Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Desorption Kinetics Characterization of Virus-Like Particles in Polyvalent HPV Vaccine Containing Aluminum Phosphate Adjuvant
*Corresponding author: Yang Wang and Liangzhi Xie, Sinocelltech Ltd., No.31 Kechuang 7th Street, BDA, Beijing 100176, China.
Received: September 25, 2024; Published: October 07, 2024
DOI: 10.34297/AJBSR.2024.24.003185
Abstract
It is of importance to explore the nature of adsorption-desorption behavior between Virus-Like Particle (VLP) proteins and adjuvant to support the development of a VLP based Human Papillomavirus (HPV) vaccines containing aluminum phosphate as the adjuvant. In this study, a home-made desorption solution and procedure were developed to desorb the HPV VLP protein adsorbed onto aluminum phosphate adjuvant. The desorbing kinetics of the VLP from adjuvant was studied. Several methods, including appearance, turbidity, ultraviolet spectrophotometry, transmission electron microscopy and ELISA-based in vitro relative potency as probed by type-specific neutralizing monoclonal antibodies, were employed for the desorption kinetics study. The kinetics of VLP morphology at various stages of desorption under transmission electron microscopy was presented for the first time. The physical and antibody-based immunological characteristics of the VLP vaccine were observed after 48hours desorption. The appearance showed that the samples with adjuvants were cleared, the turbidity showed that the samples with adjuvants were basically the same as those without adjuvants, the ultraviolet spectrophotometry revealed a complete desorption, and the transmission electron microscopy results showed that the VPLs can gradually be seen clearly during the desorption process. The particle size and particle integrity remained unchanged post desorption in comparison with those without adjuvant. The in vitro relative potency test results showed that the conformational epitopes remain intact post adjuvant desorption and the ratio of the end of dissociation to the initial dissociation was 1.41 and 1.69. In conclusion, the work demonstrates that home-made desorption procedure can fully recover adsorbed HPV VLP completely by dissolving aluminum phosphate adjuvant without structural impact, and therefore, it offers great benefits in HPV vaccine development for being able to assess the HPV VLP quality VLPs in drug product.
Keywords: Adjuvant desorption, Human papillomavirus, HPV vaccine, Aluminum phosphate adjuvant, Transmission electron microscopy.
Introduction
Cervical cancer and precancerous lesions are a major health problem for women worldwide. Clinical, molecular, and epidemiological investigations have demonstrated that Human Papillomavirus (HPV) is the major cause of cervical cancer and atypical cervical hyperplasia [1]. The use of HPV vaccine, especially the use of polyvalent HPV vaccine, can effectively prevent HPV infection and the occurrence of tumors. Currently, HPV vaccine on the market is prepared by combining Virus-Like Particles (VLP) with aluminum adjuvant in a certain proportion. VLP is the only antigen of HPV vaccine. A mature VLP has a diameter of 50~55nm and a 20-hedral symmetric structure with a triangular section fraction equal to 7 [2,3]. A VLP expressed by recombinant technique is a star-shaped pentamer [4] (Capsomere) polymerized from 360 main capsid protein (L1) monomers. With safe performance and long application history, aluminum adjuvant can activate Dendritic Cells (DCs) and inflammatory factors together with antigens to stimulate immune response. Aluminum adjuvants, usually in the form of aluminum hydroxide, aluminum phosphate, or alum, are widely used as adjuvants in vaccines. So far, aluminum hydroxide and aluminum phosphate are the adjuvants approved by WHO for human vaccines. Aluminum hydroxide adjuvant is a crystalline aluminum hydroxide with positive charge at physiological pH value (isoelectric point pI=11). Aluminum phosphate is in an amorphous form that is negatively charged at physiological pH (isoelectric pI=5~7). The reaction mechanism aluminum adjuvant in vaccines is that it is a highly efficient adsorbent, which first adsorbs an “antigen library” with protein antigen, then releases the antigen slowly, thereby increasing the efficacy of the antigen. In addition, aluminum adjuvants can promote macrophage’s response. For the adsorption between aluminum adjuvant and protein, research from Professor Stanley L. Hem’s, et al. team demonstrated that hydrophobic and electrostatic forces are major forces of aluminum adjuvant to adsorb antigen protein [5].
