Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
The Role of Monocyte Chemoattractant Protein 1 in the Diagnosis of Glomerular Pathology – A Retrospective Study
*Corresponding author: Stancescu Madalina Gabriela, Carol Davila Teaching Hospital of Nephrology and Faculty of Medicine, Carol Davila University of Medicine and Pharmacy, 010731, 050474 Bucharest Romania.
Received: August 14, 2024; Published: August 21, 2024
DOI: 10.34297/AJBSR.2024.23.003127
Abstract
Background and Objectives: The early diagnsosis off different types of glomerulonephritis remains a topic of ongoing reaserch, given the mortality and morbidity associated with this pathology.
Materials and Methods: We conducted a retrospective, unicentric study to determine the urinary concentration of MCP-1 in 50 patients who underwent renal biopsy in our tertiary nephrology service in Roumania between 2018 and 2024 and in 20 healthy volunteers.
Results: Our study indicates that urinary MCP-1 levels are significantly higher in patients with glomerular diseases of inflammatory origin compared to those with glomerular diseases due to other causes (such as hypertension or genetic conditions) and the control group (736.28 ±4 pg/mg creatinine vs 119.2 ± 58.13 pg/mg creatinine vs 104.24 ± 85.18 pg/g creatinine, p < 0.001). The highest urinary MCP-1 levels were recorded in patients with pauci-immune glomerulonephritis, although the difference was not statistically significant. Patients with MCD exhibited MCP-1 levels comparable to those in other biopsy categories, despite having < 10% chronic lesions and no prolipherative lesions on biopsy, suggesting that the presence of proteins in the tubular lumen stimulates MCP-1 synthesis in tubular epithelial cells.
Conclusions: urinary MCP-1 is elevated in inflammatory glomerular diseases and correlates with some disease processes, its role in differentiating between specific types of kidney damage remains unclear. Further studies with larger patient groups are needed to explore its potential as a non-invasive biomarker for kidney disease management.
Keywords: Urinary MCP-1, Pauci-imune glomerulonephritis, Minimal change disease
Introduction
Glomerulopathies are anatomo-clinical entities characterized by primary damage to the renal corpuscle, with secondary involvement of the tubules, vessels, and interstitium. The incidence of primary glomerulonephritis has been estimated at 57 cases per 100,000 inhabitants in the USA, with a mortality rate 2.7 times higher than in the healthy population [1]. Although relatively rare, glomerulonephritis is the third leading cause of Chronic Kidney Disease (CKD) in the USA [2]. The term "glomerulopathies" encompasses various conditions with different progression patterns (acute, subacute, and chronic) and treatment approaches. Currently, Renal Biopsy (PBR) is the gold standard for diagnosing glomerulonephritis. Key prognostic factors in glomerulonephritis include chronicity indices in PBR, creatinine levels at onset, and response to initial treatment. Identifying new diagnostic methods and prognostic factors has been a significant line of scientific research in the past two decades.
Glomerulonephritis (GN) are immune-mediated kidney diseases characterized by an imbalance between pro-inflammatory and protective factors. The etiology of these conditions is often unknown, with exceptions such as glomerulonephritis induced by infections (beta-hemolytic streptococcus, viruses), medications, or neoplasms. The Centers for Disease Control and Prevention (CDC) reported that GN was the ninth leading cause of death in the US population in 2013 [3]. Glomerular damage is a highly complex process resulting from the interaction of genetic and environmental factors. An inadequate immune response leads to pathological manifestations. Regardless of the underlying pathology, glomerular injury activates a common profibrotic pathway that ultimately leads to tubular atrophy, interstitial fibrosis, and loss of nephron mass [4,5].
Macrophages are a crucial category of cells involved in the immune process in glomerulonephritis, playing a central role in renal inflammation. Monocytes, which constitute 10% of circulating leukocytes in the blood, bone marrow, and spleen, are produced in the bone marrow by hematopoietic stem cells. They enter the bloodstream, where they remain for 1-2 days. If not recruited to inflamed tissues during this period, they die and are removed. However, if they reach the tissues, monocytes transform into macrophages, which are involved in multiple inflammatory processes. The recruitment of monocytes to the kidney occurs through interactions with deposited immunoglobulins (via Fc receptors) or various chemokines such as monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein 1 alpha (MIP-1-alpha), RANTES, and leukocyte-derived matrix metalloproteinase-9 (MMP-9).
