Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Final Methodical Research in the Surface Hydrophobicity (SHP) of Native Casein Micelles in Milk
*Corresponding author: Bärbel Lieske, 10407 Berlin Kniprodestr. 120, Germany.
Received: October 24, 2024; Published: October 28, 2024
DOI: 10.34297/AJBSR.2024.24.003219
Abstract
The development of this method goes back to 1990/91 and was refined further until 2004. The initial interest was to know the interference of the non-ionic detergent Tween 80 in the Bio-Rad Protein Assay with a variety of proteins The detergent binding seemed to be a measure of hydrophobic areas of integral proteins and thus constituting the basis for an assay. First, the methodical research was turned to analyse only isolated proteins [1]. Further development of the SHP-method was necessary to meet the demands of natural casein micelles. At the beginning double destilled water was considered to be just right for that purpose, however, its efficiency was not lasting. It seemed that the purity of water remained one crucial point for the colloid-chemical analysis. Ongoing research into the causes was promissing and integrated in the methodical description of the further developed method as described below.
Reagent And Sample Preparation
Conditioning of Water: 500 ml water (deionisized) plus Phenylmethylsulfonyl- chlorid (PMS-Cl), (Sigma, Deisenhofen, Germany) are heated at 115°C for 70 min. Then stored closed in a refrigerator overnight. The final pH of the water should be 3,7-3,8. In our case 3,6 mg PMS-Cl are required being equal to that pH.
Detergent Reagent: 0,25% Tween 80 (Sigma, Deisenhofen, Germany) is prepared with the conditioned water.
Dye Reagent: The dye reagent is purchased as a five-fold concentrate, which must be diluted and filtered through a large-pored filter prior to use.
Sample Preparation: Proteins are diluted to about 0,05-0,10% using buffer or deinonosized water. As arule, the color intensity of 50 uL diluted sample developed with 2,5 mL dye reagent should not exceed A 595nm, 1cm = 0,500 vs. pure water or buffer.
Additional Items Required
1. Spectrophotometer: allowing measurements at 595 nm
2. Water- bath: allowing the preparation of milk samples
3. Shaker : supporting the Tween 80 reaction
4. Polystyren cuvettes: 10 mm path length as semi-micro cuvettes
5. Test tubes: polystyrene test tubes /13*64 mm) each fitted with a mixing spatula
6. (Boeringer, Manheim, Germany)
7. Dispenser and microliterpipets for precise dispensing the dye reagent resp. for
8. adding the sample.
9. Rack: test tube rack to store the test tubes containing samples and blanks.
Assay Procedure
The hydrophobicity of proteins and casein micelles is calculated from two different protein assays, which are developed at the same time. Triplicates for each single measurement are necessary.
1) 1a) 50µL protein (A sample) is placed on the bottom of a dry and clean test tube whereas 50µL deionosized water or buffer are used as blank (A blank). 1b) 50 µL 0,25 % Tween 80 is placed on the bottom of a dry and clean test tube and in addition to 50µL protein is placed onto the drop of Tween 80 (B sample) whereas deionosized water or buffer and Tween 80 are prepared as blank (B blank).
2) Only the detergent-containing tubes (1b) are shaken for 10 min. (avoid foaming) to complete detergent binding at a temperature between 18-22°C.
3) Ad 2,5 mL diluted dye reagent to each test tube, insert mixing spatula by use of a pipette to avoid skin contact. Move spatula several time up and down without foaming.
4) Allow standing for 12 min. to develop the colour. The colour intensity of each tube is measured at 595 nm vs. deionosized water or buffer. Avoid any warming of cuvettes by prolonged standing inside the cuvette - department of the photometer.
Calculation
Protein Hydrophobicity (PH) is defined as following (Nakai and LiChan)
PH= (nonpolar residues) / (nonpolar residues) - (polar residues)
Using this definition on detergent binding according the proposed method, PH is calculated as
SHP (%) = (A sample- A blank) - (B sample- B blank) * 100: ( A sample - A Blank)
For analysing the casein in its native status this method was modified to protect the micelle structure in the B samples from an early dissociation. Among some other things, the purity of water has always been one crucial point for the colloid-chemical analysis of natural casein micelles.
The modified method has been proved over 5 years taking 3 different herds of cows into account. Results obtained were just right to evidence both, the hygienic status of milk and inherent effects on milk processing.