The electrostatic forces are major forces of aluminum hydroxide adjuvant to adsorb antigen protein [6], the hydrophobic and electrostatic forces are forces of aluminum phosphate adjuvant to adsorb antigen protein [7]. Stanley L. Hem’s, et al. [5] team used ethylene glycol to mitigate the hydrophobic effect between protein and aluminum adjuvant. As the content of ethylene glycol increased, the hydrophobic effect intensified. The adsorption rate of aluminum hydroxide adjuvant and BSA remained nearly unchanged, while the adsorption rate of aluminum phosphate adjuvant and lysozyme decreased significantly, which demonstrated that one of the forces for the adsorption of protein by aluminum phosphate adjuvant was hydrophobicity. The hydrophobic force was nearly nonexistent in the adsorption of aluminum hydroxide adjuvant and protein. However, the use of sodium chloride to increase the ionic strength increases the electrostatic attraction [8]. With the increase of sodium chloride content, the electrostatic force enhances gradually, and the adsorption rate of aluminum hydroxide adjuvant and aluminum phosphate adjuvant with protein decreases [9].
The desorption method of aluminum adjuvant and protein has been reported. One method of desorption is by dissolving aluminum adjuvants, including adjusting pH value and adding metal chelating agent. Adjusting the pH value can promote the desorption of aluminum adjuvant and protein: When the pH value is higher than 7.4 or lower than 4.1, ovalbumin will be eluted; When the pH is adjusted to any value other than 7.4, lysozyme will eluate from the aluminum phosphate adjuvant [10]. However, both citric acid and EDTA undergo desorption through the mechanism of chelating metal cations [11]. Structurally, citric acid contains three carboxyl groups and one hydroxyl group, which can form coordination bonds with aluminum ions [12] (Figure1a); EDTA dissolves aluminum ions by chelating with aluminum ions to form water-soluble complexes, promoting the removal of aluminum ions [13] (Figure 1b). Merck has reported that they use EDTA to dissolve the aluminum adjuvant, an equal volume of 100mM EDTA, 100mM borate, 5% sucrose and 0.05% polyssorbate 20 (PS-20) pH8.5 was mixed with the final vaccine, incubating at 25℃ for 2 hours [14]. Another method of desorption is to elute the model protein from the aluminum adjuvant using surfactant. Arnebrant [15] reported that surfactants elute the pre-adsorbed protein or lysozyme by solubilization and substitution mechanism. In a study, Sodium Dodecyl Sulfate (SDS) was used as a surfactant [16], the content of desorbed ovalbumin increases with the increase of SDS concentration. The above-mentioned methods can dissociate the aluminum adjuvant and VLP protein effectively. However, the use of EDTA or surfactant has many drawbacks, it may destroy the tertiary structure of VLP and denature it [17]. When EDTA forms a complex with a metal cation, it is not only complexed with aluminum ion, but also with other cation ions, e.g. calcium ion. There are reports that cation ions play a role in VLP assembly [18]. Therefore, EDTA may destroy the assembly of VLP protein. Although citric acid also can form a complex with metal cations, its complexation reaction conditions with different metal ions can be adjusted, the reaction condition of citric acid with calcium ion is pH4.0~4.5 and 60℃; with aluminum ion is under neutral environments.
