Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Towards the Understanding of Long-Term Effects of Glucocorticoid and Stress Hormones
*Corresponding author: Wilfried Allaerts, Biological Publishing A&O and Immunology Department, Erasmus MC Rotterdam, The Netherlands.
Received: July 09, 2024; Published: July 17, 2024
DOI: 10.34297/AJBSR.2024.23.003063
Abstract
This study originated from the observation of long-term inhibitory effects on hormone secretion in co-cultures of pituitary cell types. These long-term effects were seen in Dopamine (DA) rebound effects on Prolactin (PRL) secretion and in bi-phasic Luteinizing Hormone (LH) secretion patterns after repetitive Gonadotropin-Releasing Hormone (GnRH) administration. These observations also prompted a review of other rebound phenomena related to catecholamine and inhibitory drug withdrawal in relation to stress effects and mood disorders. Hereby, the mineralocorticoid / glucocorticoid balance hypothesis and related hypotheses, developed in the previous century by H. Selye and E.R. de Kloet, were found illuminative. The dynamics of these so-called pendulum hypotheses however remain a problematic issue, especially when the long-term effects of repeated stress are concerned, as well as in Post- Traumatic Stress Disorder (PTSD). Therefore, this review also includes a behavioral side-step towards the role of grooming and both natural and culturally defined behavioral adaptations in order to clarify the dynamic landscape of stress adaptation in humans. It is concluded that especially the culturally defined adaptive behavioral mechanisms need further concern.
Keywords: Stress hormones, Mineralocorticoid and glucocorticoid receptor, Brain effects, Grooming and Endorphin production, PTSD, Painkiller abuse and adaptive behavior
Introduction
Time is an expensive issue in the Anthropocene, but how it works and how it matters in public health, remain elusive questions. This is especially relevant in long-term health effects of stress, stress hormones and the use of medical products and substances. Despite the enormous importance of this issue, the scientific data on the mechanisms of long-term health effects are surprisingly scarce. Apparently, with the exception of epidemiological cohort studies [1], there is a huge discrepancy between the lengths of experimental research periods and the actual duration of factors affecting an individual’s health. Prolonged exposure to stress has been shown to result in a variety of health problems. It became especially notorious as Post Traumatic Stress Disorder (PTSD) after the diagnoses of U.S. military veterans returning from the Vietnam War (that according to the U.S. Department of Defense lasted from 1955 to the fall of Saigon in 1975). It was officially recognized in 1980 by the American Psychiatric Association (APA) and recorded in the third edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-III) [2]. Stress phenomena and stress signaling however are ubiquitous phenomena in the animal kingdom including our own species, as well as in the kingdom of plants [3]. In the present paper, we’ll confine ourselves to the handling of stress in so-called social mammals (in particular in humans and Non-human Primates). In Primates, an important interplay was detected in the 1990s between the social behavior of grooming, aggression and the animal’s response to stress [4]. In laboratory conditions, it had been discovered for a while that ovarian hormones (of the adults), grooming, and mother-child interactions (like nursing, feeding, cuddling…) were vital, in so far that their absence could become life-threatening for the young animals [5].
The famous ‘monkey-mother experiment’ of Harlow (1958) [6], moreover, demonstrated that ‘affection’ in the mother-child relation would even be more needed than the nutrition, in determining the closeness between the mother and the offspring. Subsequent laboratory investigations reinforced the strong connections between the activity of the Hypothalamus-Pituitary-Adrenocortical (HPA) axis and the stress response [7]. The HPA axis comprises the neuroendocrine regulation of the production and release of hormones from the adrenals. The adrenals actually are divided in cortex and medulla, the former being subdivided in a zona glomerulosa, zona fasciculata and zona reticularis [8]. All zones produce different groups of hormones, from the capsule to the medulla: mineralocorticoids (like aldosterone) in the zona glomerulosa, glucocorticoids (including cortisol and corticosterone) in the zona fasciculata, sex hormones (like progesterone) in the zona reticularis, and the stress hormones (stricto sensu) adrenaline and noradrenaline in the chromaffin cells of the medulla [8]. Whereas the latter hormones of the adrenal medulla - which are also produced by sympathetic ganglia, skin Merkel cells and the locus coeruleus in the brain - have a crucial role in the ‘flight or fight’ response, the brain receptors to mineralocorticoids and glucocorticoids are pivotal in maintaining homeostatic balance and preserving long-term health [9].
Studying the mechanisms involved in homeostatic balance and long-term health, prompted the elucidation of post-receptor effector mechanisms and delayed responses to drug administration and withdrawal too [10]. There is a well-elucidated relationship between endocrine secretion inhibition (e.g. prolactin, PRL) and rebound phenomena, for instance as shown by the catecholaminergic hormone Dopamine (DA) [11]. But, rebound phenomena also occur at the level of organisms after periods of increased neuronal activity and affecting so-called rebound sleep [12]. The relation between sleep and recovering from stress and intensive labor was found pivotal in understanding work-rest balance [13]. The notion of duration in sleep-mediated recovery was one of the crucial elements in understanding why homeostatic balance in itself is not sufficient in explaining the long-term health effects of stress. Also, it became instructive in understanding why medical substances and replacement therapies not always have been (considered) beneficial for patients suffering from PTSD [14].
