptin and key factors in PCOS, such as gonadotropins, androgens, and insulin, remains a subject of ongoing research and discussions [10]. Proposed mechanisms suggest that increased intra-follicular levels of leptin in obesity directly impact ovarian functions in PCOS, potentially inducing relative resistance to gonadotropins. Based on the current available studies between leptin, obesity, and insulin action, it is hypothesized that leptin plays a role in PCOS [11]. PCOS patients, characterized by hyperandrogenemia, elevated leutinizing hormone concentrations, hyperinsulinemia/insulin resistance, and obesity, provide a model to assess the inter-relationship of hyperinsulinemia and androgen excess with leptin concentrations beyond the association with obesity alone [12]. Several studies have attempted to clarify the detailed mechanisms and leptin-mediated pathways, addressing the gap in knowledge. A controlled, prospective, clinical study aimed to investigate epithelial Na (+) channel (ENaC) expression in the endometrium of overweight/obese women with PCOS during the implantation window, explored the mechanism connecting leptin-mediated reduction of γ-ENaC to low endometrial receptivity. Results revealed a decreased expression of γ-ENaC in the secretory phase endometrium of PCOS patients with elevated serum leptin levels. In cultured endometrial cells, leptin dose-dependently down-regulated γ-ENaC expression and reduced JAr spheroid attachment rates, mediated by the STAT3 signal pathway. Overweight/ obese PCOS patients with increased leptin levels exhibited a higher biochemical pregnancy rate, suggesting a potential role of leptin in attenuating endometrial receptivity and increasing early pregnancy loss [13].
A cross-sectional study examined the relationship between leptin levels and Body Mass Index (BMI) in Iranian women with PCOS. In the PCOS group, consisting of 40 women, and the control group of 36 healthy women, fasting blood samples were collected for the measurement of serum total leptin, blood glucose, serum insulin, follicle-stimulating hormone, and Luteinizing Hormone (LH). The findings revealed a higher mean BMI and total leptin levels in the PCOS group compared to the control group. Additionally, a positive association was observed between leptin levels, BMI, and LH among women with PCOS, emphasizing the interconnectedness of these factors in PCOS-related obesity [14]. A similar study focusing on women with PCOS, aimed to assess the association between leptin and markers of insulin resistance. Sixty diagnosed PCOS cases, as per Rotterdam criteria, were included. Insulin resistance was measured using the homeostatic model assessment-insulin resistance (HOMA-IR) in the study and serum leptin levels were determined by ELISA. The results revealed a positive correlation between serum leptin levels and markers of insulin resistance. Multiple regression analysis highlighted a statistically significant contribution of fasting insulin levels to HOMA-IR. These findings emphasize the involvement of leptin in the disruptions to carbohydrate metabolism observed in PCOS patients [15].
A study of 89 patients with PCOS and 139 individuals without PCOS, the significant elevation of serum leptin levels, particularly in subgroups characterized by hyper-fasting serum insulin, hyperandrogenism, and overweight/obesity, underscored its potential relevance in PCOS-related metabolic disorders. Assessing the diagnostic validity of leptin, both alone and in combination with total testosterone, dehydroepiandrosterone sulfate (DHEAS), and free testosterone, revealed promising outcomes, with the combined leptin and anti-Müllerian hormone (AMH) displaying the highest diagnostic accuracy. Correlations between serum leptin levels and various clinical and metabolic parameters, such as fasting serum insulin, fasting plasma glucose, homeostasis model assessment of insulin resistance, body mass index, and total testosterone, further emphasized the involved associations in PCOS. According to the study, leptin either alone or combined with anti-Müllerian hormone, shows diagnostic potential for PCOS, with substantial correlations observed with metabolic and hormonal markers [16]. In a cohort study of 40 women with PCOS and 40 age and Body Mass Index (BMI) matched controls, leptin levels were significantly higher in the PCOS group, while adiponectin levels showed a non-significant decrease. The positive correlation of leptin with fasting glucose, fasting insulin, free testosterone levels, and homeostatic model assessment of insulin resistance (HOMA-IR) values, along with the negative correlation of adiponectin with BMI, suggests distinct adipocytokine patterns in normal-weight women with PCOS compared to matched controls. These findings bring more understanding to the role of adipose tissue in PCOS pathogenesis beyond the conventional association with high BMI [17]. According to a prospective, observational study aimed to investigate the serum levels of ghrelin and leptin in Saudi women with PCOS, comparing obese and lean individuals, and examining their association with metabolic profiles. The study involved 252 women (130 with PCOS and 122 normo-ovulatory controls). While no significant differences were observed in ghrelin and leptin levels between PCOS and control groups, obese PCOS patients exhibited significantly lower ghrelin and higher leptin levels compared to lean patients. In the PCOS group, ghrelin and leptin levels correlated with various metabolic parameters, including Body Mass Index (BMI), waist-hip ratio, cholesterol levels, and insulin. Multiple regression analysis identified insulin as the primary determinant for both ghrelin and leptin levels in PCOS patients, emphasizing the potential influence of obesity, hyperinsulinemia, and insulin resistance. Their study shows the relationship between these hormones and the metabolic profile in Saudi women with PCOS [18].
