Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
TH07-A New Novel Topical Treatment for Androgenic Alopecia
*Corresponding author: Sara Bar Yehuda PhD, 21 Arbel st. Rishon Le Zion, Israel, 75474 , Email: sara@biomedwrite.co.il
Received: June 17, 2024; Published: June 20, 2024
DOI: 10.34297/AJBSR.2024.23.003033
Abstract
Objectives: Androgenic Alopecia (AGA) is common among men. Currently, topical Minoxidil and oral Finasteride are approved by the FDA for the treatment of AGA. Unfortunately, neither of them is completely effective and systemic adverse events have been reported after Finasteride administration. Triple Hair Inc. has developed a new topical treatment regimen using a combination of Finasteride, Latanoprost and Minoxidil - TH07. Each of the compounds was effective and safe as a topical treatment in animal models and in clinical studies of AGA. The aim of this proof-of-concept study was to evaluate the effectiveness of the TH07 in comparison to the 3 drugs as monotherapy on hair growth in man with AGA.
Methods: Patients with light to moderate AGA were randomized to be treated topically, once daily, for 6 months with TH07, 0.1% Finasteride, 0.03% Latanoprost or 5% Minoxidil. Data of investigators’ assessment based on pictures, as well as patients’ selfassessment and satisfaction was collected.
Results: A moderate hair re-growth in the majority of the participant treated with TH07 in comparison to the retreatment with its active components administered as monotherapy was reported by the investigators. Most of the patients treated with TH07 were satisfied with their hair appearance in comparison to the other treatments. No systemic adverse events were reported and the TH07 was well tolerated.
Conclusion: The data of the current study demonstrated that the topical administration of TH07 resulted in an improved efficacy in the treatment of the AGA compared to treatment with each of the ingredients administered separately.
Keywords: Androgenic alopecia, Topical Treatment, Finasteride, Latanoprost, Minoxidil, Combination
Introduction
Male Androgenetic Alopecia (AGA) is the most common type of baldness. AGA is characterized by progressive patterned hair loss from the scalp, starting in the frontal area and the vertex, following a defined pattern [1]. The disease is expressed by the shortening of the anagen phase (hair growth) of the hair cycle and prolongation of the telogen phase (no hair growth/quiescent follicular stage), leading to progressive miniaturization of the hair follicle [2]. The diagnosis of AGA is based on clinical presentation (i.e. Hamilton-Norwood scale progression of hair loss), clinical and family history of AGA [3,4]. Genetic and androgenic factors play roles in the pathophysiology of the disease. Androgens bind to intracellular androgen receptors, which activate various cell growth factors and calcium/K influx in the dermal papilla cells [5-7]. The metabolite 5-Dihydrotestosterone (DHT) affects the dermal papilla cells, causing progressive hair follicle miniaturization and hair cycle abnormalities leading to the development of AGA [1]. In addition, there is a cross-talk between the dermal papilla and the hair follicle cells, which results from the secretion of numerous growth factors and/or extracellular matrix factors from the dermal papilla cells, such as Insulin-like growth factor 1 (IGF-1) and the basic Endothelial Growth Factor (VEGF), that stimulate hair growth [8-10]. A variety of pharmacotherapeutic agents aiming to efficiently and safely treat AGA are being tested. Finasteride is a DTH suppressing 5-alfa-reductase inhibitor [11]. In addition, the drug was found to be involved in the protein kinase B (PKB-AKT)/β-catenin dependent cell growth mechanism, as well as up-regulating the expression of IGF-1 [12]. Finasteride has been approved by FDA since 1997 for the treatment of mild to moderate AGA as an oral drug (1 mg/day). However, complications such as depression and gynecomastia, decreased libido, erectile dysfunction and a reduction in the volume of ejaculation have been reported upon its use [13]. In order to overcome the side effects that occur upon the oral use of Finasteride, the efficacy and safety of topically administered Finasteride on various characterization of AGA have been evaluated in several human clinical studies. A significant improvement in the