The interaction between MCP-1 and macrophages has been extensively studied since the late 20th century, being considered a key point in early diagnosis and treatment guidance for patients with GN. In 1996, Tang et al. studied the expression of MCP-1 in guinea pigs injected with anti-MBG antibodies. Following anti-MBG antibody injection, glomerular MCP-1 mRNA increased within approximately 30 minutes. Subsequently, mRNA was localized in both glomerular cells and infiltrating macrophages, demonstrating that MCP-1 expression is initially stimulated in renal cells. The presence of anti-MBG antibodies in rats leads to their binding to MBG and the initiation of the complement cascade. C5a causes endothelial cell changes, which express receptors for macrophages and secrete IL-1 and TNF-alpha, further stimulating MCP-1 synthesis. IL-1 and TNF-alpha activate the coagulation cascade, leading to increased MCP-1 expression in mesangial cells. Locally synthesized MCP-1 provides an additional stimulus for macrophage recruitment and activation. Activated macrophages produce oxidants, cytokines that maintain the inflammatory cascade, and proteolytic enzymes involved in MBG lysis [6].
Activated monocytes generate free oxygen species and proteolytic enzymes, and release tissue growth factors that stimulate crescent formation and fibrogenesis processes, including TGF-beta, a molecule involved in extracellular matrix synthesis leading to glomerulosclerosis [5]. The involvement of macrophages in crescent formation is particularly significant, as crescents are renal lesions linked to disease severity and poor prognosis. In an experimental model of Goodpasture's disease, macrophages comprised 42% of the cells in early crescents and about 66% in fibrocellular or fibrous crescents [7]. The same study indicates that the macrophages involved in crescent formation likely originate from the periglomerular interstitium through Bowman’s capsule rupture, driven by inflammatory processes similar to those causing glomerular basement membrane rupture and/or cell-mediated mechanisms [7,8] Experimental studies show that inhibiting MCP-1 reduces proteinuria, crescent formation, and collagen type I deposition in the glomeruli, highlighting MCP-1's role in the development of irreversible tissue damage [9].
Macrophage migration to Bowman’s capsule is guided by the following chemoattractant molecules:
a) Monocyte chemoattractant protein 1 (MCP-1), Macrophage Migration Inhibitory Factor (MIF), macrophage inflammatory protein 1 alpha (MIP-1-alpha), and osteopontin.
b) Adhesion molecules (VCAM-1, ICAM-1) and CD44 expressed by parietal glomerular epithelial cells.
c) Kidney-derived granulocyte-macrophage colony-stimulating factor (GM-CSF), which enhances the expression of VCAM-1, MCP-1, and IL-1 beta, promoting crescent formation [10].
Macrophages also contribute to maintaining the inflammatory process and crescent proliferation by releasing the following molecules: growth factors, IL-1, and TNF (involved in stimulating adhesion molecules, cell proliferation, and recruitment of other macrophages), and TGF-beta. TGF-beta plays a crucial role in both disease activity and chronicity by favoring the transition from cellular crescents to fibrocellular and fibrous crescents. Inhibition of TGF-beta expression or activity in experimental studies has demonstrated a reduction in extracellular matrix formation [11,12]. In a study published in 2017, CCR2+ monocyte depletion significantly reduced glomerular crescents and necrosis, as well as the number of infiltrating T lymphocytes, without affecting neutrophil recruitment [13].
MCP-1 is the most well-known chemokine. It consists of 76 amino acids, has a molecular weight of ~13 kDa, and is derived from a 99-amino-acid precursor through proteolytic cleavage. MCP-1 belongs to a subfamily composed of at least four members (MCP-1, -2, -3, and -4) [14,15]. MCP-1 primarily stimulates the migration of monocytes into affected tissues, but it also exhibits chemotactic properties towards basophils, CD4+ and CD8+ lymphocytes, and T lymphocytes, being involved in inflammatory and tissue repair processes. MCP-1 performs three key functions in monocyte migration First, circulating MCP-1 recruits’ monocytes and monocytic precursors from the bone marrow into the bloodstream. Subsequently, MCP-1 secreted at inflamed tissue sites concentrates at the glycocalyx, forming a concentration gradient and recruiting monocytes from the blood into the inflamed tissue Once in the tissues, monocytes differentiate and secrete pro-inflammatory cytokines, with both processes being influenced by MCP-1 [16].