In a study involving 39 women with PCOS and 34 healthy controls matched by body mass index, the relationship between serum leptin and ghrelin concentrations and dietary macronutrient content was explored. While the groups did not significantly differ in macronutrient intake or hormone concentrations, in PCOS women, serum leptin positively correlated with total fat, total cholesterol, saturated fatty acids, and monounsaturated fatty acids intake. Conversely, serum ghrelin showed an inverse correlation with total fat, monounsaturated fatty acids, polyunsaturated fatty acids, and long-chain polyunsaturated fatty acids intake. According to the study, PCOS women exhibited a negative association between homeostasis model assessment of insulin resistance (HOMA-IR) and serum ghrelin levels, and a positive relationship with serum leptin concentration, total dietary fat, and monounsaturated fatty acids intake. These findings suggest that dietary components and insulin resistance may influence serum leptin concentrations in PCOS women, potentially impacting energy balance [19]. In a case-control study evaluating adipokines in obese adolescent girls with or without Polycystic Ovary Syndrome (PCOS), it was found that levels of adiponectin, leptin, and ghrelin were similar between obese girls with PCOS and obese controls. While hormonal markers such as LH, LH/FSH, and cortisol were higher in the PCOS group, adiponectin and leptin levels did not show significant differences between the two groups. Adiponectin negatively correlated with BMI and positively correlated with fasting glucose, while leptin positively correlated with BMI, estradiol, and TSH. These findings suggest that among obese adolescents with PCOS, the presence of PCOS itself may not significantly influence adiponectin and leptin levels, with ghrelin showing no significant correlation [20].
Different macronutrients also have an impact on serum levels of leptin and ghrelin in women with PCOS. In a case-control study with 30 PCOS patients and 30 age and BMI-matched controls, the researchers found higher levels of serum leptin, insulin, testosterone, and luteinizing hormone in PCOS women compared to healthy women, with no significant difference in ghrelin concentrations. Among PCOS women, an inverse association was observed between leptin concentration and total dietary fat and saturated fatty acid intake, independent of BMI and total energy intake. Their study suggests that certain dietary components, such as fat and saturated fatty acids, may influence serum leptin in PCOS women, while ghrelin is not affected by these factors [21]. In a study aimed to assess the variability and predictability of adiponectin, leptin, resistin, and their ratios in non-obese and obese women with Anovulatory Polycystic Ovary Syndrome (aPCOS). The results showed that the aPCOS group had lower adiponectin, Adiponectin: Leptin ratio (A: L), and Adiponectin: Resist In ratio (A: R) but higher leptin and Leptin: Resist in Ratio (L: R) compared to controls. The obese aPCOS group exhibited further decreases in adiponectin, A: L, and A: R but increases in leptin and L: R compared to the non-obese aPCOS and control group. A: L demonstrated the best discriminatory power in predicting aPCOS, followed by adiponectin alone, L: R, and leptin alone, while resistin alone had the poorest discriminatory power [22].
Genetic Insights: Leptin and Leptin Receptor Gene Polymorphisms: The human leptin (lep) gene is located in chromosome 7 and the human leptin protein is 167-. kDa in length [23]. There are several studies suggesting that mutations and any deficiencies in the leptin and leptin receptor gene lead to obesity and related disorders [24]. A study by [25] investigated the association between Single Nucleotide Polymorphisms (SNPs) of Gln223Arg and Pro- 1019Pro in the Leptin Receptor Gene (LEPR) and PCOS in a Korean population. The research found a significant association between the Pro1019Pro and Gln223Arg polymorphisms in LEPR and PCOS, suggesting that these genetic variants might contribute to the risk of PCOS in the Korean population. The study involved 379 Korean women, including 229 with PCOS and 150 healthy controls. The results indicate the association between gene polymorphisms and the presence of PCOS, shedding light on the genetic factors influencing PCOS susceptibility. Another similar study was conducted among Iranian women to investigate the association of Leptin Receptor (LEPR) polymorphisms (rs7799039 and rs1137101) and circulating levels of leptin and Soluble Leptin Receptor (sOB-R) in healthy fertile women and those with PCOS, including PCOS-infertile and PCOS-Recurrent Pregnancy Loss (RPL) subjects. Findings show significantly higher plasma leptin and sOB-R levels in PCOS, lower levels in PCOS-RPL, and distinct genotype frequencies in PCOS-infertile women for both rs7799039 and rs1137101 polymorphisms. Increased leptin levels were associated with the risk of PCOS and RPL in women with PCOS [26].