Finally, in continuation of our previous work [15], this study attempts to review the complex contextual processes affecting an individual’s health condition, as well as the role of socio-cultural factors in directing public health at the population level [16]. But first we start with a re-evaluation of our early work on the role of intercellular (so-called paracrine) communication on the immediate and delayed type of responses of pituitary cells to stimulatory and inhibitory secretagogues.
Studying Long-Term Effects in Cells and 3D Cell Cultures
Our first encounter with delayed response studies occurred in the 1980s. In that period, we studied the attenuating effects of coculturing pituitary endocrine cells with non-hormone secreting folliculo-stellate (FS) cells on the hormone production of the former cells [17]. At the time, FS-cells were described as an elusive cell type present between the endocrine parenchymal cords, with a number of still unknown functions to be discovered yet [17]. To our surprise, the effect of co-culturing the endocrine cells with FS cells not only attenuated the stimulating effects of angiotensin II on prolactin (PRL) release, but also attenuated the rebound surge of PRL secretion after inhibition with Dopamine (DA) (Figure 1) [18]. In other words, the FS-cells appeared to attenuate immediate as well as a delayed-type effect on hormone secretion.
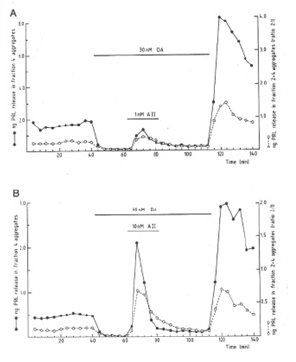
Figure 1: Influence of Folliculo-Stellate (FS) cells (open circles) on angiotensin II (A II) stimulated prolactin (PRL) secretion under dopamine (DA) inhibition and rebound effect (on PRL secretion in enriched populations of lactotroph cells (closed circles) (© Allaerts, 1989; adopted from [18]).
However, because of the coupling of the DA-induced rebound PRL secretion with the pre-inhibitory PRL release (Figure 1) [11], we turned to another model of delayed responsiveness. That model was the biphasic response of Luteinizing Hormone (LH) secretion upon repeated stimulation with Gonadotropin-Releasing Hormone (GnRH, previously named LHRH), as a model for the ovulatory LH-surge [19]. In this model, it had been shown that ovarian factors are responsible for the initial low responsiveness to GnRH [20]. In our study of co-cultures of FS cells with gonadotropin secreting cells, we demonstrated not only a cycloheximide-dependent bi-phasic LH-secretion pattern in cells from non-ovariectomized rats, cycloheximide only blocking the second, elevated LH-secretion phase [21]. Also, a diminished acceleration was shown between the two LH-secretion phases, due to the presence of the FS cells in the co-cultures with gonadotrophs (Figure 2,3).
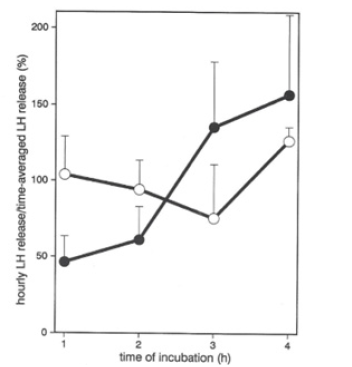
Figure 2: Effect of FS-cells on time-averaged (hourly) Luteinizing Hormone (LH) secretion after repetitive Gonadotropin Releasing Hormone (GnRH) stimulation in rat pituitary cell aggregates (ratio of stimulated over basal LH secretion) (see also Fig. 3) (© Allaerts, et al., 1994; adopted from [21]).
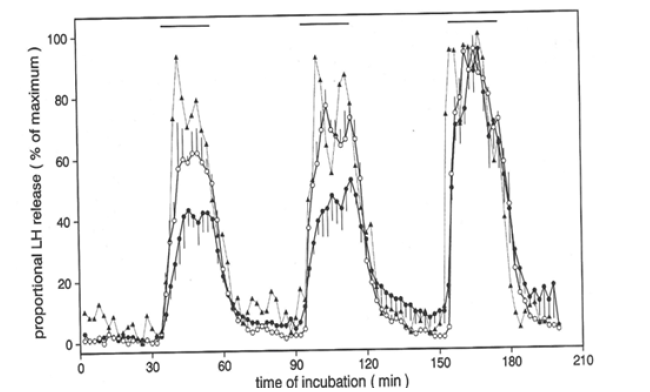
Figure 3: Effect of FS-cells on biphasic LH secretion in gonadotroph enriched cells responding to repetitive GnRH stimulation (in the presence [circles] or absence [closed triangles] of ovarian peptide feedback) (© Allaerts, et al., 1994; adopted from [21]).
This was a remarkable result, which in fact revealed a peculiar local homeostatic mechanism among pituitary cells. Although the mechanisms involved in this type of intercellular communication were still elusive [17], a possible coupling with the post-receptor adenylate cyclase system or intracellular calcium (Ca2+) remained uncertain [21]. Most remarkable however, was the finding of a prolonged effect of this intercellular communication mechanism on the responsiveness of (cultured) cells to various types of secretagogues, which forwarded the notion of spatio-temporal contingency in cellular communication systems [22].