In the kidney, MCP-1 is synthesized by both resident renal cells (endothelial cells, mesangial cells, podocytes, and mononuclear cells) and infiltrative leukocytes. MCP-1 plays a role in inflammatory processes by facilitating mobilization, localization, recruitment, and differentiation of cells. In addition, in vitro studies show that MCP-1 is synthesized by glomerular mesangial cells when stimulated by inflammatory cytokines (IL-1 and TNF-alpha), immune complexes, metabolic factors (including glucose and glycation products), Danger-Associated Molecular Patterns (DAMPs), and podocytes exposed to advanced glycation end-products, TNF-alpha, and TGF-beta [17]. Renal tubular cells also produce MCP-1 in response to IL-1, TNF, thrombin, and albumin [18-20].
The urinary expression of MCP-1 has been extensively studied in patients with renal vasculitis, a category of conditions with a reserved renal and vital prognosis. By the end of the 20th century, research was primarily conducted on experimental models and demonstrated that urinary MCP-1 levels correlate with the macrophage infiltration at the glomerular level [21], as well as with the total percentage of crescents, the percentage of fibrous crescents, and the number of CD68-positive macrophages in the interstitium [22].
Studies have shown that macrophages are actively involved not only in glomerular proliferative processes (as in pauci-immune glomerulonephritis, lupus nephritis) but also in the development and chronicity of tubulointerstitial lesions in patients with non-proliferative glomerulopathies (MCD, membranous nephropathy) that evolve with nephrotic syndrome. In this context, MCP-1 originates from tubulointerstitial sources (tubular epithelial cells, infiltrating monocytes, and peritubular capillary endothelial cells) and correlates with interstitial macrophage infiltration and progression to fibrosis. Macrophages can directly injure interstitial structures through the synthesis of reactive oxygen species, nitric oxide, complement factors, and pro-inflammatory cytokines. Additionally, macrophages can stimulate the proliferation of fibroblasts and myofibroblasts, leading to interstitial fibrosis and renal function decline [23].
A study conducted on 23 renal biopsies from patients with rapidly progressive glomerulonephritis assessed the distribution of MCP-1 and its receptor CCR-2 in affected glomeruli. MCP-1 microRNA was primarily expressed in crescents, parietal epithelial cells, tubular epithelial cells, and infiltrative monocytes in the tubulointerstitial area. MicroRNA molecules for the CCR2 receptor were predominantly described in the tubulointerstitial leukocytes and, to a lesser extent, in the glomerular tuft and crescents, with most CCR2B-expressing cells being located around the crescents [24].
In patients with IgA nephropathy, urinary MCP-1 excretion correlates with the extent of glomerular sclerosis and interstitial fibrosis, but does not correlate with the presence of endocapillary, mesangial, or extra capillary proliferation. This finding comes from a study involving 58 patients with IgA nephropathy, which examined urinary excretion of MCP-1, IL-6, and EGF at the time of renal biopsy, before treatment initiation. Additionally, the association of these urinary markers with the glomerular filtration rate more accurately identifies patients with T1-T2 fibrosis scores on renal biopsy when analyzed statistically [25].
In a 2004 study of 30 patients with membranous nephropathy who underwent renal biopsy, Yoshimoto et al. demonstrated that interstitial inflammatory infiltrates with CD68-positive macrophages at diagnosis were associated with progression to end-stage renal disease. CD68-positive interstitial macrophages express MCP-1. Consistent with studies in IgA nephropathy patients, the interstitial expression of MCP-1 is associated with progression of tubulointerstitial lesions and unfavorable disease outcomes. Moreover, the study supports that CCR-2-expressing cells, which are the main receptor for MCP-1, are found in the renal interstitium alongside MCP-1 expression, with CCR-2 being an independent risk factor for progression to end-stage renal disease [26].
Currently, renal biopsy remains the gold standard for diagnosing glomerulonephritis. Besides confirming the diagnosis, it provides prognostic information by assessing the extent of chronic lesions (such as tubular atrophy/fibrosis and glomerulosclerosis). However, it is an invasive procedure with risks and cannot be performed on all patients (e.g., those with a single kidney or coagulation disorders). Identifying urinary biomarkers that provide additional information about the type of renal damage or help triage patients for whom biopsy is necessary represents a key research focus of the past two decades.
Thus, the crucial role of MCP-1 in the inflammatory process of glomerulonephritis is evidenced by the following findings:
a) The level of MCP-1 expression correlates with the extent of macrophage infiltration in renal tissue.
b) MCP-1 production is triggered by various molecules associated with kidney damage.
c) MCP-1 levels are elevated in inflammatory lesions and fibrotic regions.
However, there are challenges to using urinary MCP-1 as a biomarker. These include variability in MCP-1 levels due to factors such as hydration status, diurnal variation, and differences in laboratory methods. Furthermore, urinary MCP-1 levels may be influenced by extrarenal conditions, limiting their specificity for renal pathologies.