Another study investigated the association of LEPR polymorphisms, specifically Lys109Arg (rs1137100) and Gln223Arg (rs1137101), with PCOS in Chinese women. The findings indicated a significant difference in the genotypic distributions of Lys109Arg between PCOS and control groups, with the AA genotype associated with a lower risk of PCOS. Furthermore, plasma soluble leptin receptor (sOB-R) levels were significantly increased in PCOS patients and were linked to PCOS independent of BMI. These findings suggest that the LEPR Lys109Arg polymorphism may be associated with PCOS susceptibility, with the AA genotype potentially acting as a protective factor. Additionally, elevated sOB-R levels could serve as a new indicator for the severity of PCOS [27]. Similarly, a retrospective case-control study conducted in Tunisian and Bahraini Arab women involving 255 PCOS cases and 253 controls from Bahrain, and 320 PCOS cases and 447 controls from Tunisia was conducted. The minor allele frequencies and genotype distribution of tested LEP variants were comparable between PCOS cases and controls in both populations. However, five-locus haplotype analysis revealed positive associations of certain haplotypes with PCOS in Bahraini women, emphasizing the importance of considering ethnic and racial background in genetic association studies [28].
Conclusion
A cumulative synthesis of evidence from published studies supporting the diagnostic potential of leptin in PCOS was achieved through this review. Elevated leptin levels, particularly in the context of obesity, consistently emerged as a notable characteristic of PCOS, and the positive correlation between leptin and the number of fat cells emphasizes the potential role of this adipokine in the pathogenesis of PCOS, especially in individuals with increased adiposity. Beyond its role in weight regulation, leptin exerts modulatory effects on the maturation of the ovum and activates ovarian enzymes involved in steroid production. These associations between leptin, gonadotropins, and insulin highlight the multi-faceted impact of leptin on both the reproductive and metabolic aspects of PCOS. Investigations into Leptin Receptor Gene (LEPR) polymorphisms provided valuable genetic insights, indicating associations between Specific Genetic Variants (SNPs) and PCOS susceptibility. These studies collectively underscore the diagnostic potential of leptin in PCOS. The significant elevation of serum leptin levels in subgroups characterized by hyperinsulinemia, hyperandrogenism, and obesity underscores its relevance in PCOS-related metabolic disorders and combining leptin with other markers, such as Anti- Müllerian Hormone (AMH), shows high diagnostic accuracy, offering potential utility in clinical assessments.
Acknowledgments
We are deeply grateful to Mr. Akshay V.P. and the Biomedical Research division of DELBIODESK- Research & Innovations for their support in creating the illustrations using Biorender.
Conflict of Interest
The authors declare no conflict of interest to disclose.
References
- Teede HJ, Tay CT, Laven JJ, Dokras A, Moran LJ, et al. (2023) Recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Eur J Endocrinol 189(2): G43-G64.
- Singh S, Pal N, Shubham S, Sarma DK, Verma V, Marotta F, Kumar M (2023) Polycystic Ovary Syndrome: Etiology, Current Management, and Future Therapeutics. J Clin Med 12(4): 1454.
- Zheng SH, Du DF, Li XL (2017) Leptin levels in women with polycystic ovary syndrome: a systematic review and a meta-analysis. Reprod Sci 24(5): 656-670.
- Namavar Jahromi B, Dabaghmanesh MH, Parsanezhad ME, Fatehpoor F (2017) Association of leptin and insulin resistance in PCOS: A case-controlled study. Int J Reprod Biomed 15(7): 423-428.
- Polak K, Czyzyk A, Simoncini T, Meczekalski B (2017) New markers of insulin resistance in polycystic ovary syndrome. J Endocrinol Invest 40(1): 1-8.
- Shetty SS, Kumari NS, Hegde P, Roopashree PG, Suhasini PC (2022) Leptin gene polymorphism Rs7799039; G2548A, metabolic and oxidative stress markers in polycystic ovarian syndrome. Journal of King Saud University-Science 34(6): 102222.
- Friedman J (2014) Leptin at 20: an overview. J Endocrinol 223(1): T1-8.
- Zhao X, Xiong Y, Shen Y (2023) Leptin plays a role in the multiplication of and inflammation in ovarian granulosa cells in polycystic ovary syndrome through the JAK1/STAT3 pathway. Clinics 78: 100265.