Rebound Phenomena, Mood Disorders and Drug-Withdrawal Symptoms
Returning to the mechanism of the DA-rebound stimulation, the finding that the DA-agonist bromocriptine did decrease PRL secretion, without causing a rebound effect after its removal, was suggestive for an involvement of Ca2+-influx [11]. The DA-rebound model of delayed responsiveness indeed became an impetus for studying also other (post-receptor) coupling mechanisms [12] and of withdrawal symptoms, as well as for the search for medical substances that would enable an uncoupling of unwanted (side) effects, such as observed in Major Depressive Disorder (MDD) [23]. The etiology of major depression for a while has been linked to levels of 5-hydroxytryptamine (5-HT or serotonin) that are too low, although definitive proof of this hypothesis is still lacking. The post-synaptic enzyme system responsible for 5-HT reuptake has played an important role in this model. A meta-analysis in human mood/anxiety disorders indeed had indicated a variety of withdrawal symptoms (like headache, dizziness, nausea, dysphoria, insomnia, and others) after 5-HT/NA Reuptake Inhibitor discontinuation [10].
DA as a neurotransmitter has been identified as another modulator of the neuronal substratum of mood and anxiety disorders [23], next to the previously known serotonergic and noradrenergic neurotransmitter systems [24]. In the latter statistical study, following a Factorial Correspondence Analysis (FCA) of patients with severe depression disorders, it was concluded that serotonergic dysfunction (measured by a test with d-fenfluramine a 5-HT release/uptake inhibitor) was associated with suicidal behavior, and that noradrenergic dysfunction (indicated by a blunted Growth Hormone [GH] response to Clonidine [CLO], a partial α2-adrenoreceptor agonist)[25], was mainly associated with severe anxiety [24].
The temporal link in the noradrenergic feedback loop system was recognized as the “noradrenergic dysregulation hypothesis” [25]. The latter hypothesis emphasized a primary diminished sensitivity or downregulation of nerve terminal α2-adrenoreceptors, leading to “impaired negative feedback on the presynaptic neuron”, which, in turn, “might induce a disinhibition of Noradrenergic (NA) output and exaggerated NA release in response to activation of the catecholaminergic system” [24]. It is well known that both serotonin and NA play an important role in regulating mood, memory and the sleep-wake cycle. Because of this interaction, so-called Serotonin and Norepinephrine Reuptake Inhibitors (SNRIs) originally were designed as being more effective than Selective Serotonin Reuptake Inhibitors (SSRIs) in treating depression symptoms, although so far, studies haven’t provided conclusive answers regarding an increased effectiveness [26]. The interaction between DA and NA on the other hand, has been shown to also impact the condition of wakefulness [27].
Finally, the role of NA adrenergic signaling in the post-neuronal activation of the brain during so-called rebound sleep effects, has been studied in a relatively new experimental animal model, the zebrafish [12]. Using CRISPR/Cas9 mediated elimination of the dopamine β-hydroxylase (dbh) gene (coding for the enzyme that transforms DA into NA) (Figure 4), these authors were able to demonstrate that elimination of the dbh-gene did not prevent additional rebound sleep following noradrenergic induction of neuronal activity, although an enhanced base-line sleep was observed in the dbh-deficient zebrafish larvae [12]. It was concluded that increased neuronal activity, as reflected by a brain-wide rise of c-fos level induction, formed a sleep pressure signal that promoted rebound sleep, independently of noradrenergic tone [12] (Figure 4,5).
In humans, both NA and 5-HT are produced at various locations in the body, but they are synthesized from two distinct essential amino acids, respectively L-phenylalanine and L-tryptophan (Figure 4,5). The distinct biochemical pathways for their synthesis involve specific hydroxylase and decarboxylase enzyme systems, explaining the distinct production sites in the body. The degradation of catecholamines and serotonin also occurs through distinct metabolic pathways, involving Monoaminoxidase (MAO) together with or without catechol-O-methyl transferase, respectively [28,29].
The Mineralocorticoid-Glucocorticoid Balance Hypothesis
In the present paragraph we focus on another hormonal group derived from the adrenals, the mineralocorticoids and glucocorticoids, both produced in the outer cortical layers. Their roles are discussed with special reference to the homeostatic balance hypothesis, that has been suggested as an important mechanism to explain the presence or absence of a healthy responsiveness to a stressful environment. In the early nineties, E.R. de Kloet [9] proposed this hypothesis for homeostatic control, based on the activation in the brain of two types of receptors, namely the Mineralocorticoid Receptors (MR) and Glucocorticoid Receptors (GR). GR are expressed at high density in brain regions involved in the organization of the stress response. Corticosterone binds to both MRs (depending on the brain region, e.g. in the limbic system) and GRs, but with a 10-fold lower affinity to GRs [9]. In the hippocampus, moreover, co-localization of MRs and GRs occurs in the CA1 neurons (which are critical in memory and self-consciousness) [30], but both receptor types differently mediate the corticosteroid actions in these neurons. De Kloet and his group found evidence that MRs were involved in ‘maintenance of excitability’, whereas GRs suppress the excitability, which was transiently raised by excitatory transmitters. Occupancy of the GRs by corticosterone (17-deoxycortisol) in the hippocampus would attenuate the MR-mediated limbic inhibition. For the long-term effect, this was a very important finding. It led to the conclusion that GR-mediated effects on (fear and food-mediated) behavior “may persist for weeks in adulthood and appear permanent during development” [9]. In animals with “an increased relative amount of limbic MRs over GRs a reduced emotional and adrenocortical reactivity” was found, as well as a “decreased ability to organize behavior with the help of external stimuli” [9]. It was concluded that “deviations of an idiosyncratic MR/GR balance” may alter the individual susceptibility to stress and may also lead “perhaps to stress-related brain diseases” [9]. The word ‘idiosyncratic’ and the absence of an emphasis on environmental stimuli were suggestive for a viewpoint with a slight preference for individualistic responsibility in a world where constant ambient stressful stimuli are the standard.
