Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Clinical Evaluation of the Efficacy of a Topical Acne Product in Improving Skin Conditions
*Corresponding author: Jonathan RT Lakey, Department of Surgery and Biomedical Engineering University of California Irvine.
Received: November 01, 2024; Published: November 07, 2024
DOI: 10.34297/AJBSR.2024.24.003230
Abstract
Acne is a common chronic inflammatory condition of hair follicles and sebaceous glands, influenced by factors like androgens, increased sebum, and immune responses. A common dermatological affecting approximately 85% of adolescents, and lesions often persist into adulthood. Even mild to moderate acne can result in scars Adipose stem cells (ASCs) have shown promise in wound healing and scar repair by promoting fibroblast activity and collagen synthesis, suggesting potential benefits for managing acne scars through enhanced dermal collagen production and fibroblast migration.
Zixeek Acne Treatment (Zixeek Inc, California) combines stem cell conditioned media (SCM) and salicylic acid to address acne in teenagers and young adults, reducing reliance on antibiotics by targeting resistant strains and hormonal imbalances. The objective of this 30-day clinical study was to evaluate the extent to which an acne cream applied twice daily for 30 days affected acne lesions and scars. Fifteen 15 participants (ages 18-43 years; mean age 29.9 years) participated.
There was a statistically significant improvement in the appearance of overall acne lesions from baseline at day 30 post-treatment interval, however, there was no statistically significant change in acne scars from baseline at day 30 post-treatment interval. According to the post-treatment questionnaire subjects generally reported favorable responses (agree, strongly agree), there was a significant favorable response to the question “My skin texture has improved since starting the treatment.”
This study provides insight into an effective way by cell therapy to treat acne lesions. However, given the small sample size, including of a larger sample in randomized controlled clinical trial is warranted.
Keywords: Acne, Stem cells, Skin treatment
Introduction
Acne, a common chronic inflammation of hair follicles and sebaceous glands, caused by multiple factors including (but not limited to) androgens, increased sebum secretion, abnormal keratinization of hair follicles and sebaceous glands, and inflammatory reactions of lymphocytes, macrophages, and neutrophils. Acne affects about 85% of adolescents. The chronic inflammation of the follicles and glands associated with moderate-to severe acne, can lead to varying degrees of scar formation. This scarring affects about 20% of adolescents globally, with an estimated >60% likelihood that it will persist in the individuals in their twenties and over 40% likelihood that acne scars persist into the third decade of life. Of note, non-Caucasians are often disproportionately affected by acne scars related to skin thickness and higher quantities of melanin with increased number of sebaceous glands [1]. After healing, acne scars are often left on the face, which may be related to abnormal degradation of facial collagen.
Almost two decades ago, it was reported that adipose stem cells (ASCs) promoted wound healing, scar repair, and fibroblasts activation [2]. More recently, mechanistic studies have demonstrated mechanistic acceleration of wound healing by ASCs by expanding the fibroblasts phenotype and increasing the supply of macrophages to the wound, enhancing granulation tissue formation, and growth of dermal fibroblasts both via intercellular cell-to-cell contact as well as paracrine activation of marginal cells [3-5]. Thus, it is plausible that ASCs with conditioned media can stimulate the synthesis of dermal collagen and promote the migration of fibroblasts into the dermis, to help moderate acne lesions and scarring.
Zixeek Acne Treatment (Zixeek Inc, California) is an innovative topical acne treatment for young adults that addresses a wide age range, providing effective results for both teenagers and young adults. Zixeek utilizes stem cell conditioned media (SCM) and salicylic acid combining the regenerative properties of SCM with the exfoliating and anti-inflammatory effects of salicylic acid. The novel formulations aim to reduce reliance on antibiotics, tackling resistant strains, while managing hormonal imbalances underlying acne, expanding therapeutic options. The product leverages the capacity of SCM to boost collagen, reduce inflammation, promoting skin renewal for acne scars, while salicylic acid penetrates pores for efficient exfoliation, preventing acne formation, with topical application, rather than injections.
The objective of this single-center, non-comparative clinical study was to evaluate the effectiveness of a topical acne product on fifteen (15) healthy male and female subjects, ages 18-43 years.
The study included a 30-day clinical efficacy protocol carried out over a two-month period in 2024 evaluating;
1. improvement in appearance of acne lesions and scars (Expert Grading of Images); and
2. Consumer Feedback.
Methods
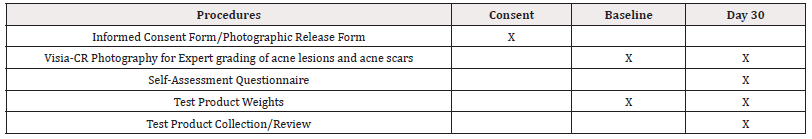
The procedures and timing of occurrence is presented in (Table 1).
At baseline subjects were provided two vials of the acne cream in a 30ml bottle at the. Subjects were instructed to apply a small amount (no more than a dime-size) of the cream evenly over the affected areas twice daily after thoroughly washing and patting dry. Subjects were instructed to report any adverse reactions or discomfort, discontinue use immediately. No adverse events were reported.