status of scalp hair was observed and the treatment was tolerable [14-18]. Latanoprost is an eye drop solution, containing prostaglandin F2α(PGF2α) analogues used in the treatment of ocular hypertension and open angle glaucoma [19]. Hypertrichosis was detected in the ipsilateral terminal and regional intermediate hairs of the upper and lower eyelids, as well as of the vellus hair of the lower eyelid skin upon treatment with the drug [20]. Latanoprost stimulates VEGF protein synthesis in human dermal papilla [21] and activates the Protein kinase C (PKC) proteins family, that are overexpressed in the mid-anagen and mature anagen stages of the hair follicle [22]. These properties could play an important role in promoting hair growth. Data from animal models and a human clinical study of patients with AGA revealed that topical treatment of Latanoprost induced hair growth, while no serious adverse events were reported [23-25]. Minoxidil, is a pyrimidine derivative that induces hair growth by prolonging anagen duration, shortening telogen, and enlarging miniaturized follicles [26]. The drug was found to increase VEGF production, induce cell growth factors secretion in dermal papilla cells, as well as increasing Ca2+ influx and opening potassium channels [27-31]. Data from clinical studies demonstrated that Minoxidil, administered topically, once or twice daily for a period up to 5 years, was safe and stimulates new non-vellus hair growth [32-35].
Taken together, the 3 drugs have safely induced hair growth when administered topically in cases of AGA. However, the efficiency of Finasteride was found to vary from 31% to 66% and Minoxidil averaged only 38% effectiveness for hair re-growth [34],[36]. In addition, the beneficial results of topical Minoxidil are evident after continuous topical application of at least 4 months and up to 12 months and if the treatment is discontinued, increased hair loss occurs within 2 or 3 months. Thus, attempts have been invested to attain improved outcomes using different topical combinations of the drugs, such as: Minoxidil with Finasteride [37-40] or with Latanoprost [41]. The data from the studies clearly demonstrated that the combination regimen was superior to either of the monotherapies. In addition, the combinations were safe and well tolerated. Following that, Triple Hair Inc. company developed the TH07 formula which contained Finasteride, Latanoprost and Minoxidil. The goal of the current clinical trial was to evaluate the ability of TH07 to promote hair re-growth, while still being safe, compared to each of the components used as a monotherapy.
Methods
Test Product
The TH07 product and all the ingredients were manufactured by Triple Hair in a cGMP manufacturing facility. The product comprises of a clear homogenous solution of 5% Minoxidil, 0.1% Finasteride and 0.03% Latanoprost dissolved in a solvent vehicle comprising on absolute alcohol, propylene glycol and diethyl glycol. The 5% strength for Minoxidil was selected as this concentration is the maximum that the FDA allows for topical use and is approved by various regulatory agencies. Latanoprost at the concentration of 0.03% is approved as an ocular drop (Latisse) to treat ocular hypertension. Finasteride tablets (1mg) taken orally daily is also approved by the US FDA, EU and Health Canada for treating AGA. A concentration of 0.1%, which represents 1 mg/cc, was chosen. The product was packaged in 60 mL opaque white bottles filleted with metred pumps, calibrated to dispense 100µL per spray. The test product was stored at temperatures between 15C0 to 30C0.
Patients
Participants were recruited through radio advertisements, meetings with managers and hair stylists in beauty salons in the Dieppe, Moncton and the Riverview areas, New Brunswick, Canada. The Triple Hair Inc. website featured a complete online registration form with specifications of the study and enrollment criteria.
Study Inclusion Criteria were: men between 19 and 65 years old; diagnosis of AGA; alopecia levels between 2 and 5 on the Norwood Scale Classification (Light to moderate hair loss); participant had to be available once a month for 15 minutes for a period of 6 months; sign a consent and medical form at the beginning of the study; could not dye their hair for the duration of the study; and had to bring their medication for the first appointment.