Objectives
Study Objective
This study aims to retrospectively analyze the correlations between urinary MCP-1 levels and various types of glomerulonephritis, as well as to evaluate the potential of MCP-1 as a biomarker for diagnosing of glomerular diseases.
Specific Objectives
1) To assess the correlation between urinary MCP-1 levels and histological findings in renal biopsies of patients with different types of glomerulonephritis.
2) To evaluate the association between urinary MCP-1 levels and clinical parameters such as proteinuria, serum creatinine, and estimated glomerular filtration rate (eGFR).
3) To explore the differences in urinary MCP-1 levels among patients with proliferative versus non-proliferative glomerulonephritis.
Materials and Methods
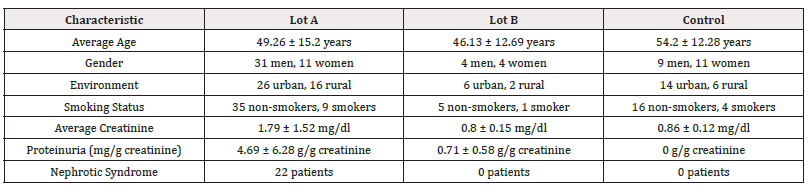
A single-center, retrospective observational study was conducted involving a group of 70 volunteers divided into three groups: Lot A – 42 patients with glomerular pathologies (IgA nephropathy, pauci-immune glomerulonephritis, membranoproliferative glomerulonephritis, NGLM, membranous nephropathy, GSFS); LotB B – 8 patients with renal conditions such as hypertensive nephropathy or thin basement membrane disease; and Lot C – the control group consisting of 20 healthy volunteers. Patients were admitted between 2018 and 2024 at the “Dr. Carol Davila Teaching Hospital of Nephrology”, Bucharest, Romania. All patients underwent renal biopsy as a diagnostic method. Sterile urine samples were collected in the morning of the renal biopsy from the midstream into sterile containers. The samples were centrifuged and stored at -20°C until processed. Clinical data, including demographics, laboratory results, and treatment information, will be collected from medical records.
Inclusion Criteria
a) Patients with a confirmed diagnosis of glomerulonephritis based on renal biopsy.
b) Availability of urinary MCP-1 levels at the time of diagnosis.
c) Complete clinical and laboratory data.
Exclusion Criteria
a) Patients with missing or incomplete data.
b) Patients with concurrent extrarenal conditions that could significantly influence urinary MCP-1 levels.
Renal Biopsy
The renal biopsy was performed under local anesthesia and with ultrasound guidance, following the patient's informed consent. This procedure was essential for diagnosing the patient's condition. The obtained samples were examined using optical microscopy, electron microscopy, and immunofluorescence to detect the following reactants: albumin, IgA, IgG, IgM, C1q, C3c, and kappa and lambda light chains.
The following histologic data were collected after the clinical assessment of pathology: date of kidney biopsy, presence of glomerulosclerosis, percentage of glomeruli affected by glomerulosclerosis, percentage of tubular atrophy and interstitial fibrosis, presence of crescents, number of affected glomeruli, presence of endocapillary proliferation, and presence of endoteliosis lesions.
Urine Sample Processing
Sterile urine samples were collected on the morning of the renal biopsy from the midstream into sterile containers. The samples were centrifuged and stored at -20℃ until processed. For determining urinary MCP-1 concentration, an ELISA assay (Quantikine R&D Systems, Minneapolis, USA) was used. Urine samples were diluted 1:2 with RD5L calibrator diluent (diluted 1:5), then incubated on a plate at room temperature for 2 hours along with standards and controls. After three washes, MCP-1 conjugate was added and incubated for 2 hours at room temperature. Following three additional washes, substrate solution was added and incubated for 30 minutes at room temperature. A stop solution was added, and optical density was measured at 450 nm wavelength. The maximum concentration determined was 2000 pg/ml. Urinary concentrations were adjusted to creatinine concentration and expressed in pg/mg creatinine (Figure 1).
Statistical Analysis
a) Descriptive statistics will be used to summarize the data.
b) Two means were compared using the t-test for independent data.
c) Correlations between urinary MCP-1 levels and clinical/histological parameters will be analyzed using Pearson or Spearman correlation coefficients, depending on data distribution.
d) Multiple linear regression analysis will be performed to identify independent predictors of urinary MCP-1 levels.
e) Statistical significance will be set at p < 0.05.