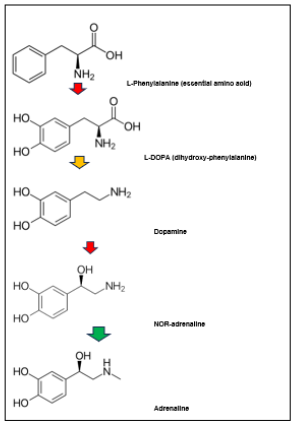
Figure 4: Biosynthesis of catecholamines derived from the essential amino acid L-phenylalanine (top). Enzymatic transformation steps include 2 hydroxylases, (red arrows), one of which is known as dbh (see main text), a decarboxylase (yellow arrow) and methyltransferase (green arrow) (Chemical structures from Wikipedia Chemistry).
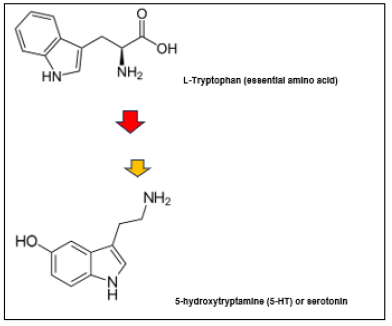
Figure 5: Biosynthesis of serotonin from the essential amino acid L- tryptophan (top). Enzymatic transformation steps include a hydroxylase (red arrow) and a decarboxylase (yellow arrow) (Chemical structures from Wikipedia Chemistry).
For de Kloet and co-workers [9,31,32], this viewpoint enabled them to construct the so-called pendulum hypothesis, which in fact was an adaptation of the pendulum hypothesis of the Hungarian Canadian endocrinologist, János (Hans) Selye (1907-1982) [33]. Selye’s pendulum hypothesis was in accordance with the use of corticosteroid therapy for anti-inflammatory and immune suppression purposes, which therapy however had serious negative side effects when used for a longer period [34]. In the version of de Kloet’s hypothesis, there is a coordinated antagonistic balance between MR- and GR-mediated effects exerted by one single adaptive hormone, corticosterone (Figure 6) [9]. However, what misses in the pendulum hypothesis is any information on the periodicity of the pendulum as a dynamic process, or of a time-dependency of the homeostatic balance. Of course, this specific information on the dynamics of the metabolic processes, is also missing in popular lifestyle and coaching theories that suggest an important role of sleep in creating healthy work-life balance. And, needless to state, sleep (and other activities than work) is/are an important tool for recovering from a stressful workload [35].
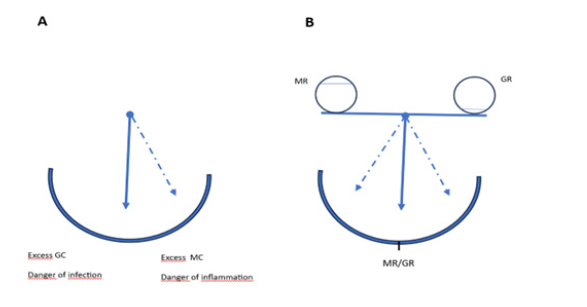
Figure 6: Pendulum hypotheses depicting the mineralocorticoid / glucocorticoid (MR/GR) balance hypotheses (adapted from de Kloet, 1991) [9]. A (left): original pendulum hypothesis proposed by H. Selye (1950); B (right): Through shifts in the MR/GR balance, the effects of corticosterone (17-deoxycortisol) on cellular excitability, neuroendocrine reactivity and behavioral adaptations create variability among individuals and between life episodes.
Stress in Humans, Non-Human Primates and Other ‘Social’ Mammals
In one of the oldest documented forms of human organization, the European vasal-sovereign relationship of the feudal system, the duties of the knighthood not only consisted of serving the king’s arms, but another important duty was to protect the feeble and the meek, the women, children and elderly so to speak. Although in feudalism, this may have been only achieved in theory, in the modern world the International Organizations and International Law Courts are designed to curtail and restrict the fiercest armies and dictators of the world, when trespassing these oldest duties of humanity. In a natural environment, social mammals have evolved with distinct behavioral adaptations as well as organizational forms to reduce the impact of stress and to reinforce the social control on trespassing individuals. One such behavioral adaptation is the process of grooming, which has been extensively studied in Higher Primates (Simiiformes) [4,5] and in particular in the Great Apes (Pongidae, called Hominidae since 1990) [36]. Grooming not only is a natural behavioral adaptation, it also has beneficial effects on heart rate (and variability) and health of laboratory non-human Primates [37]. For a considerable time, it is known that grooming behavior in monkeys is directly linked to β-endorphin production – measured by endorphin-concentrations in the cerebrospinal fluid - (see below) [38]. But also in other taxa of social mammals, including in dogs and wolves (Canidae) [39] and horses (Equidae), grooming is important for stress buffering. Moreover, grooming between the horse and its driver has a significant effect on reducing the heart rate (of the horse) after exercise [40]. Not only grooming exerted by humans with laboratory and domestic animals is beneficial to the animals, but these effects are also supposed to be reciprocal too. Moreover, as it has been suggested by the anthropologist Robin Dunbar (°1947, Liverpool, UK), gossiping too has to be understood as a human analog of grooming [41]. It remains to be seen whether the physiological effects of gossiping also extend to the lowering of the heart rate in humans (probably not!).