Study Exclusion Criteria were: the use of another hair loss prevention product in the previous year; the use of the following substances in the previous six months: Steroids, Vasodilators, Cytotoxic agents, Topical antiseptic and/or antifungals, Antibiotics, Antihypertensive agents, Diuretics, or Specifically contraindicated agents (such as spironolactone, cimetidine, diazoxide, cyclosporine, or ketoconazole); previous scalp correction surgery; presence of skin infections on the scalp; existence of an allergy or hypersensitivity to colorant and skin cancer.
Study Design
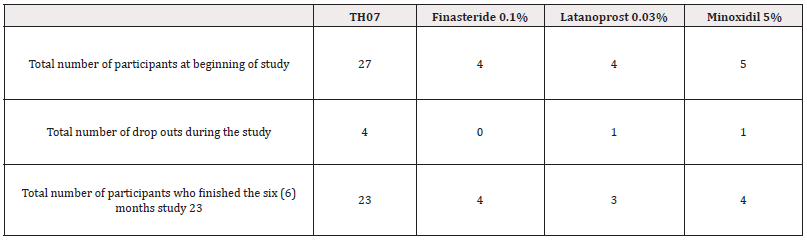
The effect of topical TH07 on hair regrowth was compared with the daily topical application of each drug in a randomized, double-blind study in men with AGA
The Patients were Randomized to 4 Topical Treatment Groups: TH07, 0.1% Finasteride, 0.03% Latanoprost and 5% Minoxidil. On the first visit, the distance between the base of the nose and the middle of the hair crown, as well as the distance separating the most distal part of the helix and the hair crown were measured. A square area of two-by-two centimeters was measured around the middle point of the hair crown (marked by a washable felt crayon). Then, pictures of the scalp from different angles and distances were taken by a professional photographer.
The patients were instructed to apply a dosage of 1ml (10 metered dose sprays of 0.1ml) to the scalp once a day after cleansing. The same scalp-measuring and photo-taking method was repeated in the following five sessions in order to properly evaluate the product’s efficacy. At the end of the treatment, the participants were asked to fill out a self-assessment questionnaire about the treatment’s success and their satisfaction regarding the change in their appearance. This questionnaire has been validated by Barber, et al. [42].
The Investigators' assessment of hair growth was conducted from the pictures taken from each patient before and at the end of the treatment. A growth sufficient to achieve a less pronounced pattern of baldness was classified as minimal improvement of hair growth. Moderate growth meant that there was a visible change. As for the dense level, it was described as sufficient growth to be cut and combed.
Statistical Analysis
Patients' self-assessment and satisfaction and investigators' assessment are presented in the percentage of subjects who specified their agreement with each of the questionnaires' declarations.
A One-Way Analysis of Variance (ANOVA) was used to test the difference in means between the four groups. This test was used to determine whether there was any significant difference between the mean values of the four groups. In addition, the Tukey HSD test was used to assess the difference in results between two groups. Lastly, a comparison of the mean values to determine which group offered the best results was conducted by weighting the test subject’s responses using the following scale: a status of "High Growth" was scaled as 3, a status of "Moderate Growth" was scaled as 2, a status of " Minimal Growth " was scaled as 1 and a status of " No Growth " was scaled as 0.
Results
Drop Outs
Out of a total of 40 patients recruited initially for the study, 6 subjects withdrew from the study. Overall, 34 patients completed the study (Table1).