Results and Discussion
The colloid-chemical status of casein micelles in 6 milk samples is presented in Figure 1-6 in the following order: Figure 1- skimmed milk of high quality; Figure 2 herd milk on mastitis; Figure 3 antibiotics in skimmed milk and Figure 4a and Figure 4b herbicide in skimmed milk from a joung herd a) and after 6 weeks b) Two colloid-chemical critera are compared with the overall surface hydrophobicity (SHP) and the corresponding BIO-RAD protein assay; both criteria are interrelated with the natural pH-depended structural changes of casein micelles in raw milk [5]. Here it is applied to assess the effects of oxidative stress, of toxic substances like natural penicillin (vaccination) and herbicide in green-stuff on the micelle structure and inherent complexation.
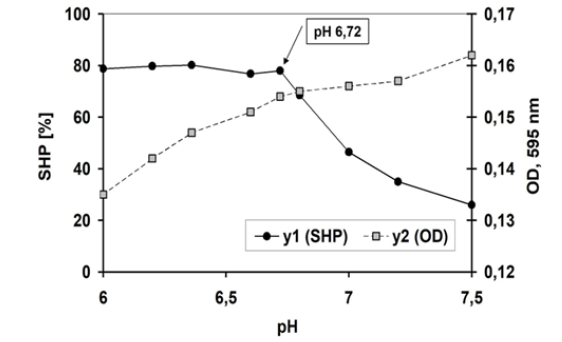
Figure 1: Effect pH on micellar surface hydrophobicity and dye binding characteristics of a first-class skimmed raw milk (SHP, ●ꟷ●) and dye binding (□---□) were determined from pH 6 to 7,5; orig. pH 6,72. N =3.
First, the profile of raw skim-milk (Figure 1) will be reviewed. It is a typical example for a first-class raw milk. At natural pH of milk, casein micelles are very voluminous and exhibit both a great surface area (O.D.) and a high hydrophobic potential (SHP) of the protein, too. The micellar structure is built on non-covalent binding forces. A very hydrophilc surface layer, developed by the C-terminal glycosylated residues of k-casein, is protecting the hydrophobic layer core of micelles to get collapsed in contact with other micelles. On lowering the pH, the colloidal calcium phosphate becomes increasingly dissolved. This is paralleled to a gradual decrease in micellar surface area and thus to a denser protein structure. In the profile of milk (Figure 1) two minima of dye binding (O.D.) are detected, one at natural pH and the other at pH 6,0 and both are pointing to some denser micelle structure [4]. At pH above 6,82 the SHP decreases constantly due to the beginning of micellar disintegration with a stronger binding of calcium to individual caseins and it is paralleled with the dissociation of k-Cn from the micellar surface at pH 6,9 [5] and with advanced disintegration (pH 6,9) the dye binding increases showing intersection with the graph of SHP. This intersection is a common feature of the natural colloidal status of casein micelles in raw milk (Figure 1).
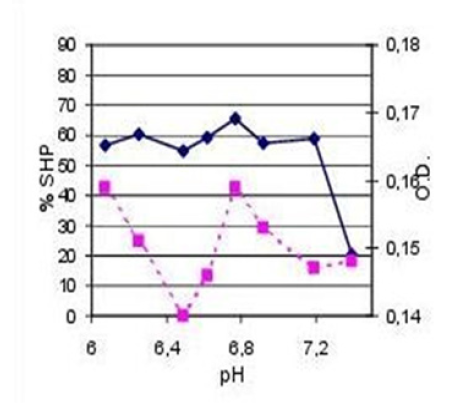
Figure 2: Effect pH on micellar surface hydrophobicity and dye binding characteristics of skimmed raw milk representing elevated oxidative stress resulting from increased incidence of herd mastitis.(SHP; ●ꟷ●) and dye-binding (□---□) were determined from pH 6 to 7,5; orig. pH 6,72. N =3.
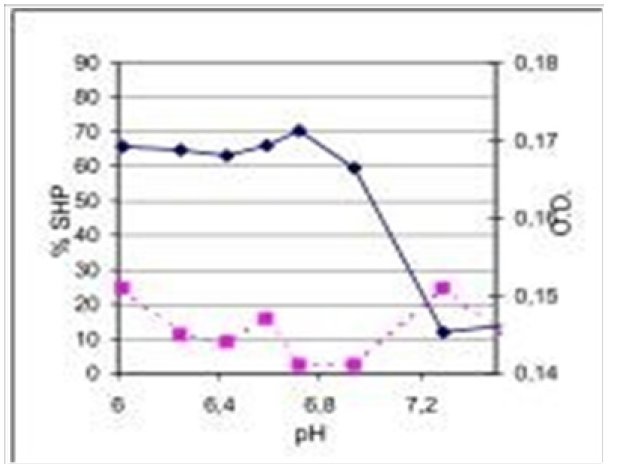
Figure 3: Effect pH on micellar surface hydrophobicity and dye binding characteristics of skimmed raw milk representing elevated oxidative stress resulting from increased incidence of herd mastitis.(SHP; ●ꟷ●) and dye-binding (□---□) were determined from pH 6 to 7,5; orig. pH 6,72. N =3.