Results
The baseline characteristics for patients are presented in (Table 1, Figure 2).
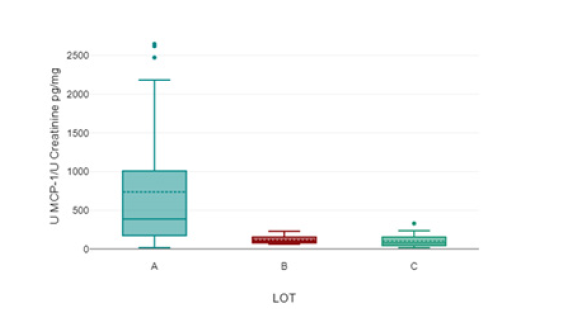
Figure 2: Concentration of urinary MCP-1/urinary creatinine. LOT A – the study LOT, LOT B- patients with hypertensive nephropathy and thin basement membrane disease; LOT C – control group.
A one-way analysis of variance revealed a significant difference in urinary MCP-1/creatinine concentrations among the groups. Patients in Group A had a concentration of 736.28 ± 775.4 pg/mg creatinine, which was significantly higher compared to patients in Group B (119.2 ± 58.13 pg/mg creatinine) a Group C (104.24 ± 85.18 pg/g creatinine), with a p-value of <.001.
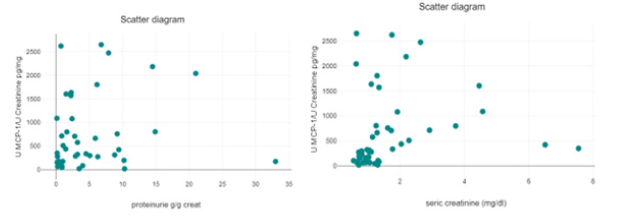
The distribution of patients after seric creatinine and proteinuria was not statistically significant (Figure 3, Table 2).
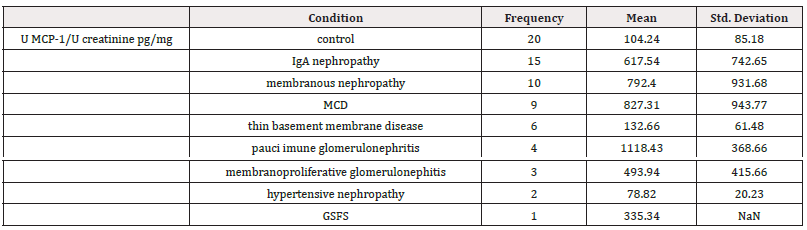
Table 2: Distribution of Patients by Renal Biopsy Results and Mean Urinary MCP-1/Creatinine Concentration.
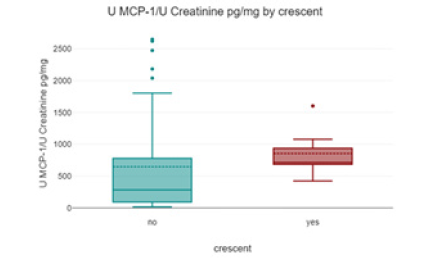
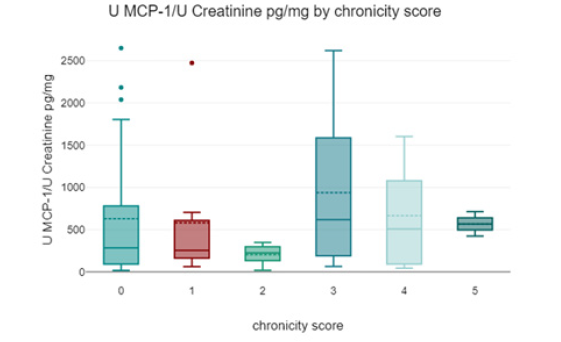
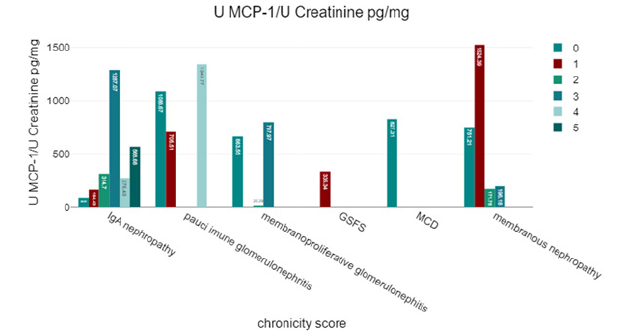
Patients with pauci-immune glomerulonephritis had the highest urinary MCP-1 levels (1118.43 ± 368.66 pg/mg creatinine) compared to other categories of glomerular damage; however, the difference was not statistically significant (Figure 4).