All procedures and methods were performed according to the approved protocol. This clinical study was conducted in accordance with the International Conference of Harmonization Tripartite Guideline in compliance with Good Clinical Practices, applicable FDA regulations/guidelines set forth in 21 CFR Parts 11, and 50 and standard practices of ALS.
Data quality is assured, personnel are trained according to the study to be carried out, equipment is duly calibrated, and the methods used are recognized and/or validated. The Quality Assurance Department oversees auditing the Management System with availability for any specific study monitoring. Responsibility for the identity, purity, strength, safety, composition, and stability of the test articles remained with the Sponsor. An adequate amount of test article was supplied for use by the testing facility to ensure quantity would last for the maximum number of subjects for the entire study duration. Upon arrival at the test facility, test articles were weighed, and the weights recorded prior to distribution, at each visit and upon collection at the final visit (or earlier, as outlined in the study plan). Sample was retained for a minimum of 30 days beyond submission of final report. Sample disposition will be conducted in compliance with appropriate federal, state, and local ordinances.
Statistical analyses were performed to test the hypothesis that the pre-treatment values of each parameter were statistically different from its post-treatment values. Statistical analysis was performed using a z-test. Statistical significance was declared if the p-value is ≤ 0.05. All subjects did not respond to questions #21-#25 on the post-treatment self-assessment questionnaire. There were no adverse events reported during the study period.
Results
The subject demographics are presented in Table 2. Mean age was 29.9 years. Of the 15 subjects, one was Caucasian, two were African American, eight were Hispanic, two were bi-racial, and two were Native American. Two were ≤20 years of age, 5 were 21-29 years of age, five were ≥30 years, and three were ≥40 years.
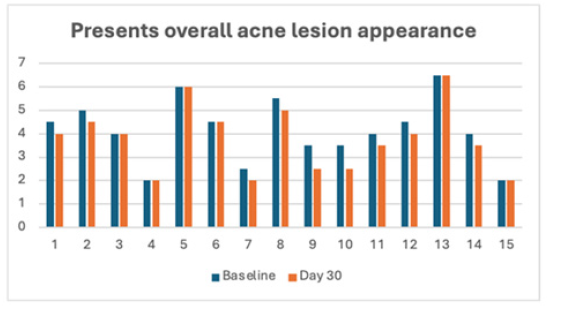
Table 3 provides the mean precent difference of overall acne lesion appearance and percentage of subjects improve. There was a statistically significant improvement in the appearance of overall acne lesions from baseline at day 30 post-treatment interval.
As displayed in Table 4, there was no statistically significant change in acne scars from baseline at day 30 post-treatment interval
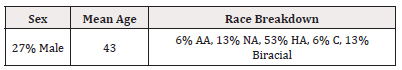
Table 2: Subject Demographics.
Note*: AA=African American, C= Caucasian, H=Hispanic, NA= Native American.

Table 3: Clinical Grading (Overall Acne Lesion Appearance).
Note*: Negative difference indicates improvement. Bold values indicate statistical significance (p≤0.05). NS= Not Significant.

Table 4: Clinical Grading (Acne Scars Appearance).
Note*: Negative difference indicates improvement. Bold values indicate statistical significance (p≤0.05). NS= Not Significant.
Post-Treatment Questionnaire
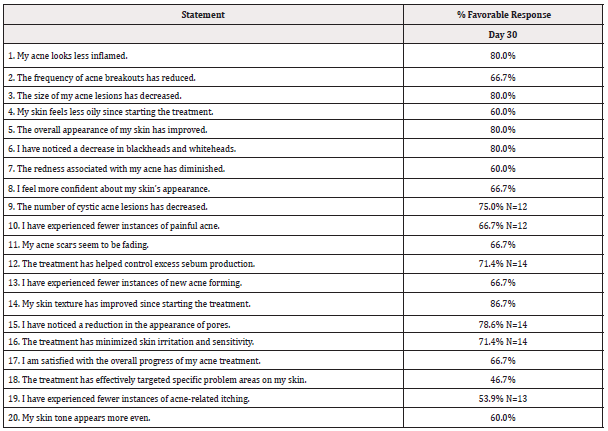
The responses to the post-treatment questionnaire are illustrated in Table 4. According to the scale: Strongly Agree, Agree, Disagree, Strongly Disagree, Does not apply. Individuals that opted not to complete a question are excluded and the n is presented as appropriate. While subjects generally reported favorable responses (agree, strongly agree), there was a significant favorable response to the question “My skin texture has improved since starting the treatment.”
On average, subjects presented with moderate appearance of acne lesions (4.13±1.33) with a range of 2.0-6.5 at baseline. On Day 30, the average appearance was 3.77 ± 1.41. Subjects with more severe acne (e.g. >6.0) or milder (e.g. ≤2.0) did not appear to benefit from the treatment. On average, subjects presented with mild to moderate appearance of acne scars (3.47 ± 1.87) with a range of 1.5-6.5 at baseline with no change among any subject at Day 30 (Figure 1).