Patients' Self-Assessment
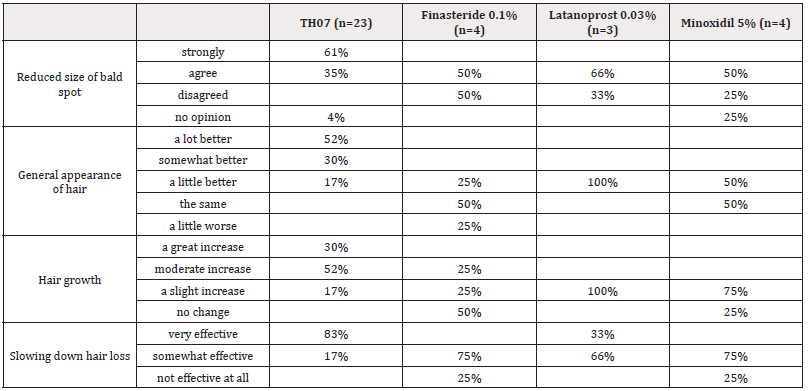
Most of the patients treated with TH07 (61%) strongly agreed, while most of the patients in the Latanoprost (66%) and Minoxidil (50%) agreed that the size of their bald spot has been reduced. Half (52%) of the TH07 group reported that the general appearance of the hair seems a lot better, whereas 100% of the Finasteride group felt it was a little better. The appearance of the hair looked the same for half of the patients in the Finasteride and Minoxidil groups. The majority of the TH07 treated patients (52%) saw a moderate increase in their hair growth, a slight change was reported from the Latanoprost (100%) and the Minoxidil (75%) groups and no change by the finasteride group (50%). A very effective slowing down of hair loss was reported by the TH07 treated patients (83%), while a somewhat effective slowing down was achieved in the Finasteride (75%), Latanoprost (66%) and Minoxidil (75%) groups (Table 2).
Patients' Satisfaction
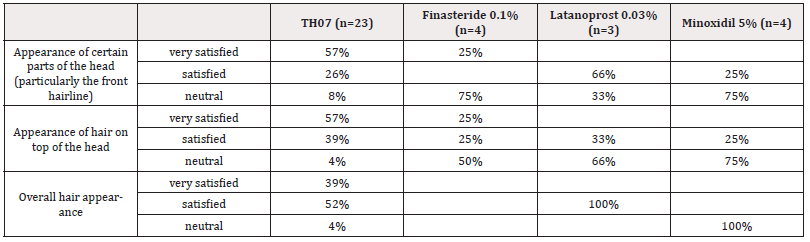
When asked about their satisfaction concerning the appearance of front hair line compared to before treatment, 57% of the respondents in the TH07 treatment group said that they were very satisfied. Among the responders in the Latanoprost treatment group, 66% declared that they were satisfied with their front hairline. Of the respondents in the Minoxidil treatment group, 75% were neutral with regards to the front hairline.
When describing their satisfaction with their hair on top of their head 57% of responders in the TH07 treatment group said they were very satisfied and 66% of the Latanoprost treatment group were neutral. Of the respondents in the Minoxidil treatment group, 75% were neutral with regards to the effect of treatment on the hair at the top crown of their head.
Concerning the overall appearance and their hair, 52% of the responders in the TH07 treatment group were satisfied. When asked about their satisfaction with their overall hair appearance, all participants of the Latanoprost treatment group were satisfied. When asked what they thought about the appearance of parts of their head before and after treatment, all respondents in the Minoxidil treatment group said they were neutral about their hair growth overall.
The data are presented in (Table 3).
Investigators' Assessment
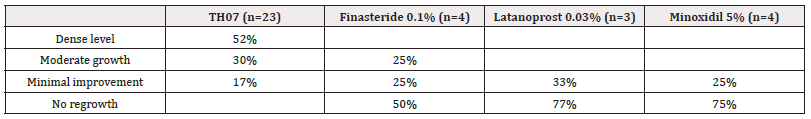
In the TH07 treatment group 52% of the patients demonstrated a dense level of hair growth and 30% moderate growth. No growth was observed in most of the subjects in the Finasteride, Latanoprost and Minoxidil treatment groups (50%, 77% and 75%, respectively).
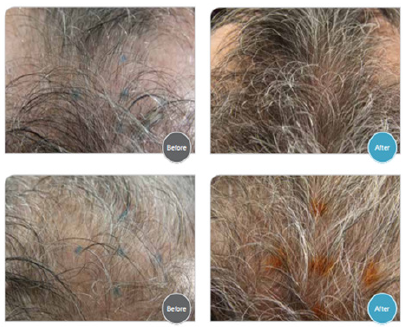
A representative picture of two patients, before and after the treatment with TH07 is presented in Figure 1 and the data are presented in Table 4.
