Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Impact of the Biochemical Nutrition Constituents as a Treatment Strategy for Patients with Renal Anemia: A Narrative Review
*Corresponding author: Samy H Khwaiter, Department of Medical Biochemistry, Faculty of Medicine, University of Al-Butana, Sudan.
Received: September 12, 2024; Published: September 20, 2024
DOI: 10.34297/AJBSR.2024.24.003169
Abstract
Renal Anemia is a common complication of chronic kidney disease (CKD), which gradually increased morbidity and mortality. CKD is a life-threatening disease, and most of cases are treated with Hemodialysis, while, most of them will be suffering of many complications as Renal Anemia. Renal anemia will be controlled by clinical nutritional management which is now a fundamental treatment strategy, therefore we aimed in the current study to discuss biochemical nutrient constituentss and amount of energy metabolism to decline morbidity, mortality, and complications appearance progression. Medical nutrition therapy is divided into many factors includes; proteinuos and non proteinuos molecules with blood minerals, vitamin D, and Erthroepotein hormone. However, in many studies it were reported the deficiency in one biochemical constituent or more than one. In many studies it was observed a strong relation with renal anemia appearance and nutrients deficiency in contrast to other anemic cases were treated for nutrients biochemical constituents’ deficiency the renal anemia progression, morbidity and mortality were declined.
Keywords: Biochemical, Constituents, Nutrition, Patients, Remal anemia, Treatment strategy
Introduction
Renal anemia is a common complication of chronic kidney disease (CKD), impacting long-term outcomes such as mortality and morbidity [1], while, is clinically defined as having low levels of hemoglobin concentration, which is the iron-rich protein in red blood cells (RBCs), it is responsible for the transport of O2. And the kidney disease: Improving Global Outcomes (KDIGO) anemia work group guidelines defined anemia in CKD as a hemoglobin concentration of <13.0 g/dL. In men and <12.0 g/dL. In women [2]. In addition, the disruptions in the iron homeostasis can lead to iron-deficiency anemia [2].The most of CKD cases may be suffering of renal anemia manly due to many factors but the most common factor is the deficiency of nutritional constituents leads to increase the morbidity and mortality [3]. So, the Statistics have revealed an increase in renal anemia diseases is responsible for an estimated 41 million people dying annually, constituting 74% of all deaths worldwide [4].
Chronic kidney disease (CKD) is a pathophysiologic process loss function 80% with abnormalities of kidney structure or renal function that persist for over three months [5]. It is a multiple etiology. It is defined as a decreased glomerular filtration rate (GFR) of less than 60 mL/min per 1.73 m2 for more than 3 months, [6]. According to the Kidney Disease Improving Global Outcome (KDIGO) 2024 clinical practice guidelines. CKD classified into 5 stages according to GFR, whereas stages (5) considered as severe End Stage of Renal Disease (ESRD), and new a day is used the value of serum of albumin for determination of stage severity [8]. CKD is considered a serious public health problem in worldwide [8]. It is a life-threatening outcome of CKD [9].
It was estimated that the worldwide prevalence of CKD is 13.4% [1,9], and over 2 million people have progressed to ESRD and most of them are going to HD treatment [10]. In 2021 a survey study was reported that the CKD affected 1 to 7 in the US, and it is often undiagnosed due to a lack of apparent symptoms in early stages because CKD interferes with the body’s physiological and biological mechanisms, such as fluid electrolyte and pH balance, blood pressure regulation, excretion of toxins and waste, vitamin D metabolism, and hormonal regulation. Many CKD patients are at risk of hyperkalemia, hyperphosphatemia, chronic metabolic acidosis, bone deterioration, blood pressure abnormalities, and edema. These risks may be minimized, while the disease’s progression may be slowed through careful monitoring of protein, phosphorus, potassium, sodium, and calcium, relieving symptoms experienced by CKD patients [1].
Nutritional treatment strategy the best treatment strategy for along therapy for survival is the renal replacement process [11] but it is very cost, while most of patients are going to Hemodialysis as a quick and available treatment ways. While most of these patients will be suffering of many complication as renal anemia [12]. In recent studies in renal anemia complication treatment and stopping it progression, it was examined the Mediterranean diet and the whole foods plant-based diet for their potential role in delaying CKD progression. Biological explanations for why the whole foods plant-based diet may benefit CKD patients compared to diets that include animal products are examined [10]. There was strong evidence continued to support the importance of diet meeting the daily requirement in the prevention and progression of kidney disease, and medical nutrition therapy with a registered dietitian is a critical aspect in medical intervention for CKD [10]. To slow the development of Renal Anemia progression it should be assessment of biochemical nutrition constituents, according to numerous studies that also include dialysis patients, the prevalence of malnutrition in CKD ranges from 12% to 80% [3].