- Chakrabarti J (2013) Serum leptin level in women with polycystic ovary syndrome: correlation with adiposity, insulin, and circulating testosterone. Ann Med Health Sci Res 3(2): 191.
- Rizk NM, Sharif E (2015) Leptin as well as free leptin receptor is associated with polycystic ovary syndrome in young women. Int J Endocrinol 2015: 927805.
- Al yasiry RZ, Jwad MA, Hasan MF, Alsayigh HA (2022) How obesity affects female fertility. Medical Journal of Babylon 19(2): 111-114.
- Aversa A, La Vignera S, Rago R, Gambineri A, Nappi RE, et al. (2020) Fundamental concepts and novel aspects of polycystic ovarian syndrome: expert consensus resolutions. Front Endocrinol 11: 516.
- Lin XH, Liu ME, Xu HY, Chen XJ, Wang H, et al. (2015) Leptin down-regulates γ-ENaC expression: a novel mechanism involved in low endometrial receptivity. Fertil Steril 103(1): 228-35.e3.
- Jalilian N, Haghnazari L, Rasolinia S (2016) Leptin and body mass index in polycystic ovary syndrome. Indian J Endocrinol Metab 20(3): 324-328.
- Nasrat H, Patra SK, Goswami B, Jain A, Raghunandan C (2016) Study of association of leptin and insulin resistance markers in patients of PCOS. Indian J Clin Biochem 31: 104-107.
- Peng Y, Yang H, Song J, Feng D, Na Z, et al. (2022) Elevated serum leptin levels as a predictive marker for polycystic ovary syndrome. Frontiers in Endocrinology 13: 845165.
- Gözüküçük M, Yarcı Gürsoy A, Destegül E, Taşkın S, Şatıroğlu H (2020) Adiponectin and leptin levels in normal weight women with polycystic ovary syndrome. Horm Mol Biol Clin Investig 41(4).
- Daghestani MH, Daghestani M, Daghistani M, El Mazny A, Bjørklund G, et al. (2018) A study of ghrelin and leptin levels and their relationship to metabolic profiles in obese and lean Saudi women with polycystic ovary syndrome (PCOS). Lipids Health Dis 17(1): 195.
- Polak AM, Krentowska A, Łebkowska A, Buczyńska A, Adamski M, et al. (2020). The association of serum levels of leptin and ghrelin with the dietary fat content in non-obese women with polycystic ovary syndrome. Nutrients 12(9): 2753.
- Kale Gurbuz T, Akhan SE, Bastu E, Telci A, Iyibozkurt AC, et al. (2013) Adiponectin, leptin and ghrelin levels in obese adolescent girls with polycystic ovary syndrome. J Pediatr Adolesc Gynecol 26(1): 27-30.
- Pourghassem Gargari B, Houjeghani S, Farzadi L, Houjeghani S, Safaeiyan A (2015) Relationship between Serum Leptin, Ghrelin and Dietary Macronutrients in Women with Polycystic Ovary Syndrome. Inter J Fertil Steril 9(3): 313-321.
- Obirikorang C, Owiredu WK, Adu Afram S, Acheampong E, Asamoah EA, et al. (2019) Assessing the variability and predictability of adipokines (adiponectin, leptin, resistin and their ratios) in non-obese and obese women with anovulatory polycystic ovary syndrome. BMC research notes 12: 1-8.
- Green ED, Maffei M, Braden VV, Proenca R, DeSilva U, et al. (1995) The human obese (OB) gene: RNA expression pattern and mapping on the physical, cytogenetic, and genetic maps of chromosome 7. Genome Res 5(1): 5-12.
- Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, et al. (1997) Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 387(6636): 903-908.
- Li L, Lee KJ, Choi BC, Baek KH (2013) Relationship between leptin receptor and polycystic ovary syndrome. Gene 527(1): 71-74.
- Kargasheh FB, Ansaripour S, Borumandnia N, Moradi N, Zandieh Z, et al. (2021) Association of leptin G2548A and leptin receptor Q223R polymorphisms and their serum levels with infertility and recurrent pregnancy loss in Iranian women with polycystic ovary syndrome. PloS One 16(8): e0255920.
- Tu X, Yu C, Gao M, Zhang Y, Zhang Z, et al. (2017) LEPR gene polymorphism and plasma soluble leptin receptor levels are associated with polycystic ovary syndrome in Han Chinese women. Per Med 14(4): 299-307.
- Dallel M, Sghaier I, Finan RR, Douma Z, Hachani F, et al. (2019) Circulating leptin concentration, LEP gene variants and haplotypes, and polycystic ovary syndrome in Bahraini and Tunisian Arab women. Gene 694: 19-25.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.