Analyses of the hair growth statues according to the weighting scale (ranging from 0 for "No Growth" to 4 for "High Growth") revealed that TH07 had the highest average of 2.35±0.78, demonstrating a moderate hair growth. The cooperative ingredients demonstrated a low statue of minimal growth (0.1% Finasteride - 0.25± 0.50, 0.03% Latanoprost 0.33±0.58 and 5% Minoxidil 0.75±0.96).
The ANOVA analysis resulted with a significant difference between the four groups of treatments (P<0.0001). The Tukey HSD test results showed a significant difference between the TH07 treatment and the 5% Minoxidil treatment (P<0.05, as well as with the 0.1% Finasteride treatment (p<0.01) and the 0.03% Latanoprost treatment (p<0.01). However, comparisons between the 5% Minoxidil and the 0.03% Latanoprost treatments, between the 5% Minoxidil and the 0.1% Finasteride treatments, as well as between the 0.03% Latanoprost and the 0.1% Finasteride treatments were not statistically significant.
Safety
None of the participants in the TH07 treatment group noticed any change in their sexual activities. Only 3 participants had itching and eruption problems which were deemed a reaction to propylene glycol, 2 after 1 month and 1 after more than 5 months of treatment.
Discussion
The rationale for the development of TH07 came from cumulative evidence, demonstrating that the efficacy of each of the ingredient was limited. The data from the current study indicate that the topical use of TH07 is more effective at promoting hair growth than the component administered as monotherapy. No systemic side effects were reported by participants. Pharmacokinetic studies report a low rate of absorption in a topical route of administration than when taken orally, which results in fewer systemic side effects while still being effective at promoting hair growth [17], [43,44]. It is suggested that the TH07 formula's mechanisms of action (direct and an indirect) affects simultaneously some elements of the disease's pathophysiology (Figure 2). The direct mechanism of action of the combined treatment includes: an anti-androgenic effect and increasing the levels of growth factors. Minoxidil was found to hamper the activity of androgen receptors in the dermal papilla cells [29]. Furthermore, increased IGF-1 expression in Minoxidil-treated mice was observed compared to saline-treated mice [45]. Increased levels of MAPK and Akt phosphorylation were detected post-treatment with Minoxidil in dermal papilla cells cultures [46]. Finasteride alters the conversion of testosterone to DHT in the dermal papilla cell, leading to the decreased androgenic activation and to the reversal of the follicular miniaturization of the hair [47]. Increased levels of IGF-1 were detected in patients who demonstrated a moderate hair growth improvement after 12 months of treatment with Finasteride [48]. Finasteride treatment significantly raised the levels of phosphorylated AKT in dermal papilla cell line and in 2 human primary dermal papilla cells [12]. The addition of Latanoprost to human ciliary muscle cells cultures increased ERK1/2 levels [22]. The in-direct mechanism of action of the combined treatment includes: induction off VEGF production, increased supply of oxygen, blood and nutrients to the hair follicle, opening of the Ca2+/K channels and increased absorption and penetration of the drugs. Both VEGF mRNA and protein were significantly elevated in Minoxidil-treated dermal papilloma cells [27]. Minoxidil enhanced the growth of cultured isolated red deer anagen follicle [49] and raised the amount of intracellular Ca2+ and VEGF production in cultures [50]. It might also be suggested these activities of the Minoxidil could enhance the absorption of the Finasteride, resulting in the improved efficiency of hair growth and density. VEGF protein synthesis was elevated in a dose-dependent manner in Latanoprost treated human dermal papilla cells [21]. Taken together, it seems that when the 3 drugs are applied simultaneously, various pathophysiological process of the diseased are affected. Figure 2 presents a scheme of the suggested TH07mechanism of action.
Conclusion
The data of the current study demonstrated that the topical treatment of TH07 resulted in an improved efficacy in the treatment of the AGA compared to each of the ingredients administered separately, as monotherapy. The superiority of the TH07 might be due affecting simultaneously various mechanisms, playing a role in the development of AGA, resulting with an additive/synergistic effect on hair growth. TH07 offers an option to meet the unmet demand for safe and effective treatments for AGA, as this new drug combination has a potential of hair growth with very little potential of side effects.
