Case Report 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Endoscopic Treatment of Hydrocephalus Secondary to Pediatric Thalamus Lesion and Arachnoid Cyst: A Case Report and Technical Note
*Corresponding author: Guoqiang Xie, Department of Neurosurgery, Nuclear Industry 215 Hospital of Shaanxi Province, China 712000, Xianyang Weiyang west road 35, Shaanxi Province, China.
Received: October 29, 2024; Published: November 04, 2024
DOI: 10.34297/AJBSR.2024.24.003225
Abstract
Background: Thalamic tumors are relatively rare in children and frequently cause obstructive hydrocephalus. The main symptoms of thalamic tumors include high intracranial pressure, visual problems, motor deficits, seizures, central pain and behavioral problems. Because of the vital surrounding structures and the high risk of surgery, the strategy of treating pediatric thalamic tumors remains controversial until now.
Case Presentations: In this report, we describe a child presented with symptoms of High Intracranial Pressure (ICP). Pre-operative Magnetic Resonance Imaging (MRI) revealed obstructive hydrocephalus secondary to pediatric thalamic tumor and sub-tentorial arachnoid cyst. Based on the MRI data, the Virtual Endoscopy (VE) was reconstructed with 3D Slicer software. The dilated Moron holes, the narrow space between the lesion and fornix and the opening of the arachnoid cyst were confirmed with VE. The surgical approach was chosen as Endoscopic Third Ventriculostomy (ETV), Endoscopic Tumor Biopsy (ETB) and Ommaya reservoir insertion subsequently. The child improved remarkably after the operation and had a normal neurological status and psychomotor development during follow-up of 16 months. The histopathological examination was low-grade glioma. Post-operative MRI revealed contraction of the enlarged ventricles, shrinkage of the arachnoid cyst, and diminishment of periventricular edema.
Conclusion: Endoscopic treatment may play a significant role in the management of patients with obstructive hydrocephalus caused by pediatric thalamic tumors and arachnoid cysts.
Keywords: Thalamic tumors, Hydrocephalus, Endoscopy, Virtue endoscopy, Biopsy
Abbreviations: CSF: Cerebral Spinal Fluid; ETV: Endoscopic Third Ventriculostomy; ETB: Endoscopic Tumor Biopsy; ICP: Intracranial Pressure; MRI: Magnetic Resonance Imaging; 3D FIESTA: Three-Dimensional Fast Imaging Steady Acquisition; VE: Virtual Endoscopy
Introduction
Thalamic tumors account for approximately 1~5% of brain tumor pathologies and frequently lead to obstructive hydrocephalus in pediatric series [1-3]. The main clinical presentations of thalamic tumors include High Intracranial Pressure (ICP), visual problems, motor deficits, seizures, sensory dysfunctions, central pain and behavioral problems [4]. The types of pathology included gliomas, neuronal tumors and embryonal tumors. Moreover, astrocytes arising from gliomas are the most common tumors and high-grade tumors predominate [5,6]. According to their anatomical location, the tumors were divided into three groups: unilateral thalamic, bilateral thalamic, and thalamopedunclular lesions. Due to the deep location, vital surrounding structures and high risk of surgery, the management of pediatric thalamic tumors remains controversial [3]. We report our experience with endoscopic treatment of obstructive hydrocephalus caused by thalamic lesions.
Case Presentation
A 22-month-old boy with a history of headache and vomiting who was presented with signs and symptoms of high intracranial pressure for two weeks was referred to our hospital. The Magnetic Resonance Imaging (MRI) revealed an isointense lesion on a T1-weighted image without enhancement following contrast agent administration in the left thalamus. There was a big cyst located above the cerebellum and inferior to the tentorial region. Because of the obstruction of the Moron holes and the distortion of the aqueduct, the ventricles were enlarged, and the periventricular edema was obvious bilaterally. The sagittal view of Three-Dimensional Fast Imaging Steady Acquisition (3D FIESTA) revealed the sank of the third ventricular floor because of the obstructive hydrocephalus and a big cyst located inferior to the tentorial (Figure 1). Virtual Endoscopy (VE) based on 3D FIESTA data made from 3D Slicer software revealed the dilated Moron hole bilaterally, the narrow space between the lesion and the fornix and the opening of the cyst superior to the cerebellum (Figure 2).
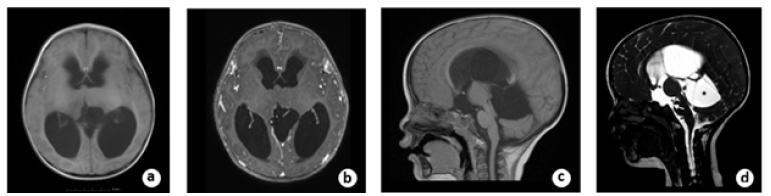
Figure 1a: pre-operative axial view of T1-wighted MRI showed the tumor located in the left thalamic region and the ventricles were enlarged bilaterally. B. The tumor was not enhanced after contrast material administration. C. The aqueduct was distorted by the compression of the cyst located superior to the cerebellum. D. pre-operative sagittal view of 3D FIESTA showed the sink of the third ventricle floor (white arrow) and the big cyst (black star) located superior to the cerebellum.