Patients with crescents on renal biopsy had higher urinary MCP-1 concentrations compared to those without crescents, but the difference was not statistically significant.
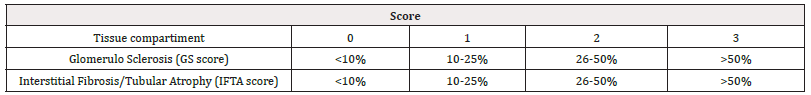
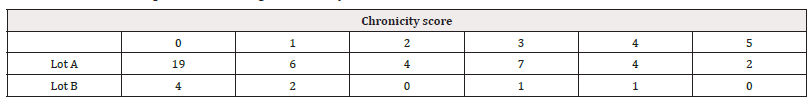
Based on the biopsy results, we calculated a chronicity index using the following model (Table 3,4) (Figure 5,6).
Discussions
Our study indicates that urinary MCP-1 levels are significantly elevated in patients with glomerular diseases of inflammatory origin compared to those with glomerular diseases due to other causes (such as hypertension or genetic conditions) and the control group. This suggests that MCP-1 could be a marker for inflammation in glomerular diseases, potentially aiding in distinguishing between inflammatory and non-inflammatory causes. The study corroborates previous research by F. Tam and others, who found significantly higher urinary MCP-1 levels in patients with active renal vasculitis compared to inactive vasculitis, those with vasculitis without renal involvement, and healthy volunteers. In your study, the highest urinary MCP-1 levels were recorded in patients with pauci-immune glomerulonephritis, although the difference was not statistically significant.
MCP-1 may originate from macrophages infiltrating glomerular and tubulo-interstitial lesions, especially in patients with nephrotic syndrome. However, the urinary levels of MCP-1 did not allow differentiation between glomerular and tubulo-interstitial damage types. Similar MCP-1 levels were observed in patients with nephrotic syndrome and those with proliferative glomerular pathologies, indicating that MCP-1 might not differentiate between these types of damage.
Another study involving patients with Membranous Nephropathy (NM), Membranoproliferative Glomerulonephritis (NMP), IgA Nephropathy, and Focal Segmental Glomerulosclerosis (GSFS) found that MCP-1 expression in renal biopsies correlates with the intensity of interstitial macrophage infiltration. Although these diseases primarily affect the glomerulus, they frequently involve tubulo-interstitial damage characterized by inflammatory infiltrates (mainly macrophages and T lymphocytes), tubular atrophy, and interstitial fibrosis. The presence of MCP-1 in tubular epithelial cells is therefore explained by local production, rather than increased reabsorption, indicating that these cells actively produce MCP-1 in response to injury or inflammation [18].
Patients with MCD exhibited MCP-1 levels comparable to those in other biopsy categories, despite having a chronicity score of 0. This indicates that even in the absence of significant chronic damage (less than 10% global or partial glomerular sclerosis, less than 10% tubular atrophy, and interstitial fibrosis) or prolipherative lesions, MCP-1 levels can still be elevated. The elevated MCP-1 levels in MCD may originate from increased synthesis in the tubulo-interstitial compartment. This could be due to:
Tubular Epithelial Cells: These cells may produce more MCP-1 in response to massive proteinuria, which is characteristic of MCD.
Infiltrating Macrophages: Macrophages in the interstitial spaces might also contribute to MCP-1 production, potentially as an early response to injury before chronic, irreversible lesions develop.
Proteinuria is a significant factor in the progression of kidney diseases. When large amounts of protein pass through the glomerular filter and are reabsorbed by the renal tubules, this process can have toxic effects on the tubular cells. The reabsorbed proteins can exacerbate tubulo-interstitial damage, which in turn can accelerate the progression of kidney disease. This damage can lead to increased production of MCP-1 as part of the inflammatory response. MCP-1 excretion is notably higher in patients with IgA nephropathy and diabetic nephropathy compared to diabetic patients without nephropathy. This was demonstrated in a study published in 2009. The study suggests a direct relationship between proteinuria and elevated urinary MCP-1 excretion, regardless of the underlying cause of proteinuria. This implies that MCP-1 levels rise in response to proteinuria itself, rather than the specific etiology of the kidney disease [27].