Limitation
Our study has some limitations as well. The size of the samples was small, especially in the single drugs treatments. There is no vehicle arm, even though all 3 drugs and the TH07 were prepared with the same vehicle. A longer period of treatment may have further refine the differences between the treated groups. Larger and longer clinical studies are needed to establish the advantage of the TH07 combined treatment.
Acknowledgment
None.
Conflicts Of Interest
None.
References
- Rathnayake D, Sinclair R (2010) Male androgenetic alopecia. Expert Opin Pharmacother 11(8): 1295-1304.
- Bergfeld WF (1995) Androgenetic alopecia: an autosomal dominant disorder. Am J Med 98(1A): 95S-98S.
- Norwood OT (1975) Male pattern baldness: classification and incidence. South Med J 68(11): 1359-1365.
- Ishino A, Takahashi T, Suzuki J, Nakazawa Y, Iwabuchi T et al. (2014) Contribution of hair density and hair diameter to the appearance and progression of androgenetic alopecia in Japanese men. Br J Dermatol 171: 1052-1059.
- Tsai MJ, O’Malley BW (1994) Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem 63: 451-486.
- Von Ledebur EICF, Almeida JP, Loss ES, Wassermann GF (2002) Rapid effect of testosterone on rat Sertoli cell membrane potential. Relationship with K+ATP channels. Horm Metab Res 34(10): 550-555.
- Foradori CD, Weiser MJ, Handa RJ (2008) Non-genomic actions of androgens. Front Neuroendocrinol 29(2): 169-181.
- Itami S, Kurata S, Takayasu S (1995) Androgen induction of follicular epithelial cell growth is mediated via insulin-like growth factor-I from dermal papilla cells. Biochem Biophys Res Commun 212(3): 988-994.
- Botchkarev VA, Kishimoto J (2003) Molecular control of epithelial-mesenchymal interactions during hair follicle cycling. J Investig Dermatology Symp Proc 8(1): 46-55.
- Inui S, Fukuzato Y, Nakajima T, Yoshikawa K, Itami S (2003) Identification of androgen-inducible TGF-beta1 derived from dermal papilla cells as a key mediator in androgenetic alopecia. J Investig Dermatology Symp Proc 8(1): 69-71.
- Dhurat R, Sharma A, Rudnicka L, Kroumpouzos G, Kassir M, Galadari H, et al. (2020) 5-Alpha reductase inhibitors in androgenetic alopecia: Shifting paradigms, current concepts, comparative efficacy, and safety. Dermatol Ther 33(3): e13379.
- Rattanachitthawat N, Pinkhien T, Opanasopit P, Ngawhirunpat T, Chanvorachote P Finasteride Enhances Stem Cell Signals of Human Dermal Papilla Cells. In Vivo 33(4): 1209-1220.
- Lolli F, Pallotti F, Rossi A, Fortuna MC, Caro G, Lenzi A, et al. (2017) Androgenetic alopecia: a review. Endocrine 57(1): 9-17.
- Mazzarella F, Loconsole F, Cammisa A, Mastrolonardo M VG (1997) Topical finasteride in the treatment of androgenic alopecia. Preliminary evaluations after a 16-month therapy course. J Dermatolog Treat 8: 189-192.
- Charuwichitratana S, Krisdapong P, Sumethiwit R et al. (2003) Randomized double-blind placebo-controlled trial in the treatment of male androgenetic alopecia with 0.1% finasteride solution. Jpn J Derm 113: 881.
- Sitticharoenchai P (2006) Clinical evaluation of topical formulation of finasteride in male androgenetic alopecia. Bangkok.
- Hajheydari Z, Akbari J, Saeedi M, Shokoohi L (2009) Comparing the therapeutic effects of finasteride gel and tablet in treatment of the androgenetic alopecia. Indian J Dermatol Venereol Leprol 75(1): 47-51.
- Piraccini BM, Blume Peytavi U, Scarci F, Jansat JM, Falqués M, Otero R, et al. (2022) Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia: a phase III, randomized, controlled clinical trial. J Eur Acad Dermatol Venereol 36(2): 286-294.