Surgery
Because of the limited space on the right side, the entry point was chosen on the left frontal incision. After general endotracheal tube anesthesia, the patient was secured in supine position with head on a horse-shaped headrest. Endoscopic Third Ventriculostomy (ETV), Endoscopic Tumor Biopsy (ETB) and Ommaya reservoir insertion were performed successively through the left frontal burr hole (LOTTA Endoscope; Karl Storz, Tuttlingen, Germany) (Figure 3). A catheter was inserted at the opening of the cyst and an Ommaya reservoir was anchored subcutaneously beside the incision. The post-operative course was uneventful. The result of the histopathological examination was low-grade glioma (Figure 4).
Follow-Up
During the 16 months follow-up, the boy improved remarkably with a recovery of normal neurological status and psychomotor development. Post-operative MRI revealed contraction of the enlarged ventricles, shrinkage of the arachnoid cyst, and diminishment of periventricular edema. On the sagittal view of the 3D FIESTA, the floor of the third ventricle was elevated and vacant locally. The arachnoid cyst superior to the cerebellum shrank, and the compression of the aqueduct was relieved (Figure 5). The child is scheduled for annual follow-up currently.
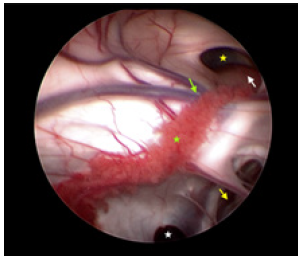
Figure 3: Under the vision of neuroendoscopy, the dilated Moron hole (yellow star), the tumor (white arrow), the penetration of the Septum pellucidum (yellow arrow), the thalmostriate vein (green arrow), choroid plexus (yellow star) and the opening (white star) of the cyst of superior to cerebellums were confirmed.
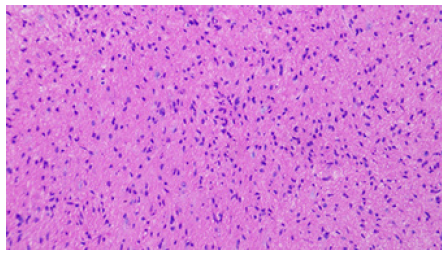
Figure 4: The histological diagnosis was low grade glioma (fibrillary astrocytoma) and the immunohistochemistry results revealed Vim (+), GFAP (+), S-100 (+), Olig-2 (+), CD34 (+), H3K27Me3(+), Braf (-), EMA (-),Syn (+), MGMT (+), Ki-67 (+).
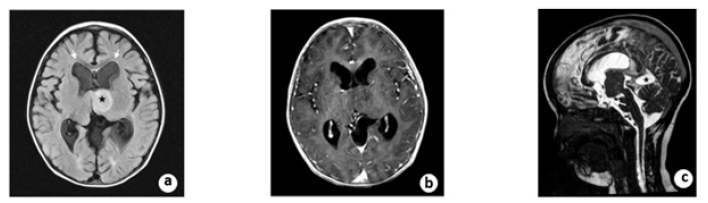
Figure 5: A. Post-operative MRI scanning showed an intensive FLAIR signal (black star) and the diminishment of paraventricular edema (white arrow). B. The tumor was still not enhanced after contrast administration at follow-up. C. On the sagittal view of 3D FIESTA, the floor of the third ventricle was elevated and vacant locally. The arachnoid cyst (black star) superior to the cerebellum shrinks and the compression of aqueduct relieved.
Discussion
Thalamic tumors are relatively rare in pediatric patients and frequently lead to obstructive hydrocephalus [7]. The most common presentation was symptoms of high ICP usually caused by obstructive hydrocephalus. Because of the deep location and the surrounding vital structures of thalamic tumors, surgical procedures for these tumors remain challenging with high mortality and morbidity. Several reports have described total or subtotal (>90%) resection of thalamic gliomas, but the surgical morbidity was found in 12-80% of these patients [3,8,9] Moreover, compared with the extent of surgical resection, there was no significant difference in overall survival between patients who underwent gross total resection and patients with residual tumors after subtotal resection, biopsy, or no resection [10,11]. Therefore, the necessity, feasibility and safety of the surgical procedure remain controversial. The treatment of pediatric thalamic tumors will gradually evolve along with the development of medical imaging, the usage of pre-operative plan, the advancement of surgical techniques and the utility of intraoperative monitoring.
The primary principle of thalamic tumors management should be Cerebral Spinal Fluid (CSF) diversion for ICP reduction and histological diagnosis for advanced therapy. With the advancement of endoscopic equipment and techniques, neuro-endoscopy offers opportunity for histological diagnosis and CSF diversion [12-15].