In theory, citric acid can be chosen as the desorbent. In practice, Transmission Electron Microscopy (TEM) was used in this study to investigate the morphology and structure of VLP antigen protein dissociated from citric acid and EDTA, respectively. It was proved that after citric acid desorption, VLPs remain intact. Then, a citric acid desorbent was used to desorb the VLP antigen with aluminum phosphate adjuvant. The specific citric acid desorption solution is: 10mM histidine, 0.01% polysorbate 80, 320mM sodium chloride, 400mM citric acid dehydrate sodium, 120mM sodium dihydrogen phosphate dihydrate, pH=6.0. In the initial state, the VLP antigen is coated to form an adjust-antigen adsorbent as the high adsorption capacity of aluminum phosphate, so that most of the specific binding sites of the antigen are deeply buried in the precipitation of aluminum adjuvant particles. With the increased time of the reaction between the desorbent and the adjuvant, the aluminum phosphate adjuvant began to dissolve, and the VLP was gradually precipitated, and it was fully precipitated after 48 hours (Figure 2). This study represents the inaugural exploration of the kinetic characterization of adsorption. The appearance, turbidity, Ultraviolet (UV) method, Transmission Electron Microscopy (TEM) and In Vitro Relative Potency (IVRP) characterization methods were used to prove that the citric acid desorption method could separate the protein from the adjuvant, and the protein morphology remained intact. This study is the first to show the morphological changes of VLP under TEM.
Materials
The serotypes 6, 11, 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 recombinant human papillomavirus like particles (made by Sinocelltech Ltd., Recombinant plasmid containing various HPV type L1 genes was constructed, and recombinant rod DNA was obtained in DH10Bac bacteria. The VLP assembled in the Insect High Five (HF) cell lines was cleaved and purified to obtain high-purity monovalent VLP); Aluminum phosphate adjuvant (made by Sinocelltech. Ltd.). Turbidity standard storage solution (1% hydrazine sulfate solution mixed with 10% ulotropine solution of equal volume); Uranyl ac etate dye; 0.18M histidine solution (0.18M histidine, 0.32M NaCl, 0.01% polysorbate 80, pH7.0); citric acid desorption solution: 10mM histidine, 0.01% polysorbate 80 (PS80), 320mM sodium chloride, 400mM citric acid dehydrate sodium, 120mM sodium dihydrogen phosphate dihydrate, pH=6.0; VLP diluent (19.1mg/mL sodium chloride, 2.8mg/mL histidine, 0.1mg/mL Polysorbate 80). 96-well cell culture plate (Costar, USA); Circular oscillator (MS 3 basic, IKA); 30mL sample container with lid (2084900, HACH); ultramicro spectrophotometer (NanoDrop2000C, Thermo); desktop turbidity meter (TL2360, HACH); Gelex second-level turbidity standardization Kit (HACH); transmission electron microscope (Tecnai Spairit, FEI).
Method
Sample Preparation Method
Adsorption Sample Preparation
Serotype 33 was selected as the univalent representative subtype and 14 mixed valence protein was selected as the polyvalent representative. After the samples were prepared according to the following methods, the samples were all left for adsorption at 4℃ overnight, and then part of the supernatant (excess target volume) was taken for protein content detection (UV method). If no protein was detected in the supernatant, it proved that the adsorption was successful. If the protein was detected in the supernatant, it indicated that the adsorption was incomplete, or desorption was present. (Aluminum phosphate adjuvant diluted with normal saline, VLP diluted with VLP diluent, aluminum phosphate adjuvant: protein=1.35).
1) Subtype 33 antigen (Sample 1): 20mL of subtype 33 antigen with a final concentration of 0.74mg/mL.
2) 33 subtype antigens +aluminum phosphate adjuvant (Sample 2): 10mL of 33 subtype monovalent antigen, 10mL of aluminum phosphate adjuvant, and 1mL of 0.18M histidine were mixed with the final concentration of 0.74mg/mL for antigen and 1mg/mL for aluminum phosphate adjuvant.
3) 14-valent mixed antigen (Sample 3): 20mL of 14-valent mixed antigen ( the final concentration of 60μg/mL for subtype 6, 80μg/mL for subtype 11 and subtype 18, 120μg/mL for subtype 16, 40μg/mL for all other subtypes).
4) 14-valent mixed antigen +adjuvant (Sample 4): 10mL of 14-valent mixed antigen, 10mL of aluminum phosphate adjuvant, and 1mL of 0.18M histidine (the final concentration of antigen were 60μg/mL for subtype 6, 80μg/mL for subtype 11 and subtype 18, 120μg/mL for type 16, 40μg/mL for all other subtypes, and the final concentration of aluminum adjuvant was 1mg/mL).