- Alexander CL, Miller SJ, Abel SR (2002) Prostaglandin analog treatment of glaucoma and ocular hypertension. Ann Pharmacother 36(3): 504-511.
- Johnstone MA (1997) Hypertrichosis and increased pigmentation of eyelashes and adjacent hair in the region of the ipsilateral eyelids of patients treated with unilateral topical latanoprost. Am J Ophthalmol 124(4): 544-547.
- Zhang H, Zhu H FW (2006) Effects of latanoprost on vascular endothelial growth factor protein synthesis in human dermal papilla cells. J Clin DERMATOLOGY 35(5): 283-285.
- Husain S, Jafri F, Crosson CE (2005) Acute effects of PGF2alpha on MMP-2 secretion from human ciliary muscle cells: a PKC- and ERK-dependent process. Invest Ophthalmol Vis Sci 46(5): 1706-1713.
- Uno H, Zimbric ML, Albert DM, Stjernschantz J (2002) Effect of latanoprost on hair growth in the bald scalp of the stump-tailed macacque: a pilot study. Acta Derm Venereol 82(1): 7-12.
- Sasaki S, Hozumi Y, Kondo S (2005) Influence of prostaglandin F2alpha and its analogues on hair regrowth and follicular melanogenesis in a murine model. Exp Dermatol 14(5): 323-328.
- Blume Peytavi U, Lönnfors S, Hillmann K, Garcia Bartels N (2012) A randomized double-blind placebo-controlled pilot study to assess the efficacy of a 24-week topical treatment by latanoprost 0.1% on hair growth and pigmentation in healthy volunteers with androgenetic alopecia. J Am Acad Dermatol 66(5): 794-800.
- Messenger AG, Rundegren J (2004) Minoxidil: mechanisms of action on hair growth. Br J Dermatol 150(2): 186-194.
- Lachgar S, Charveron M, Gall Y, Bonafe JL (1998) Minoxidil upregulates the expression of vascular endothelial growth factor in human hair dermal papilla cells. Br J Dermatol 138(3): 407-411.
- Otomo S (2002) [Hair growth effect of minoxidil]. Nihon Yakurigaku Zasshi 119(3): 167-174.
- Hsu CL, Liu JS, Lin AC, Yang CH, Chung WH, et al. (2014) Minoxidil may suppress androgen receptor-related functions. Oncotarget 5(8): 2187-2197.
- Goren A, Naccarato T, Situm M, Kovacevic M, Lotti T, et al. (2017) Mechanism of action of minoxidil in the treatment of androgenetic alopecia is likely mediated by mitochondrial adenosine triphosphate synthase-induced stem cell differentiation. J Biol Regul Homeost Agents 31(4): 1049-1053.
- York K, Meah N, Bhoyrul B, Sinclair R (2020) A review of the treatment of male pattern hair loss. Expert Opin Pharmacother 21(5): 603-612.
- Roberts JL (1987) Androgenetic alopecia: treatment results with topical minoxidil. J Am Acad Dermatol 16(3 Pt 2):705-710.
- Shupack JL, Kassimir JJ, Thirumoorthy T, Reed ML, Jondreau L (1987) Dose-response study of topical minoxidil in male pattern alopecia. J Am Acad Dermatol 16(3 Pt 2): 673-676.
- Olsen EA, Dunlap FE, Funicella T, Koperski JA, Swinehart JM, Tschen EH, et al. (2002) A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. J Am Acad Dermatol 47(3): 377-385.
- Blume Peytavi U, Issiakhem Z, Gautier S, Kottner J, Wigger Alberti W, et al. (2019) Efficacy and safety of a new 5% minoxidil formulation in male androgenetic alopecia: A randomized, placebo-controlled, double-blind, noninferiority study. J Cosmet Dermatol 18(1): 215-220.
- Mella JM, Perret MC, Manzotti M, Catalano HN, Guyatt G (2010) Efficacy and safety of finasteride therapy for androgenetic alopecia: a systematic review. Arch Dermatol 146(10): 1141-1150.