In the present case, the tumor was found located in the left thalamus without definite boundary. The obstructive hydrocephalus was confirmed by bilateral tumor blockage of the Moron holes and the distorted aqueduct caused by the presence of a cyst superior to the cerebellum. According to the VE reconstruction, the limited pathway for ETV on the right side and the opening of the cyst were defined. Therefore, the entry point was chosen on the left frontal lobe, and ETV and ETB were performed successively. Because the biopsy had little effect on tumor debulking and the Moron holes maybe occluded with tumor progression, Ommaya reservoir insertion was performed during the operation and intermittent reservoir puncture was used to deal with acute hydrocephalus in the future. During the follow-up of 16 months, the child had a satisfied outcome with normal psychological and motor function.
Conclusion
These results indicate that endoscopic treatment, including ETV and ETB, is a feasible and safe approach for treating pediatric thalamic tumors accompanied with hydrocephalus and deserves further investigations with more research.
Acknowledgement
Not applicable.
Compliance with Ethical Standards
Ethics Approval
The study protocol was approved by our institutional ethic committee on Mar 20,2023.
Consent to Participate
The family of the patient had provided written consent to participate.
Consent for Publication
The family of the patient had provided written consent for publication.
Funding
No funding was received for this research.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Authors' contributions
Xie performed the surgery and was the major contributor in writing the manuscript. All authors read and approved the final manuscript.
References
- Sharaf AF, ES Hamouda, JG Teo (2016) Bilateral Thalamic and Right Fronto-temporo-parietal Gliomas in a 4 Years Old Child Diagnosed by Magnetic Resonance Imaging. J Radiol Case Rep 10(1): 1-13.
- Rajput DK, A Mehrotra, AK Srivastav, Raj Kumar, Ashok K Mahapatra (2010) Bilateral thalamic glioma in a 6-year-old child. J Pediatr Neurosci 5(1): 45-48.
- Puget S, DW Crimmins, MR Garnett, Jacques Grill, Ricardo Oliveira, et al., (2007) Thalamic tumors in children: a reappraisal. J Neurosurg 106(5 Suppl): 354-362.
- Bernstein M, HJ Hoffman, WC Halliday, EB Hendrick, RP Humphreys (1984) Thalamic tumors in children. Long-term follow-up and treatment guidelines. J Neurosurg 61(4): 649-656.
- Wong, TT, HH Chen, ML Liang, Kevin Li Chun Hsieh, Yi Shan Yang, et al., (2016) Clinical considerations and surgical approaches for low-grade gliomas in deep hemispheric locations: thalamic lesions. Childs Nerv Syst 32(10): 1895-1906.
- Renedo D, F Ferraro, AR Johnson, Romina Argañaraz, Sebastian Giovannini, et al., (2021) Thalamic tumors in children: case series from our institution and literature review. Childs Nerv Syst 37(2): 457-463.
- Carrabba G, G Bertani, F Cogiamanian, Gianluca Ardolino, Barbara Zarino, et al., (2016) Role of Intraoperative Neurophysiologic Monitoring in the Resection of Thalamic Astrocytomas. World Neurosurg 94: 50-56.
- Steiger HJ, C Götz, R Schmid Elsaesser, W Stummer (2000) Thalamic astrocytomas: surgical anatomy and results of a pilot series using maximum microsurgical removal. Acta Neurochir (Wien) 142(12): 1327-1336.
- Bandopadhayay P, G Bergthold, WB London, Liliana C Goumnerova, Andres Morales La Madrid, et al., (2014) Long-term outcome of 4,040 children diagnosed with pediatric low-grade gliomas: an analysis of the Surveillance Epidemiology and End Results (SEER) database. Pediatr Blood Cancer 61(7): 1173-1179.
- Niu X, T Wang, Y Yang, Youjun Gan, Jiaoming Li, et al., (2018) Prognostic Factors for the Survival Outcome of Bilateral Thalamic Glioma: An Integrated Survival Analysis. World Neurosurg 110: e222-e230.
- Jenkinson MD, C Hayhurst, M Al Jumaily, Jothy Kandasamy, Simon Clark, et al., (2009) The role of endoscopic third ventriculostomy in adult patients with hydrocephalus. J Neurosurg 110(5): 861-866.
- Yurtseven T, Y Erşahin, E Demirtaş, S Mutluer (2003) Neuroendoscopic biopsy for intraventricular tumors. Minim Invasive Neurosurg 46(5): 293-299.
- Grunert P, P Charalampaki, N Hopf, Filippi (2003) The role of third ventriculostomy in the management of obstructive hydrocephalus. Minim Invasive Neurosurg 46(1): 16-21.
- Jones RF, WA Stening, M Brydon (1990) Endoscopic third ventriculostomy. Neurosurgery 26(1): 86-91.
- Vogel TW, B Bahuleyan, S Robinson, Alan R Cohen (2013) The role of endoscopic third ventriculostomy in the treatment of hydrocephalus. J Neurosurg Pediatr 12(1): 54-61.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.