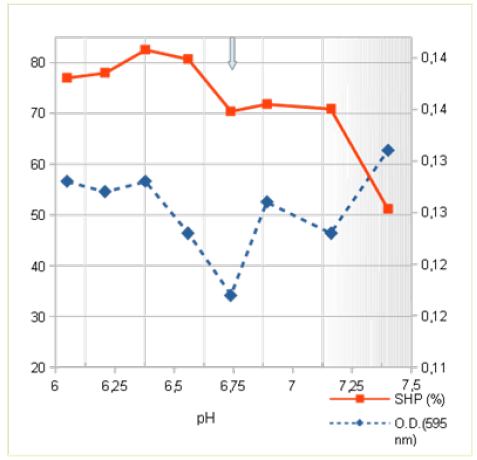
Figure 4a: Effect of pH on micellar surface hydrophobicity and dye binding characteristics of skimmed raw herd milk containing protein-bound herbicide. Micellar surface hydrophobicity (SHP: ■ꟷ■) and dye-binding (□-□) were determined from pH 7,5; orig pH 6,74. N = 3)At natural pH of the skimmed milk the casein micelles are on a point of lowest voluminosity with a middling OFH of 71 %. Next to the natural pH the course of OFH is indicating a maximum at pH 6,4 but a slight denser micellar structure (O.D.) Between pH 6,74 and 7,12 the micellar SHP remained on equal level between 72-73 %, it is paralleled with a gradual increase of O.D. in two steps. At pH 7,3 both curves are showing still interception.
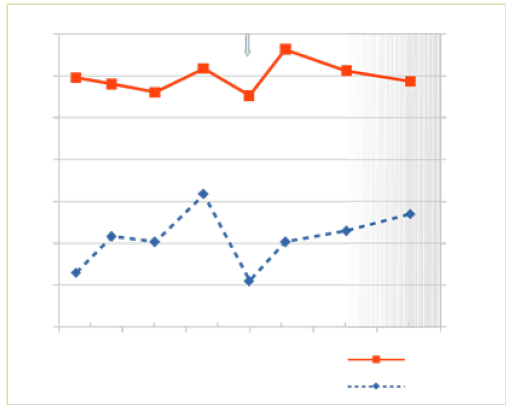
Figure 4b: Effect of pH on micellar surface hydrophobicity and dye binding characteristics of skimmed raw herdmilk containing protein-bound herbicide, collected 6 weeks later. Micellar surface hydrophobicity (SHP; ꟷ) and dye- binding were determined from pH 7,5; orig pH 6,75. N =2).
Mastitis
In comparison to that it is sufficiently known about slow-coagulating herd milk with disturbed gel formation and syneresis related to. The scientific proof about the reasons of deficit gel formation in milk becomes feasible using the colloid-chemical assay presented in Figure 2. The herd milk was sampled at the time of increased udder infections. The natural pH was determined at pH 6,77. At this point a partial dissociation of k-casein from the micellar surface as well as the intersection of the two graphs -SHP and O.D.- occurred later at pH 7,4 pointing to an exceptionally high oxidative potential in this milk sample. In this case, the dissociation of k-casein happened in two stages and is to put down to the fact that the herd milk was collected in one tank, comprising both, first grade raw milk and milk taken from infected mammary glands. In practice, all casein micelles mixed in a tank milk influencing the oxidative potential. This oxidation is the reason of poor/ non renneting milk with insufficient gel formation (Figure 2).
In the following the profile of sample No. 2 will be viewed. The herd milk was sampled at the time of increased udder infections and has been confirmed by the values of Somatic Cell Counts (SCC) and Colony Forming Units (CFU). The natural pH was determined at pH 6,77. After that, the courses of both, OFH and O.D., decrease up to pH 6,9. Between pH 6,9 and 7,2 only the values of O.D are decreasing further; whereas the hydrophobic potential increases. The high surface hydrophobicity comes from an increased dissociation of k-casein released from the inside of micellen. The point of interseption in that graph at pH 7,2 pointing to an exceptionally high oxydative potential in that milk. This oxidation is the reason for poor/non renneting milk with insufficient gel matrix formation and instability on heating [2-5].