5) Aluminum phosphate adjuvant (Sample 5): 20mL of aluminum phosphate adjuvant with a final concentration of 1mg/mL.
Desorption Sample Preparation
Use 96-well plates on the super clean bench, add 100μL of citric acid desorption solution to each well. Add all samples (Sample 1 to Sample 5) into the corresponding hole with citric acid desorption solution at the rate of 100μL/well, make a mark, cover the plate, and seal the plastic wrap. Fixed on a circular oscillator with 400rpm at room temperature.
Desorption Experiment
100μL of Sample 4 was added to the same volume of EDTA desorption solution and citric acid desorption solution and dissolved at room temperature for 48h. negative transmission electron microscopy.
Desorption Kinetics Experiment
All samples (Sample 1 to Sample 5) were desorbed for 2h, 4h, 6h, 24h, 30h, 48h, 100h, the samples status at each time point were investigated by appearance, turbidity, UV, IVRP, TEM. The trend of test results at each time point was investigated.
Appearance
The appearance test was carried out for all samples (Sample 1 to Sample 5), and the differences of samples at different dissociation times were compared. Take photos of samples at 2h, 4h, 6h, 24h, 30h, 48h, 100h.
Turbidity
The turbidity of all samples (Sample 1 to Sample 5) was detected, and the differences of samples at different dissociation times were compared. Turbidity is performed using a turbidity analyzer, which uses the mechanism of light scattering for the analysis of particles in the sample. When the light passes through the sample, the particles will scatter the light, and the light generated by the scattering is captured in the detector. The turbidity value of sample is determined according to the ratio of the scattered light intensity to the incident light intensity. The detection needs to be calibrated with a standard sample to ensure the accuracy of the measurement results. All samples were diluted 4 times respectively, the VLPs concentration of Sample 1 to Sample 4 was 0.185mg/mL, and the aluminum concentration of Sample 2, Sample 4 and Sample 5 was 0.25mg/mL. Then, the samples were added to a clean micro-empty sample container, wiped and put into the sample container rack, and tested in parallel three times.
UV
UV detection was carried out on all samples (Sample 1 to Sample 5), and the differences of samples at each time were compared. The test is carried out at 280nm wavelength, because protein molecules often contain tyrosine, tryptophan, phenylalanine and other benzene ring structure, there is a maximum absorption peak at 280nm wavelength, the absorption value is proportional to the protein concentration. Therefore, the absorption value at a wavelength of 280nm can be used to determine protein content. 2μL of VLP diluent was taken into the detection base, the blank control was obtained, 2μL of samples (5000rpm, 5min centrifuge, take supernatant) were pipetted, and added to the detection base, the corresponding scan spectra can be obtained. Record the absorbance of the sample at wavelength 280nm.
TEM
The TEM detection was performed on all samples (Sample 1 to Sample 5), and the differences of samples at each dissociation times were compared. TEM is the electron beam emitted by the tungsten filament electron gun, which passes through the condenser along the optical axis of the mirror body in the vacuum channel, and converges into a thin, bright and uniform light spot through the condenser, which irradiates on the sample in the sample room. After passing through the sample, the electron beam carries the internal structure information of the sample, and through the convergent focusing and comprehensive amplification of the objective lens, the enlarged electron image is finally projected on the fluorescent screen in the observation room. A fluorescent screen converts an electronic image into a visible image. 30μL of the sample was pipetted to the carbon support film of copper mesh for 2min, and filter paper was used to absorb excess solution from the edge part. Dye for 90s with acetic acid dioxyuranium dye solution, pointed filter paper was then used to absorb the excess dye solution and dry. The copper mesh was placed on the operating rod configured with the electron microscope, and the voltage was set to 120kV for shooting.