So of renal anemic patients may include a good clinical nutrition strategy contain suitable constituents of macroeconomics (Carbohydrate, Protein, and fat) and micromolecules as (minerals as Iron, phosphor, calcium and vitamin as alfa calcidol (vitamin D), C), with Epoetin alfa instead to endogenous Erthroepotein hormone. In some cases, need to blood transfusion and others medical medicines. Medical nutrition therapy is imperative for CKD patients because it may slow the progression of the disease through careful monitoring of protein, calcium, phosphorus, potassium, and sodium [14]. In other it was empirically confirmed that the positive impact of nutrition education on hemodialysis patients, addressing several critical aspects. And These findings underscore the importance of healthcare providers incorporating nutrition education into the standard treatment for Hemodialysis patients [4].
Epidemiologic evidence about the direct association between dietary mineral intake and CKD is limited, and has been conducted in small sample sizes [5]. Most studies examining the association between dietary mineral intake and CKD have focused on the terminal stages of CKD or on patients on dialysis [5], or did not distinguish the stages of CKD. The complications related to reduced eGFR would be abnormal sodium balance causing hypertension, a blunted erythropoietin axis causing anemia, and disturbed calcium and phosphate balance causing secondary hyperparathyroidism and metabolic acidosis [4]. And in advanced stages of CKD and in the dialysis population anemia [1].
Renal anemia is present in as high as 90% of patients. In addition, Patients with advanced chronic kidney disease (ACKD) have a high prevalence of Malnutrition [3]. Therefore, the nutritional and dietary management is fundamental in the treatment of patients with chronic kidney disease (CKD) [3]. The aim of this study was to determine the most effective medical treatment protocol for stooping or slowing of renal anemia complication appearance and progression in renal anemic patients by study the role of the most important Biochemical nutrient constituents and its relation with disease development.
Literature Review
Renal Anemia
Renal Anemia is the commonest Renal Failure complication, it may be the initial laboratory sign of an underlying medical problem. Consequently, a complete blood count, including the hemoglobin (Hb) concentration, is routinely part of global health assessment in most adults, whether or not they have chronic kidney disease (CKD). The anemia should be evaluated independently of CKD stage in order to identify any reversible process contributing to the anemia [20]. It was first linked to CKD in 1836 by Richard Bright [16]. And this condition is highly prevalent but treatable and frequency increases in the more advanced stages of the disease. It can be found in More than 50% of patients with the diagnosis of CKD In stages 4 and 53 [1]. Kidneys control many biological mechanisms such as fluid, electrolyte, pH balance, blood pressure, excretion of toxins and waste, vitamin D metabolism, and hormone synthesis [18].
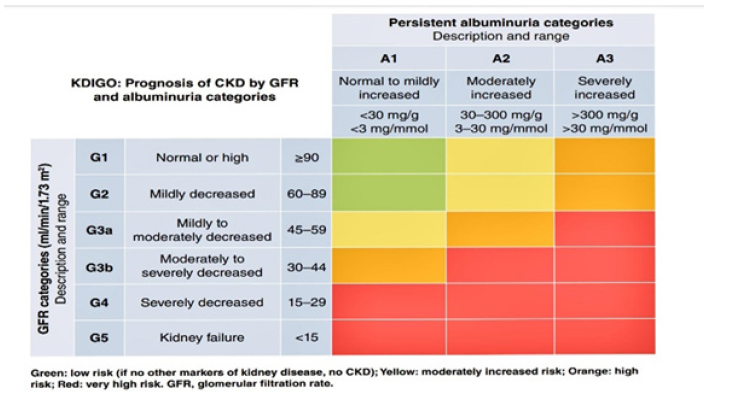
Chronic Kidney Disease: Many recent and previous studies were defined the CKD as a 80% renal Failure of cases with abnormalities of kidney structure or function, present for a minimum of 3 months, with implications for health. The prevalence of CAD is increased gradually particularly in Palestinian Gazans, in addition, the Korea National Health and Nutrition Examination Survey (KNHANES) in 2011-2013, reported that the prevalence of CKD for adults aged ≥20 in the Republic of Korea in 2013 was estimated to be 8.2% [44]. In other study it was reported About 37 million US adults are estimated to have (CKD) [17]. CKD is diagnosed and classified based on Glomerular filtration rate (GFR) category (G1-G5), and Albuminuria category (A1-A3), abbreviated as CGA [7]. As shown in the following table (Table: 1).
In addition, very low levels of serum creatinine (SCr) often represent poor health status, this biological feature of creatinine (i.e., relation to muscle mass) has limited its prognostic utility and results in reducing the risk associations for eGFRcr 45-60 ml/min per 1.73 m2 and elevating risks for eGFRcr >110 ml/min per 1.73 m2. These limitations are not observed when risk is estimated using eGFRcr-cys or cystatin C-based eGFR (eGFRcys) [7]. (Table 1)
It was mentioned that Globally people with hypertension, diabetes, or CVD are at high risk for CKD [7], and many studies was established main CKD risk factors for including hypertension, diabetes mellitus, poor glycemic control, and dyslipidemia [1], recent studies and guidelines suggested that mineral metabolism is associated with the pathogenesis or progression of CKD [12]. In addition, other high-risk people may be identified through genetic risk factors or by varying exposure to environmental pollution, pesticides, water, and nephrotoxic medications including significant analgesic use and herbal medications, depending on geographical region [7]. However, most of CKD will need to HD as a fast treatment and patient care. Renal anemia is the most common of CKD complication, and many studies the overall prevalence of CKD associated with renal anemia is noticed, while and the factors for renal anemia progression include: poor dietary intakes, nutritional deficiency, Abnormal Iron metabolism, Blood loss during dialysis, Inflammation, Shortened of RBC survival due to Oxidative stress, Gastrointestinal bleeding, Hyperparathyroidism (SHIP) [19]. So, the most of them are depended on patients nutrition program, however, malnutrition in CKD implies an increase in morbidity and mortality [3].