Considering the duration of the grooming activities, and its beneficial effects on health, extensive studies have been devoted to the reciprocity and individual variability in duration of the grooming behavior in particular in Chimpanzees [42]. The allogrooming (= not self-grooming) in chimpanzees, moreover, has been considered an important heuristic tool in approaching the analog of altruistic behaviour in our closest relatives in nature [42,43] (Table 1).
Also, other behavioral adaptations and activities (or the absence of activity during sleep) have been suggested to have a beneficial effect on reducing stress and improving health in the civilized world. A number of ‘natural’ behavioral adaptations is listed in (Table 1). Several of these activities, such as long-distance running, have also been linked to endorphin production, in particular from the brain prefrontal and limbic regions. It is well-known (at least among runners) that long-distance running may cause a so-called runner’s high (as well as serious injuries, obviously) [47]. Endorphins are fragments of the proopiomelanocortin (POMC) peptide, that is primarily produced in the pituitary gland. POMC is cleaved by a number of enzymes, to start with by pro-peptide convertase 1 (PC1) resulting in the formation of Adrenocorticotropin (ACTH) and β-lipotropin (β-LPH). The latter hormone is further processed by the peptide convertase 2 (PC2) into α-, β-, and γ-endorphin. NA – the fight or fight mediator - is known to increase the endorphin production in inflammatory tissues, causing an analgesic effect (through binding to the opioid μ-receptor in the dorsal root and inhibiting the release of substance P from the spinal cord, which reduces the frequency of pain signals to the brain) [48] In the CNS, blocking of the GABA neurotransmitters results in the increased production of dopamine, sometimes designated as the feel-good drug [49]. The interconnectedness of β-endorphin production, blocking of pain sensations and the ‘feeling-good’ mood, reveal an antagonistic balance between pain, the physiological protection towards trauma and the inflammatory response. The vulnerability to acute traumatic stress of β-endorphin metabolism is invoked to explain differences in individual reactivity too [50].

Table 1: Behavioral adaptations in various mammalian species in relation to brain size (number of neurons) and daily sleeping hours. There is not a simple relation between animal size and duration of sleep, although large herbivorous mammals tend to sleep less than the small and carnivorous mammals. Bigger mammals (animals in general) have a higher number of neurons, but the Great Apes obviously display a divergent evolutionary track in this respect. (x) The duration of (allo-, polyadic) grooming in Primates is highly variable and an important determinant of an animal’s position in the hierarchy of the social group, but numerical data aren’t easily compared between species (also other than Primates) (indicated with x) (Data obtained from multiple sources) [42-46].
Consequences for Post Traumatic Stress Disorder (PTSD)
The problematic, antagonistic relationships between physical stress, analgesic effects (pain relief), hedonic mood alleviation and addiction became painfully exposed in the popular TV/Netflix series ‘Painkiller’ created by Micah Fitzerman-Blue and Noah Harpster [51]. The series was based on the reports and newspaper articles of Patrick Radden Keefe [52] and the book of Barry Meier (°1949, New York) [53]. The case presented in this paper, book and TV-series, reflects the non-fictitious story of the Sackler family, that with the Purdue Pharma gained a fortune on the saless of the OxyContin™ drug (estimated benefit: some 30 billion/year) [53]. This was a novel drug, developed and patented by Purdue Pharma LP. in 1996, based on the activity of the opioid oxycodone (Figure 7). In 2007, the pharmaceutical company pleaded guilty on the charge of ‘misleading marketing/advertising’ and paid a 635 million USD fine, followed by civil trials in 2020 and subsequent procedures. The very high addiction liability of oxycodone, comparable to that of heroin, was deceitfully denied for a very long period, which allegedly caused the enormous opioid addiction epidemic in the US (and allegedly causing more than 15000 deaths per week in the USA and beyond) [53].
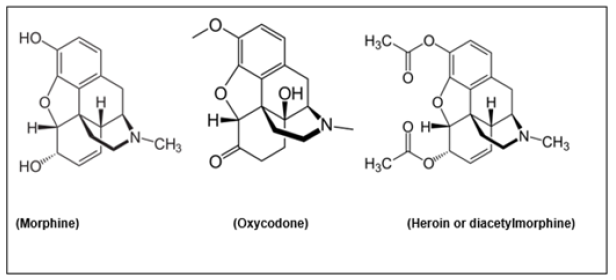
Figure 7: Chemical structures of three heavy painkillers: morphine (left) and closely related derivatives, oxycodone (middle) formed by (among others) a methyltransferase step, and heroin (right), formed by a twofold acetylation of morphine (Chemical structures from Wikipedia Chemistry).