IVRP
The IVRP test was carried out for all samples (Sample 1 to Sample 5), and the differences of samples at each dissociation time were compared. The detection was performed by double-antibody sandwich Enzymed-Linked Immunosorbent Assay (ELISA). The recombinant human papillomavirus neutralizing antibody was combined with solid phase carrier to form solid phase antibody, and the unbound antibody and impurities were removed by washing. The solid phase antigen-antibody complex was formed by adding samples and control products and binding with solid phase antibody; Then, enzyme-labeled recombinant human papilloma virus neutralizing antibody is added, the antigen on the solid phase immune complex binds to the enzyme-labeled antibody, and the substrate is added, and the substrate catalyzed by the enzyme on the solid phase becomes a colored product. Through colorimetry, the absorbance of the sample with different dilution at 450nm is different, and the EC50 value of the control product and the sample is calculated. The relative efficacy ratio of recombinant human papillomavirus vaccine solution in vitro was investigated.
Results and Discussion
Results of Desorption Experiment
The morphology of VLPs in 14-valent mixed antigen containing aluminum phosphate adjuvant (Sample 4) by different desorption solution was observed under TEM. The results are shown in Figure 2. After dissolving in the citric acid solution for 48h, the aluminum phosphate adjuvant was completely dissolved, the VLP was released. The particle integrity was good, with almost identical particle size (about 50nm, consistent with the theoretical particle size). After dissolving in the EDTA solution for 48h, the aluminum phosphate adjuvant was also completely dissolved, but the VLP particles were obviously disassembled or destroyed. Experimental results have shown that the EDTA desorption mechanism can disrupt the VLP structure, while the citric acid system used in this study maintained the integrity of the VLP structure (Figure 2).
Results of Desorption Kinetics Experiment
Result of Appearance
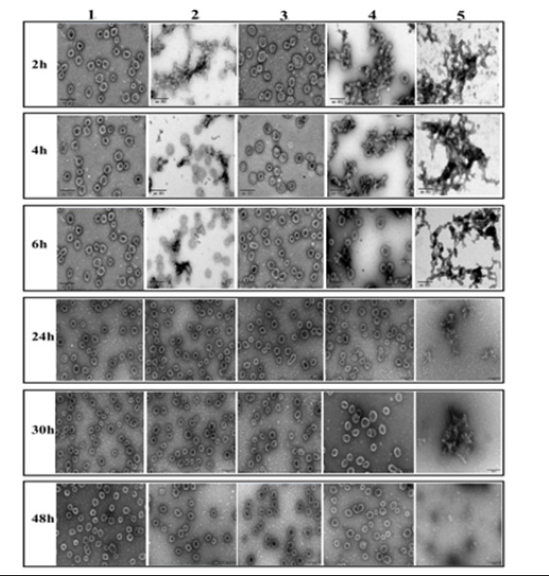
During the initial period (2h~6h), the appearance of samples containing adjuvant was turbidity and emulsion. After 24h of dissociation, the appearance of the sample containing adjuvant was slightly cloudy on sample 2, sample 4, and sample 5 and gradually clarified. After 48h of dissociation, the appearance of samples with adjuvant was all clarified, which was almost consistent with the appearance of samples without adjuvant (Figure 3).
Result of Turbidity
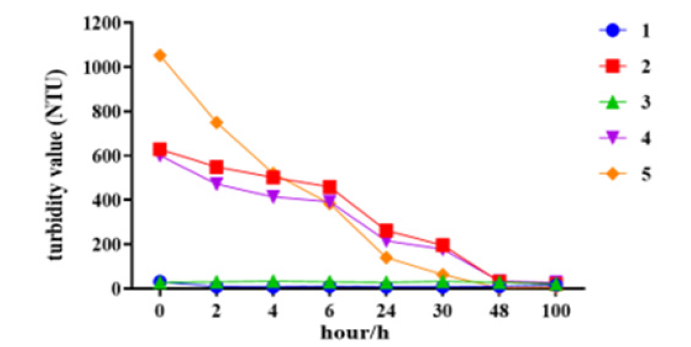
The turbidity of samples at beginning time, the turbidity of samples containing aluminum phosphate adjuvant was high (Figure 4), and the turbidity values of all samples containing adjuvant showed a downward trend with the extension of desorption time. After 48h of desorption, samples with aluminum phosphate adjuvant (Sample 2, Sample 4, Sample 5) were clarified. For samples without adjuvant (Sample 1, Sample 3), the turbidity value of T0 point is low, and it does not change much in the desorption process. For aluminum phosphate adjuvants themselves are emulsions. The desorption process can reduce the turbidity and clarify the appearance of citric acid, which proves that the desorption mechanism of citric acid system is carried out by dissolving aluminum adjuvant. Sample 2, Sample 4 also showed low turbidity and clear appearance after desorption, which proved that the desorption mechanism of citric acid system is that aluminum adjuvant is dissolved and protein and gradually released (Figure 4).