Usually, the course of micellar dissociation is associated with a significant increase in protein-dye binding due to the occurrence of a vast number of submicellar particles (see Figure 1); in Figure 2 , the value of O.D. at 7,4 is even below that determined at pH 6,0. From this it can be drawn that the dissociation of casein micelles results in larger particles than in high-quality milk.
The udder health problems on herd level (Figure 2) were regulated by a medical treatment with Antibiotics (AB). The milk samples used for the colloid-chemical analysis were collected some days after the legitimated waiting period. At that time the „PR“-Test reacted still positively on antibiotics. The results obtained with the colloid-chemical analysis are shown in Figure 3.
Mastitis and Antibiotics
(Figure 3) The profile of the raw milk in Figure 3 shows maximum hydrophobicity at natural pH (6,72) and thereafter decreases steadily coming up to a total dissociation of micelles at pH 7,4. The overall graph of SHP decreases steadily coming up to total dissociation at pH 7,4. The overall graph of O.D. is on low level indicating to a denser micelle structure. The peak of dye binding at pH 7,2 has been turned out to be a distinguishing-feature of protein-bound AB's in raw milk and appeared never in the profiles of high- grade milk samples.
Several species of pathogenic bacteria may cause inflammation of the udder if they have succeeded in entering it; such an inflammation is called mastitis. It causes a decrease in milk yield and a change in milk composition and a number of somatic cells in milk (especially polymorphonuclear leucocytes). A cell count of 5 x105 often is taken as criterion of mastitis, but it is known to be not precise owing to other factors affect the cell count too, notably the individual cow, the stage of lactation and the age of the cow with their own mastitis history.
The incidence of udder infections may be influenced by use of a milking machine in different way (4):
a) mechanical transfer of pathogenic microorganisms from animal to animal
b) back-flow of milk inside the milking system
c) back-flow of the milk between the cows
d) damage of the udder tissue particularly in the region of the teat head and the teat duct.
The inherent percentage of decreased udder quarters has been proposed to be smallest at a critical milk flow between 2 and 3 kg/ min and additional desinfection from time to time [4].
Clinical Udder Infections are changing the milk clearly requiring medical treatment. The known pathogens clearly requiring treatment. The known pathogens are Escherichia coli, Bacillus cereus, Klepsiella, Pseudomonas aerugginosa, clostridien, nocardien, Streptococcen (Serogruppen A, D, C, G, L), Pneumococcen, Staphylococcen, Corynea pyogenes, yeasts, mycoplasmen) [4].
Subclinical Mastitis might be developed from a clinical mastitis or remained on the subclinical status from the begin. The most widespread pathogens of subclinical mastitis are streptococcen and staphylococcen.
Judging the appearance it is often difficult to recognize a raw milk sample on subclinical mastitis although the chemical effects are gradually varying. All in the mammary gland were absolutely but in addition to, some of the constituents are coming unchanged from the blood.
On subclinical mastitis the total casein, phosphor, riboflavin and ascorbinic acid were reduced. It is paralleled with an increase of catalase and chlor but with decreased lactose.
Diagnostically usable for an early test of mastitis might be an increase of catalase and chlorine as well the alteration of lactose on mastitis. The technological significance from cows on mastitis is affected due to:
1. an increased content of chlor and natrium
2. reduction of the fat-free dry matter (cheese production)
3. an impaired chymosin coagulation of milk plus a reduced discharge of whey
4. decreased heat stability of milk protein (increased values of serum albumin and immunglobulin)
5. the risk of occurrence of a last bit antibioticum as one inhibitor after therapeutic treatment.
Herbicide
Last of all, another group of foreign toxins in raw milk are protein-bound herbicide will be viewed. For that, effects of herbicide in skimmed raw milk obtained from a young herd were chosen to demonstrate inherent effects on the micellar status. The milk samples were collected in an interval of six weeks. Results obtained are seen in the graphs of SHP and O.D. in Figure 4a and 4b.
The profile in Figure 4b points to never-ending oxidative stress due to the deposit of herbicide in the tissue of the mammary gland. Here all immune reactions of the mammary gland have no effect, and thus all micellar SH-groups were available to get involved in a non reversible complexation up to an obliteration of teats.