The simple slogan used in the TV-series that “human behavior is essentially comprised of two things: ‘run from pain’ towards ‘run to pleasure’ (…)” [51], illustrates the danger of an oversimplified approach to the evaluation of human behavior in public health. This may perhaps explain the cautious and possibly also restrictive/negative advises of official instances like the FDA against the use of MDMA (3,4-methylenedioxymethamphetamine, also known as ecstasy) therapy for PTSD, explained as “the lack of crucial psychological and physiological safety data” [14].
The analogy with the phenomenon of β-endorphin production during long-distance running, suggests an important role of endogenous analgetic substances that diminish the painful effects of physical injury and inflammation. This was also reflected in the pendulum hypothesis of Selye and followers. where glucocorticoids affect the balance between a dangerous infection and the danger or negative effects of inflammation (Figure 6). What misses in these binary schematic models, however, is the important role of the factor time (duration). Inflammation is a natural response against harmful stimuli of all kinds, of pathogenic nature or simply innocent or irritating substances (like annoying, urticating sting of the nettle family, Urticaceae). The immune system reacts to these stimuli within a certain, limited time period (varying from a number of hours to days).
Repeated exposure to stressful stimuli, however, may form a far greater risk for health. This is not only seen in PTSD. Following the suppression of the immune system, repeated exposure to high stress levels may aggravate several (auto) immune disorders (e.g. in lupus erythematosus), some of which moreover are typically linked with PTSD [54], and possibly in other systemic autoimmune disorders (like in rheumatoid arthritis) [55]. Also, in some ‘rare’ neurologic disorders, such as Stiff Person’s Syndrome (SPS), the disease appears to reveal an important role of stress in its onset, together with an (auto)immune etiology, as SPS is associated with the presence of anti-glutamic acid decarboxylase antibodies [56].
Unlike the relatively short half-life time of a typical inflammatory response, at least when in absence of an enhanced systemic response (such as during a septic shock) [57], the dynamic aspects of restoring a disbalance (or skewness) of the immune system as a whole, are far less obvious [57,58]. Also here, it is not only a matter of a binary balance between Th1- or Th2-mediated inflammatory responses, and of a mix of cytokines tuned accordingly. Also, an individual’s Th1/Th2 bias and immunological history with exposure to environmental antigens, may direct the individual towards a ‘skewed’ immune system, as seen in allergic disorders [58].
In analogy, it is well-known among (para)medical and social workers, that restoring a distorted work-life balance is a much more difficult, say a slow and dragging process, than the easy solutions offered by pharmaceutical companies and so-called ‘recreative’ drug suppliers. Also, other fast rewarding feedback loops appear to lead to a focus shift, rather than to a persistent solution of health problems (e.g. in bulimia and eating disorders) [15]. Whereas in ‘natural’ environments, many animal species found a ‘natural’ balance between several time-consuming (not only long-distance running, or walking, but also long hours spent on chewing or sleeping) (Table 1) as well as stress alleviating activities (such as grooming), this occupational balance has hardly survived in our human fast-life economy. Therefore, more emphasis is needed for the dynamic aspects of these processes, in order to understand the slow dynamic processes at work in a non-natural, but culturally dominated society.
Concluding Remarks
At the onset, this review started from the observation of delayed-type effects of intercellular communication related to DA-rebound PRL- and biphasic LH-secretion in cell cultures. The rather ill-defined notion of a homeostatic control of cellular responsiveness (in particular in multicellular 3D-cultures), however, could be explained in terms of the dynamics of long-term intercellular communication (paracrine or otherwise) [17,18]. The rebound phenomena observed after DA withdrawal can also be extrapolated to the effects seen at the organism level after withdrawal of certain mood- affecting drugs. The interconnectedness of mood impairment and long-term effects of repeated stress stimuli, such as observed in PTSD, prompted us to re-evaluate some historic balance hypotheses related to the brain response to glucocorticoids and stress hormones [9]. An important observation is the lack of dynamic understanding of the binary balance hypotheses developed so far. When compared to the immune response in inflammation, being limited in time and local effects (at least during the initial response), the dynamics of systemic aberrations in chronic immune diseases as well as in long-term stress-related dysfunction are less well understood. A comparative study of natural behavior observed in our closest relatives in mammals and the analysis of culturally imprinted behavior, such as in the human analogs of grooming (see e.g. Dunbar [1996]) [41], forms an interesting lead for future research.
Not surprisingly, individual differences in coping strategy (both susceptibility to stressful stimuli and behavioral adaptations) and individual variability in endogenous endorphin production and breakdown play a decisive role [50]. This variability makes it more important to protect the more vulnerable, especially the children, because the effects of too high levels of glucocorticoids in particular affecting the limbic system, are most notable (and, both practically and ethically, unpredictable!) at young age [9]. In a world that is flooded with war zones and impending climate catastrophes on top of that, protecting the most vulnerable should be also a top priority of the global political leaders.
Acknowledgements
None.
Conflict of Interest
None.