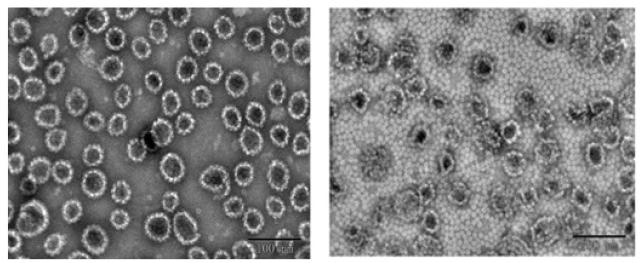
Figure 2: TEM images of Sample 4 dissolved in EDTA and citric acid respectively for 48 hours. The left image shows the citric acid solution dissolved Sample 4 for 48h; The right image shows the EDTA solution dissolved Sample 4 for 48h.
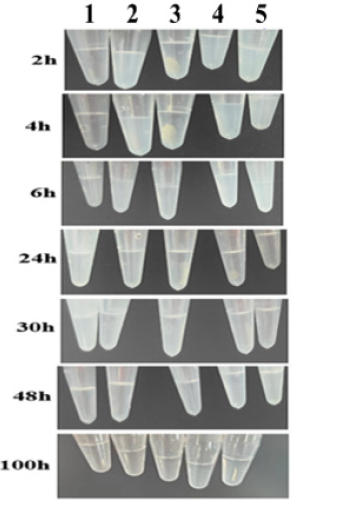
Figure 3: Appearance of desorption samples. samples from left to right are: sample 1(subtype 33), sample 2 (subtype 33+aluminum phosphate adjuvant), sample 3(14-valent mixed antigen), sample 4(14-valent mixed antigen+ aluminum phosphate adjuvant), sample 5(aluminum phosphate adjuvant).
Result of UV
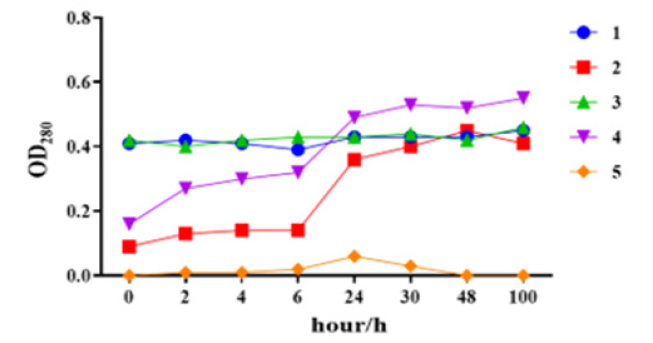
The absorbance value of samples without aluminum phosphate (Sample 1, Sample 3) at UV 280nm was basically unchanged before and after desorption. Before desorption (T0 point), the absorbance value of samples with aluminum phosphate (Sample 2, Sample 4, Sample 5) at UV 280nm wavelength was low, and the value gradually increased with the desorption time, and became stable at 48h, which was basically consistent with the absorbance value of Sample 1 and Sample 3. It was confirmed that the deep embedding of VLP antigen in the adsorptive aluminum phosphate adjuvant particles in Figure 1 affected UV detection until the dissolved aluminum phosphate state, the VLP antigen was completely released and accurately detected (Figure 5).