Discussion
We report positive results, with no adverse outcomes from a small pilot study evaluating the effectiveness of a novel approach to acne treatment using SCM and salicylic acid. Unlike many current acne treatments such as topical ointments (e.g. benzoyl peroxide, salicylic acid, retinoids), which target pore-clearing, skin renewal, and bacterial reduction or systemic treatments (e.g. oral antibiotics, hormonal therapies, isotretinoin) regenerative options with stem cell conditioned media (SCM) promotes healing and reduce scarring. The acne treatment evaluated herein is based on combining treatments, with a focus on minimizing side effects, as the most effective way to manage acne. Recent studies have shown that ACSs have key features effective in repairing various tissue injuries, with relevance in treating acne scars. According to the methodology used to assess the efficacy of the investigational product Acne Care, there was a statistically significant improvement in the appearance of overall acne lesions from baseline at day 30 post-treatment interval. We report a statistically significant improvement in the appearance of overall acne lesions from baseline at day 30 post-treatment interval, however, there was no statistically significant change in acne scars from baseline at day 30 post-treatment interval. According to the post-treatment questionnaire subjects generally reported favorable responses (agree, strongly agree), there was a significant favorable response to the question “My skin texture has improved since starting the treatment.”
Kim, et al. were the first to report ASCs induced wounds healing, scar repair, and fibroblasts activation. In recent years, studies have shown that ASCs exhibit significant therapeutic effect on chronic lesions compared to bone marrow mesenchymal stromal cells. Using an animal model to investigate the effect of subcutaneous injection of the ASCs with conditioned media in the treatment of acne vulgaris scar, [6] observed a significant curative effect on acne vulgaris scar concluding the application as a therapeutic approach to regenerate to treat acne vulgaris scar in humans.
Li, et al. explored the use of facial autologous fat transplant of ASCs to dramatically reduce acne lesions [7]. Their findings suggested that ASCs altered acne-induced inflammatory responses. Similarly, Behrangi, et al (2022) reported ASC treatment of patients with acne scars accelerates the improvement of volume, area and depth of the scar by increasing collagen content and the dermal thickness, so it can be used as a potentially effective treatment for these patients [8]. Notably, these studies used injections and were carried out over a longer duration than this one. The application of cream rather than use of injections is likely to be a preferred approach.
Participants in this study reported high level of satisfaction with the treatment.
Limitations: While this pilot study demonstrated strengths and potential to improve acne lesions, the sample size was relatively small. In addition, a patient's physical condition, genetics, age, and skin type can all affect their response to treatment and their ability to produce collagen in the skin, thus affecting treatment outcomes. Future studies will include a larges sample size, i.e., n ≥ 25, for obtaining statistically significant results. In addition, the lack of a control group presents a limitation. To serve as reference for normal biological and environmental changes, a control group (untreated or placebo) will be included in future clinical studies. Further, based on the scope of the study, images of subjects’ lesions and scars with analytical measurement capacity would be of great benefit for future trials. For example, as described by Liu L, et al, the “AcneGrader,” provides deep learning base models, offering a novel approach to effectively grade acne severity [9].
References
- Davis EC, Callender VD (2010) A review of acne in ethnic skin: Pathogenesis, clinical manifestations, and management strategies. J Clin Aesthet Dermatol 3(4): 24-38.
- Kim WS, Park BS, Sung JH, Yang JM, Park SB, et al (2007) Wound healing effect of adipose-derived stem cells: A critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci 48(1): 15-24.
- Zaleski Larsen LA, Fabi SG, McGraw T and Taylor M (2016) Acne scar treatment: A multimodality approach tailored to scar type. Dermatol Surg 42(Suppl 2) S139-149.
- Hong SJ, Jia SX, Xie P, Xu W and Leung KP, et al (2013) Topically delivered adipose derived stem cells show an activated-fibroblast phenotype and enhance granulation tissue formation in skin wounds. PLoS One 8(1): e55640.
- Kim WS, Park BS, Park SH, Kim HK and Sung JH (2009) Antiwrinkle effect of adipose-derived stem cell: Activation of dermal fibroblast by secretory factors. J Dermatol Sci 53(2): 96-102.
- Shan X, Choi JH, Kim KJ, Lee YJ, Ryu YH, et al (2018) Adipose stem cells with conditioned media for treatment of acne vulgaris scar. Tissue Eng Regen Med 15(1): 49-61.
- Li X, Luo S, Chen X, Li S, Hao L, et al (2022) Adipose-derived stem cells attenuate acne-related inflammation via suppression of nlrp3 inflammasome. Stem Cell Res Ther 13(1): 334.
- Behrangi E, Moradi S, Ghassemi M, Goodarzi A, Hanifnia A et al (2022) The investigation of the efficacy and safety of stromal vascular fraction in the treatment of nanofat-treated acne scar: A randomized blinded controlled clinical trial. Stem Cell Res Ther 13(1): 298.
- Liu S, Fan Y, Duan M, Wang Y, Su G, et al (2022) Acnegrader: An ensemble pruning of the deep learning base models to grade acne. Skin Res Technol 28(5): 677-688.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.