References
- Houterman S, Janssen Heijnen MLG, van de Poll Franse LV, Brenner H, Coebergh JWW (2006) Higher long-term cancer survival rates in southeastern Netherlands using up-to-date period analysis. Ann Oncol 17(4): 709-712.
- Friedman MJ (2013) Finalizing PTSD in DSM-5: getting here from there and where to go next. J Trauma Stress 26(5): 548-556.
- Zhang H, Zhao Y, Zhu JK (2020) Thriving under Stress: How Plants Balance Growth and the Stress Response. Deve Cell 55(5): 529-543.
- Kruk MR, Westphal KGC, van Erp AHM, van Asperen J, B J Cave, et al. (1998) The hypothalamus: cross-roads of endocrine and behavioural regulation in grooming and aggression. Neurosci Behav Rev 23(2): 163-177.
- Michael RP, Herbert J, Welegalia J (1966) Ovarian hormones and grooming behaviour in the rhesus monkey (Macaca mulatta) under laboratory conditions. J Endocrinol 36(3): 263-279.
- Harlow HF (1958) The nature of love. American Psychologist 13(12): 673-685.
- Herman JP, McKlveen JM, Ghosal S, Kopp B, Walsin A, et al. (2016) Regulation of the Hypothalamic Pituitary-Adrenocortical Stress Response. Compr Physiol 6(2): 603-621.
- Standring S (ed.) (2005) Gray’s Anatomy. London, New York, Sydney, Toronto: Elsevier Ltd 1248-1249.
- De Kloet ER (1991) Brain Corticosteroid Receptor Balance and Homeostatic Control. Front Neuroendocrinol 12(2): 95-164.
- Fava GA, Benasi G, Lucente M, Offidani E, Cosci F, et al. (2018) Withdrawal Symptoms after Serotonin-Noradrenaline Reuptake Inhibitor Discontinuation: Systematic Review. Psychother Psychosom 87(4): 195-203.
- Chen C, Zhang J, Israel JM, Clarke IJ, Vincent JD (1993) Mechanism of the prolactin rebound after dopamine withdrawal in rat pituitary cells. Am J Physiol 265(1 Pt 1): E145-152.
- Benoit E, Lyons DG, Rihel J (2024) Noradrenergic tone is not required for neuronal activity-induced rebound sleep in zebrafish. J Comp Physiol B 194(3): 279-298
- Clayton R (2020) Sleep: a missing ingredient in balancing work and life. Psychology Today.
- Reardon S (2024) MDMA therapy for PTSD rejected by FDA panel. Nature News.
- Allaerts W (2020) Facts and myths about neuropeptide Y. Curr Trends Endocrinol11: 13-22.
- Allaerts W (2024) Life as a dissipative structure. A metabletic exploration of public health research. Communication Cognition 57(1-2): 105-126.
- Allaerts W, Vankelecom H (2005) History and perspectives of pituitary folliculo-stellate cell research. Eur J Endocrinol 153: 1-12.
- Allaerts W (1989) Involvement of folliculo-stellate cells in inhibitory interactions in rat anterior pituitary. A morpho-functional study in vitro (PhD thesis) (Series: Acta Biomedica Lovaniensia) 91.
- De Koning J, van Dieten JAMJ, van Rees GP (1980) The pattern of LH-release of rat pituitary glands during long-term exposure to LHRH in vitro. In: M Jutisz, KW McKerns (eds), Synthesis and release of adenohypophyseal hormones. New York: Plenum Publishing 639-657.
- De Koning J, Tijssen AMI, van Rees GP (1987) The involvement of ovarian factors in maintaining the pituitary glands of female rats in a state of low LH responsiveness to GnRH. J Endocrinol 112(2): 265-273.
- Allaerts W, Tijssen AMI, Jeucken PHM, Drexhage HA, De Koning J (1994) Influence of folliculo-stellate cells on biphasic luteinizing hormone secretion response to gonadotropin-releasing hormone in rat pituitary cell aggregates. Eur J Endocrinol 130(5): 530-539.
- Allaerts (1992). Inquiry into the spatio-temporal contingency of cellular communication systems. Communication & Cognition, 25 (4): 277-294.
- Wong AHC, Liu F (2011) Uncoupling the dopamine D1-D2 receptor complex: a novel target for antidepressant treatment. Clin Pharmacol Ther 91(2): 298-302.
- Duval F, Mokrani MC, Bailey P, Corrêa H, Crocq MA, et al. (2000) Serotonergic and noradrenergic function in depression: clinical correlates. Dialogues Clin Neurosci 2(3): 299-308.
- Siever LH, Uhde TW (1984) New studies and perspectives on the noradrenergic receptor system in depression: effects of the α2-adrenergic agonist clonidine. Biol Psychiatry 19(2): 131-156.
- (2023) Cleveland Clinic SNRIs (Serotonin and Norepinephrine Reuptake Inhibitors).
- Wisor JP (2019) Dopamine and Wakefulness: Pharmacology, Genetics, and Circuitry. Handb Expe Pharmacol 253: 321-335.
- Kopin IJ, Axelrod J, Gordon E (1961) The metabolic fate of H3-epinephrine and C14-metanephrine in the rat. J Biol Chem 236(7): 2109-2113.