Result of Transmission Electron Microscopy
For the TEM detection of all samples (Sample 1 to Sample 5) after dissociation, the results are shown in Figure 6. The samples without aluminum phosphate (Sample 1, Sample 3), clear VLP particles with uniform particle size can be seen under TEM, and the particle morphology does not change with the extension of desorb time. For aluminum phosphate adjuvant (Sample 5), the aluminum phosphate adjuvant formed a network, and the longer the desorption time, the smaller the network. For VLPs samples with aluminum phosphate adjuvant (Sample 2, Sample 4), the adjuvant can be adsorbed, because there is accumulation on the background
(aluminum adjuvant), the staining solution is difficult to stay on the copper network, and the protein clarity is not high. After 30~48 hours of desorption, VLP particles are clear and uniform, which is due to the dissolution buffer dissolving the aluminum phosphate adjuvant and releasing the protein. Therefore, 48 hours was opted as the desorption time to dissociate the protein (Figure 6). In summary, after 48 hours of desorption in the citric acid solution, the aluminum phosphate adjuvant can be completely dissolved, and the morphology, structure, and size of the VLPs remain intact afterwards. To further validate this conclusion, the Sample 2 and Sample 4 were selected for IVRP detection.
Result of IVRP
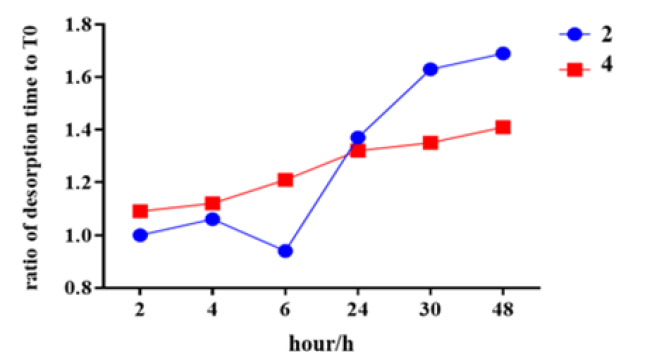
IVRP analysis was performed on Sample 2 and Sample 4, and the results were shown in Figure 7. The results showed that, for samples containing adjuvant, the IVRP ratio of desorption time to T0 time was gradually increased with the increased desorption time. After 48 hours of dissociation, the ratio of Sample 2 to T0 was 1.69, and the ratio of Sample 4 to T0 was 1.41, respectively. It is proved that the desorption method can completely remove VLP virus-like particles from the precipitation of aluminum phosphate, and the binding activity of VLP protein can be restored, that is, the protein is not destroyed (Figure 7).
Conclusion
For aluminum adjuvant adsorbent anti-prototype vaccines, vaccine quality control testing methods are often challenged due to the presence of aluminum adjuvant. Therefore, it is necessary to provide an adjuvant system with good adsorption effect, and to release the antigen absorbed on the adjuvant in a certain way for detection and analysis. There are many studies on the adsorption of subunit protein antigens by aluminum adjuvants, but there are few reports on the kinetic characterization of the desorption of viral particles by aluminum adjuvants. In this study, the state changes of different samples after desorption were investigated by appearance, turbidity, UV and TEM. The results showed that the sample containing adjuvant had a slightly cloudy appearance and became transparent after 48 hours of dissociation, the appearance of the samples containing adjuvants was clarified. Turbidity indicates that the sample containing adjuvant has a low turbidity during the desorption process, which proves that the desorption mechanism of citric acid system is carried out by dissolving aluminum adjuvant. UV indicates that the absorbance value of the aluminum-phosphate adjuvant containing the binding protein in the sample was low at 280nm wavelength before desorption (T0) but increased with the desorption time and tended to be stable at 48h, which was basically consistent with the absorbance value of the sample without aluminum-phosphate adjuvant. TEM indicates that under electron microscope, it was more intuitive to observe that the VLP protein was gradually clear during the desorption process, and the particle size and particle integrity remained unchanged. IVRP indicates the ratio of IVRP to T0 of the sample containing adjuvant gradually increased with the increased desorption time, proving that the potency of the protein recovered.