Experimental studies that investigate the role of proteinuria in the activation of NF-kB and the expression of MCP-1 in kidney diseases, particularly in two models of progressive proteinuric nephropathies: 5/6 nephrectomy and Passive Heymann Nephritis (PHN) have demonstrated that the rise in proteinuria was strongly associated with increased NF-kB activity. The activation of NF-kB was paralleled by an upregulation of MCP-1 mRNA, indicating that increased NF-kB activity promotes the production of MCP-1, a key chemokine involved in recruiting monocytes/macrophages to sites of inflammation [20]. Even though our study did not demonstrate a statistically significant correlation between urinary MCP-1/creatinine concentration and proteinuria, it nonetheless supports the hypothesis that the presence of proteins in the tubular lumen stimulates MCP-1 synthesis in tubular epithelial cells. This suggests a direct effect of proteinuria on MCP-1 production, with albumin acting as a trigger for increased MCP-1 synthesis. This connection underscores the role of proteinuria in promoting a pro-inflammatory response in the kidney, contributing to the progression of renal damage. The findings suggest that MCP-1 could be an early marker of tubular injury in MCD, even before chronic lesions are evident on biopsy.
Additional studies are needed involving a larger number of patients with MCD to evaluate the role of urinary MCP-1 in diagnosing and predicting relapses in this patient category. Can measuring urinary MCP-1 predict the risk of relapse in patients with MCD before the onset of proteinuria or clinical manifestations, and can it influence the management of these patients? This is an important question because early identification of relapses could allow for timely therapeutic interventions, thus optimizing the prognosis and reducing complications associated with severe nephrotic syndrome in MCD. Urinary MCP-1 concentrations could help identify patients for whom a kidney biopsy is necessary (i.e., those with different inflammatory glomerular conditions), as opposed to those for whom biopsy results would not alter disease management. No significant difference was found between the urinary MCP-1 levels in patients with hypertensive nephropathy or thin basement membrane disease (Lot B) and the control group (Lot C), suggesting MCP-1 may not be elevated in these conditions. No statistically significant correlations were found between urinary MCP-1 levels and the chronicity score derived from renal biopsy or active glomerular lesions (such as crescents, endothelial proliferation, and mesangial proliferation). This suggests that urinary MCP-1 might not reflect the severity or chronicity of glomerular damage directly.
Conclusions
In summary, while urinary MCP-1 is elevated in inflammatory glomerular diseases and correlates with some disease processes, its role in differentiating between specific types of kidney damage remains unclear. Further studies with larger patient groups are needed to explore its potential as a non-invasive biomarker for kidney disease management. Monitoring MCP-1 levels could provide insights into the extent of tubular injury and the inflammatory response in the kidney, which could be crucial for guiding treatment strategies. This makes it a potentially valuable biomarker for assessing kidney damage and disease progression.
Acknowledgement
None.
Conflict of Interest
None.
References
- Wetmore JB, Guo H, Liu J, Collins AJ, Gilbertson DT, et al. (2016) The incidence, prevalence, and outcomes of glomerulonephritis derived from a large retrospective analysis. Kidney Int 90(4): 853-860.
- United States Renal Data System. 2014 Annual Data Report: Epidemiology of Kidney Dis-ease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2014.
- Heron M Deaths (2016) Leading Causes for 2013. Natl Vital Stat Rep 65(2): 1-95.
- Worawichawong S, Worawichawong S, Radinahamed P, Muntham D, Sathirapongsasuti N, et al. (2016) Urine Epidermal Growth Factor, Monocyte Chemo-attractant Protein-1 or Their Ratio as Biomarkers for Interstitial Fibrosis and Tubular Atrophy in Primary Glomerulonephritis. Kidney Blood Press Res 41(6): 997-1007.
- Ramòn GB Bonegio, Mechanisms of immune injury of the glomerulus www.uptodate .com
- Tang WW, Qi M, Warren JS (1996) Monocyte chemoattractant protein 1 mediates glomerular macro-phage infiltration in anti-GBM Ab GN. Kidney Int 50(2): 665-671.
- Lan HY, Nikolic Paterson DJ, Mu W, Atkins RC (1997) Local macrophage proliferation in the pathogene-sis of glomerular crescent formation in rat anti-glomerular basement membrane (GBM) glo-merulonephritis. Clin Exp Immunol 110(2): 233-240.
- Boucher A, Droz D, Adafer E, Noël LH (1987) Relationship between the integrity of Bowman's capsule and the composition of cellular crescents in human crescentic glomerulonephritis. Lab Invest 56(5): 526-533.