Both, the presence of protein-bound antibiotics in milk and of protein-bound herbicides, are serious problems in various aspects. Antibiotics are given for a limited time and the inherent immune response may recover thereafter. Whereas the presence and the depositing of herbicide is persisting and cows are strict vegetarians depending on green food daily.
Aflatoxins
Another group of foreign substances in milk (e.g., toxins of chemical and microbial origin) are remaining on the hydrophobic surface of casein micelles occurring protein-bounded as well and are recognizable by their typical signs in the colloidal profiles. Mycotoxins are very surface-active and were taken up by the micellar surface and thus evident at each pH of the photometric analysis. Mycotoxins are competing increasingly with the non-ionic detergent Tween 80 for the places on the casein micelle surface to dock with on gentle warming, e.g; already a few seconds of standing inside the cuvette- department of the photometer.
The aflatoxins have closely related structures and form a unique group of highly originated heterocyclic compounds. They are produced by Aspergillus flavus and Aspergillus parasiticus ,which are grown on foods (e.g. nuts cereals, oilseeds and beans) under favorable temperatures and humidy conditions, before or during harvest or inproper storage. AFM1, a metabolit of AFB1 in mammals may be found in the milk of animals ingesting feed contaminated with AFB1, Afs B1,B2 and G2 are classified as group1human carcinogens, whereas AFM1 is classified as a Group2B AF probable human carcinogen, (Food and Agriculture Organization of UN and World Health Organization(1995). Codex Comitee on Food Additives and Contaminants, 28. Session, Manila Philippines, March 18-22, 1996, p.1-2.
The biological activities of aflatoxins enable them to function as potent toxins, cacinogens, tertogens and/or mutagens: being harmful to both kidneys and liver, development of cancer and wreck, the immune system of humans and animals. In the colloid-chemical method to determine the micellar SHP of native casein micelles in milk Tween 80 may selectively block the hydrophobic area on the micellar surface allowing the differentation between hydrophobic and hydrophilic areas, reliably. All aflatoxins are surface-actively and thus are interfering with Tween 80 the assay procedure The question whether aflatoxins are present in milk or not being answered in the photometer by never-ending deviations during the reading.
Mastitis and Antibiotics
Antibiotics have been used in the dairy industry since the 1940`s, mainly for the prevention or treatment of mastitis in lactating cattle [6]. However one of the major problems for the dairy industry is associated with antibiotic use has been the inhibitory effect of residues on acid production by starter lactic acid bacteria. For example, penicillin residues at 0,1 and 0,05 units/mL have been found to cause a deterioration in cheese quality and delayed acid production during cheese manufacture, respectively. In order to overcome potential residue problems for antibiotic detection have been developed into the industry.
It is evident from newer studies that many antibiotics used in the dairy industry significantly inhibit starter bacteria at concentrations below the detectable limit of standard antibiotic screening assays. Sub-detectable levels of antibiotic residues in milk fo cheese making may be suffient to inhibit starter activity in the cheese vats resulting in unexplained non-phage inhibition.
A milk-based diäteticum developed for hospitalised premature babies caused the death of one baby due to the presence of antibiotics. In that dairy, each delivery of bulked tank milk has been proved on residues of antibiotics before use. Antibiotics are protein-bound toxins and thus with standard antibiotic screenings not accessible.
References
- Lieske B, Konrad G, Faber W (1994) A new approach to estimate surface hydrophobicity of proteins. Milchwissenschaft 49: 663-666.
- Lieske B (1998) Effects of aging of raw milk on some structural properties of natural casein micelles. Milchwissenschaft 53: 562-565.
- Lieske B (2012) Complexation Between Casein Micelles and Whey Proteins by Indirect UHT-Processing of Milk / Influence of Surface Hydrophobicity and Dye Binding Characteristics of Micelles in Relationship with Their Physico- Functionality. Milk Protein 6: 159-167.
- Lieske B, Valbuena R (2008) Variation in colloid-chemical status of casein micelles with influence on the chymosin coagulating properties of raw milk samples. Milchwissenschaft 63: 237-250.
- Kielwein G (1985) Bedeutung des maschinellen Milchentzuges für die Entstehung Euterkrankungen. Verl. Paul Parey-Berlin und Hamburg: 111-113.
- Hady PJ, Lloyd JW, Kaneene JB (1993) Antibacterial use in lactating cattle. J Am Med Ass 203: 210-216.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.