In this study, a variety of characterization methods were used to carry out kinetic studies on monovalent and multivalent HPV vaccines containing aluminum phosphate adjuvants. The results fully proved that citric acid desorption method can release VLPs by completely dissolving the aluminum adjuvant, without compromising the integrity of VLP antigens, which is consistent with the mechanism that the chelation of citric acid with aluminum adjuvant cannot damage the structural integrity of VLP.
Acknowledgements
The authors are also grateful to Dr. He (from Sinocelltech Ltd.,) for the writing and editorial support.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The intellectual property rights belong to Sinocelltech Ltd., Beijing., China.
References
- JM Walboomers, MV Jacobs, MM Manos, FX Bosch, JA Kummer, et al. (1999) Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 189(1): 12-9.
- Li Z, Song S, He M, et al. (2018) Nat Commun 9(1): 1.
- Lin K, Doolan K, Hung C F, et al. (2010) J Formosan Med Assoc 109(1): 4.
- Faulstich F (2014) Generation and evaluation of chimeric particles consisting of HPV16 L1 and p16INK4a for second generation HPV vaccines.
- Al Shakhshir RH, Regnier FE, White JL, Hem SL (1995) Contribution of electrostatic and hydrophobic interactions to the adsorption of proteins by aluminium-containing adjuvants. Vaccine 13(1): 41-44.
- Iyer S, Robinett R S R, HogenEsch H (2004) Mechanism of adsorption of hepatitis B surface antigen by aluminum hydroxide adjuvant. Vaccine 22(11-12): 1475-1479.
- HogenEsch H, O’Hagan DT, Fox CB (2018) Optimizing the utilization of aluminum adjuvants in vaccines: you might just get what you want. npj Vaccines 3(1): 51.
- Rinella JV, White JL, Hem SL (1998) Effect of pH on the elution of model antigens from aluminum-containing adjuvants. J Colloid Interface Sci 205(1): 161-165.
- Hem SL, Hogenesch H (2006) Aluminum‐Containing Adjuvants: Properties, Formulation, and Use.
- Seeber SJ, White JL, Hem SL (1991) Predicting the adsorption of proteins by aluminium-containing adjuvants. Vaccine 9(3): 201-203.
- Vanessa Jully, Frédéric Mathot, Nicolas Moniotte, Véronique Préat, Dominique Lemoine, et al. (2016) Mechanisms of antigen adsorption onto an aluminum-hydroxide adjuvant evaluated by high-throughput screening. J Pharm Sci 105(6): 1829-1836.
- Müller B (2004) Citric acid as corrosion inhibitor for aluminium pigment. Corrosion science 46(1): 159-167.
- Yang L, Mei X, Xie S (2023) The role of EDTA-2K in the chemical mechanical polishing of aluminum. ECS Journal of Solid-State Science and Technology 12(2): 024002.
- William J Smith, Rachel Thompson, Patricia M Egan, Yuhua Zhang, Lani Indrawati, et al. (2023) Impact of aluminum adjuvants on the stability of pneumococcal polysaccharide-protein conjugate vaccines. Vaccine 41(35): 5113-5125.
- Arnebrant T, Wahlgren MC (1995) ‘‘Proteins at Interfaces II’’. American Chemical Society, Washington pp: 239-254.
- Rinella JV, Workman RF, Hermodson MA, White JL, Hem SL (1998) Elutability of proteins from aluminum-containing vaccine adjuvants by treatment with surfactants. J Colloid Interface Sci 197(1): 48-56.
- Yuhua Li, Jingliang Li, Ying Wang (2021) Method for desorption of antigen and detection of antigen content in aluminum-salt adsorbent vaccines. Jilin Province.
- Paintsil J, Müller M, Picken M, Gissmann L, Zhou J (1998) Calcium is required in reassembly of bovine papillomavirus in vitro. J Gen Virol 79(Pt 5): 1133-1141.




 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.