- Bartolato M, Chen K, Shih JC (2010) The degradation of serotonin: role of MAO (Chapter 2.4). Handb Behav Neurosci 21: 203-218.
- Bartsch T, Döhring J, Rohr A, Jansen O, G. Deuschl (2011) CA1 neurons in the human hippocampus are critical for autobiographical memory, mental time travel, and autonoetic consciousness. Proc Natl Acad Sci USA 108(42): 17562-17567.
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joëls M (1998) Brain corticosteroid receptor balance in health and disease. Endocr Rev 19(3): 269-301.
- De Kloet ER, Oitzl MS, Joëls M (1999) Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci 22(10): 422-426.
- Selye H (1950) Stress. The physiology and pathology of exposure to stress. Montreal: Acta Medica.
- Buchman AL (2001) Side effects of corticosteroid therapy. J Clin Gastroenterol 33(4): 289-294.
- Chatterjee R (2024) “Feel Better, Live More” (Podcast series).
- Hemelrijk CK, Wantia J (2005) Individual variation by self-organisation. Neurosci Biobehav Rev 29(1): 125-136.
- Grandi LC, Ishida H (2015) The physiological effect of human grooming on the heart rate and the heart rate variability of Laboratory non-human primates: a pilot study in male rhesus monkeys. Front Vet Sci 2:50
- KeverneEB, Martensz ND, Tuite B (1989) Beta-endorphin concentrations in cerebrospinal fluid of monkeys are influenced by grooming relationships. Psychoneuroendocrinology 14(1-2): 155-161.
- Cimarelli G, Marshall Pescini S, Range F, Berghänel A, Virányi Z (2021) Relationship quality affects social stress buffering in dogs and wolves. Animal Behaviour 178: 127-140.
- Normando S, Haverbeke A, Meers LL, Tallegan MI, F Ödberg (2002) Heart rate reduction by grooming in horses (Equus caballus).
- Dunbar R (1996) Grooming, Gossip and the Evolution of Languages. Cambridge (MA).
- Phelps S, Ng WL, Musolesi M, Russell YI (2018) Precise time-matching in chimpanzee allogrooming does not occur after a short delay. PLoS One 13(9): e0201810.
- Girard Buttoz C, Surbeck M, Samuni L, Boesch C, Fruth B, et al. (2020) Variable use of polyadic grooming and its effect on access to social partners in wild chimpanzees and bonobos. Animal Behaviour 168: 211-224.
- Markham AC, Gesquiere LR, Alberts SC, Altmann J (2015) Optimal group size in a highly social mammal. Proc Natl Acad Sci USA (PNAS) 112(48): 14882-14887.
- Siegel JM (2022) Sleep function: an evolutionary perspective. Lancet Neurol 21(10): 937-946.
- Herculano Houzel S (2009) The human brain in numbers: a linearly scaled-up primate brain. Front Hum Neurosci 3: 31.
- Fetters KA (2024) What causes the ‘runner’s high’ and how can you achieve it?
- Luan YH, Wang D, Yu Q, Chai XQ (2017) Action of β-endorphin and nonsteroidal anti-inflammatory drugs, and the possible effects of nonsteroidal anti-infammatory drugs on β-endorphin. J Clin Anesth 37: 123-128.
- Bekhbat M, Li Z, Mehta ND, Treadway MT, Lucido MJ, et al. (2022). Functional connectivity in reward circuitry and symptoms of anhedonia as therapeutic targets in depression with high inflammation: evidence from a dopamine challenge study. Mol Psychiatry 27(10): 4113-4121.
- Kavushansky A, Kritman M, Maroun M, Klein E, Richter Levin G, (2013). β-endorphin degradation and the individual reactivity to traumatic stress. Eur Neuropsychopharmacol 23(12): 1779-1788.
- Fitzerman Blue M, Harpster N (2023) Painkiller. TV Miniseries, IMDb (https://m.imdb.com) and Netflix series (released 2023) (https://www.netflix.com).
- Keefe PR (2017) The Family that built an Empire of Pain.
- Meier B (2018) Pain Killer: An Empire of Deceit and the Origin of America’s Opioid Epidemic.
- Goldschen L, Ellrodt J, Amonoo HL, Feldman CH, Case SM, et al. (2023) The Link between Post-Traumatic Stress Disorder and Systemic Lupus Erythematosus. Brain BehavImmun 108: 292-301.
- Allaerts W, Drexhage HA (1994) Dendritic Cells in Autoimmune Disease. In: CAFM Bruijnzeel Koomen and ECM Hoefsmit (eds.), Immunopharmacology of Macrophages and Other Antigen-Presenting Cells 117-134.
- Rakocevic G, Floeter MK (2012) Autoimmune stiff person syndrome and related myelopathies: understanding of electrophysiological and immunological processes. Muscle Nerve 45(5): 623-634.
- Rossi JF, Lu ZY, Massart C, Levon K (2021) Dynamic Immune/Inflammation Precision Medicine: The Good and the Bad Inflammation in Infection and Cancer. Front Immunol 12: 595722.
- Allaerts W, Chang TW (2017) Skewed exposure to environmental antigens complements Hygiene Hypothesis in explaining the rise of Allergy. Acta Biotheor 65(2): 117-134.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.