- Lloyd CM, Dorf ME, Proudfoot A, Salant DJ, Gutierrez Ramos JC (1997) Role of MCP-1 and RANTES in inflammation and progression to fibrosis during murine crescentic nephritis. J Leukoc Biol 62(5): 676-680.
- Charles D Pusey, MD, A. Richard Kitching, MBChB, PhD Mechanisms of glomerular crescent formation. Uptodate.com
- Isaka Y, Nakamura H, Mizui M, Takabatake Y, Horio M, et al. (2004) DNAzyme for TGF-beta suppressed extracellular matrix accumulation in experimental glo-merulonephritis. Kidney Int 66(2): 586-590.
- Border WA, Ruoslahti E (1990) Transforming growth factor-beta 1 induces extracellular matrix for-mation in glomerulonephritis. Cell Differ Dev 32(3): 425-431.
- Rousselle A, Kettritz R, Schreiber A (2017) Monocytes Promote Crescent Formation in Anti-Myeloperoxidase Antibody-Induced Glomerulonephritis. Am J Pathol 187(9): 1908-1915.
- Deshmane SL, Kremlev S, Amini S, Sawaya BE (2009) Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 29(6): 313-326.
- Graves DT, Jiang Y (1995) Chemokines, a family of chemotactic cytokines. Crit Rev Oral Biol Med 6(2): 109-118.
- Haller H, Bertram A, Nadrowitz F, Menne J (2016) Monocyte chemoattractant protein-1 and the kid-ney. Curr Opin Nephrol Hypertens 25(1): 42-49.
- Tam FWK, Ong ACM (2020) Renal monocyte chemoattractant protein-1: an emerging universal bi-omarker and therapeutic target for kidney diseases? Nephrol Dial Transplant 35(2): 198-203.
- Prodjosudjadi W, Gerritsma JS, Klar Mohamad N, Gerritsen AF, Bruijn JA, et al. (1995) Production and cytokine-mediated regulation of monocyte chemoattractant protein-1 by hu-man proximal tubular epithelial cells. Kidney Int 48(5): 1477-1486.
- Grandaliano G, Monno R, Ranieri E, Gesualdo L, Schena FP, et al. (2000) Regenerative and proinflammatory effects of thrombin on human proximal tubular cells. J Am Soc Nephrol 11(6): 1016-1025.
- Donadelli R, Abbate M, Zanchi C, Corna D, Tomasoni S, et al. (2000) Protein traffic activates NF-kB gene signaling and promotes MCP-1-dependent interstitial inflamma-tion. Am J Kidney Dis 36(6): 1226-1241.
- Tam FW, Karkar AM, Smith J, Yoshimura T, Steinkasserer A, et al. (1996) Differ-ential expression of macrophage inflammatory protein-2 and monocyte chemoattractant pro-tein-1 in experimental glomerulonephritis. Kidney Int 49(3): 715-721.
- Wada T, Furuichi K, Segawa Takaeda C, Shimizu M, Sakai N, et al. (1999) MIP-1alpha and MCP-1 contrib-ute to crescents and interstitial lesions in human crescentic glomerulonephritis. Kidney Int 56(3): 995-1003.
- Sean Eardley K, Cockwell P (2005) Macrophages and progressive tubulointerstitial disease. Kidney Int 68(2): 437-455.
- Segerer S, Cui Y, Hudkins KL, Goodpaster T, Eitner F, et al. (2000) Expres-sion of the chemokine monocyte chemoattractant protein-1 and its receptor chemokine re-ceptor 2 in human crescentic glomerulonephritis. J Am Soc Nephrol 11(12): 2231-2242.
- Segarra Medrano A, Carnicer Caceres C, Valtierra Carmeno N, Agraz Pamplona I, Ramos Terrades N, et al. (2017) Value of urinary levels of interleukin-6, epider-mal growth factor, monocyte chemoattractant protein type1 and transforming growth factor β1 in predicting the extent of fibrosis lesions in kidney biopsies of patients with IgA nephropa-thy. Nefrologia 37(5): 531-538.
- Yoshimoto K, Wada T, Furuichi K, Sakai N, Iwata Y, et al. (2004) CD68 and MCP-1/CCR2 expression of initial biopsies reflect the outcomes of membranous nephropathy. Nephron Clin Pract 98(1): c25-34.
- Tsukasa Morii, Hiroki Fujita, Takuma Narita, Jun Koshimura, Takashi Shimotomai, et al. (2003) Increased Urinary Ex-cretion of Monocyte Chemoattractant Protein-1 in Proteinuric Renal Diseases. Renal Failure 25(3): 439-444.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.