- Tanglertsampan C (2012) Efficacy and safety of 3% minoxidil versus combined 3% minoxidil /0.1% finasteride in male pattern hair loss: a randomized, double-blind, comparative study. J Med Assoc Thai 95(10): 1312-1316.
- Sheikh S, Ahmad A, Ali SM, Ahmad MU, Paithankar M, et al. (2015) A New Topical Formulation of Minoxidil and Finasteride Improves Hair Growth in Men with Androgenetic Alopecia. J Clin Exp Dermatol Res 6(1): 2-6.
- Suchonwanit P, Srisuwanwattana P, Chalermroj N KS (2018) A randomized, double-blind controlled study of the efficacy and safety of topical solution of 0.25% finasteride admixed with 3% minoxidil vs. 3% minoxidil solution in the treatment of male androgenetic alopecia. J Eur Acad Dermatol Venereol 32(12): 2257-2263.
- Marotta JC, Patel G, Carvalho M, Blakeney S (2020) Clinical Efficacy of a Topical Compounded Formulation in Male Androgenetic Alopecia: Minoxidil 10%, Finasteride 0.1%, Biotin 0.2%, and Caffeine Citrate 0.05% Hydroalcoholic Solution. Int J Pharm Compd 24(1): 69-76.
- Bloch LD, Escudeiro CC, Sarruf FD, Valente NYS (2018) Latanoprost and minoxidil: Comparative double-blind, placebo-controlled study for the treatment of hair loss. Surg Cosmet Dermatology 10: 39-43.
- Barber BL, Kaufman KD, Kozolff RC, Girman CJ, Guess HA (1998) A hair growth questionnaire for use in the evaluation of therapeutic effects in men. J Dermatolog Treat 9: 181-186.
- Caserini M, Radicioni M, Leuratti C, Annoni O, Palmieri R (2014) A novel finasteride 0.25% topical solution for androgenetic alopecia: pharmacokinetics and effects on plasma androgen levels in healthy male volunteers. Int J Clin Pharmacol Ther 52: 842-849.
- Monti D, Tampucci S, Burgalassi S, Chetoni P, Lenzi C, Pirone A, et al. (2014) Topical formulations containing finasteride. Part I: in vitro permeation/penetration study and in vivo pharmacokinetics in hairless rat. J Pharm Sci 103(8): 2307-2314.
- Park KS, Park DH (2016) Comparison of Saccharina japonica-Undaria pinnatifida Mixture and Minoxidil on Hair Growth Promoting Effect in Mice. Arch Plast Surg 43(6): 498-505.
- Dastan M, Najafzadeh N, Abedelahi A, Sarvi M, Niapour A (2016) Human platelet lysate versus minoxidil stimulates hair growth by activating anagen promoting signaling pathways. Biomed Pharmacother 84: 979-986.
- Motofei IG, Rowland DL, Baconi DL, Tampa M, Sârbu MI, Păunică S, et al. (2018) Androgenetic alopecia; drug safety and therapeutic strategies. Expert Opin Drug Saf 17(4): 407-412.
- Tang L, Bernardo O, Bolduc C, Lui H, Madani S, Shapiro J (2003) The expression of insulin-like growth factor 1 in follicular dermal papillae correlates with therapeutic efficacy of finasteride in androgenetic alopecia. J Am Acad Dermatol 49(2): 229-233.
- Davies GC, Thornton MJ, Jenner TJ, Chen YJ, Hansen JB, Carr RD, et al. (2005) Novel and established potassium channel openers stimulate hair growth in vitro: implications for their modes of action in hair follicles. J Invest Dermatol 124(4): 686-694.
- Li M, Marubayashi A, Nakaya Y, Fukui K, Arase S (2001) Minoxidil-induced hair growth is mediated by adenosine in cultured dermal papilla cells: possible involvement of sulfonylurea receptor 2B as a target of minoxidil. J Invest Dermatol 117(6): 1594-1600.




 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.