Diagnosis and assessment of anemia: Diagnose anemia in adults and children >15 years with CKD when the Hb concentration is <13.0 g/dl. (<130 g/l) in males and <12.0 g/dl. (<120 g/l) in females. (Not Graded). Diagnose anemia in children with CKD if Hb concentration is <11.0 g/dl. (<110 g/l) in children .5-<5 years, <11.5 g/dl. (115 g/l) in children 5-12 years, and <12.0 g/dl (120 g/l) in children 12-15 years. (Not Graded) [20]. While the increase in mortality occurs mainly when Hb≤ 8 g/dL [21]. In addition, the diagnosis of iron-deficiency anemia (IDA) is essential to ensure prompt treatment to correct the deficiency and improve the accompanying anemia. All individuals with CKD, particularly those with an estimated glomerular filtration rate (eGFR) of <60 mL/min/1.73 m2 (stage ≥3 CKD), should be screened for anemia as part of their initial evaluation after CKD diagnosis. The screening includes measurements of proteins involved in iron metabolism, with serum ferritin, hemoglobin level, and TSAT being the main tests used [2]. Main couses is the reduction in the production of erythropoietin significantly contributes to anemia in CKD with iron deficiency as observed in many previous studies [22].
Renal anemia treatment: The ESRD patients are treated with many strategy, which include dialysis (hemodialysis or peritoneal dialysis), Erthroepotein hormone supplementation, suitable medical Nutrition (macromolecules as Carbohydrates, Protein, and Fat, and micromolecules as blood electrolytes as Iron, calcium, and phosphor, and vitamin D, and C), Blood transfusion, and Kidney translation which is good treatment. However, Hemodialysis emerges as the primary treatment modality for end-stage renal failure worldwide [4]. However, before the start of treatment with erythropoietin analogs, lower hemoglobin levels were common among patients on dialysis- which exposed them to the risk of multiple blood transfusions and reduced functionality [23-25].
In other hand, according on many recent studies they were referred that the kidney disease awareness remains low, and worldwide only 6% of the general population and 10% of the high-risk population are aware of their CKD status. Important to note is that patient advocates with CKD strongly argue for earlier CKD screening and diagnosis [7]. Beside to biochemical nutrition treatment the protocol should be include ERO hormone, the first erythropoietin analog used was epoetin alfa. Subsequently, darbepoetin was also approved. Both can be administered subcutaneously or parenterally. The use of erythropoietin analogs is not devoid of risks [26,27].
Biochemical Nutritional Treatment Strategy
It’s a suitable treatment protocol is used for supply the essential biochemical molecules with calculate the amount of released and consumed energy. The biochemistry parameters values of micronutrients and macronutrients (carbohydrate, protein, and fat), and energy intakes, are related to the progression of renal failure so the obtaining of data about patient nutrition biochemical parameters And other medical recorded is very important for patient nutrition assessment [3]. Nutritional advice is essential in the treatment of CKD since malnutrition associated with kidney disease is associated to a worse prognosis and increased morbidity and mortality.
However, renal patients have usually been labeled as having protein calorie malnutrition; a Spanish study was reported that, after performing a subjective global assessment (GSA), protein energy wasting criteria (PEW), a three-day dietary record, anthropometric parameters and bioimpedance, a 27.9% of patients had values in the malnutrition range. In addition, the majority of studies are in dialysis patients that report a prevalence of malnutrition-malnutrition ranging from 12% to 80% In 2018 [3]. In recent years, the term protein energy wasting (PEW), which is defined as the pathological state in which there is a decrease i then protein and energy stores, gives equal importance to catabolism and malnutrition [3]. 50% of dray weight of human body building muscle are consist of protein (15%of cell components), heal, fight infection, and stay healthy. Protein needs vary based on age, gender and overall general health. However, Proteins come from both animal and plant sources. In CKD serum albumin and protein are pass in urine in increased concentration due to renal failure, but the restrictions in protein intake are recommended, since there are studies that show that they reduce proteinuria and can improve GFR.
The federal Dietary Guidelines for Americans recommend an amount of 0.8 g/kg/body wt/d dietary protein intake for healthy adults [28].
Exceeding the recommended dietary allowance (RDA) may increase the risk of health complications even for healthy adults [29]. And protein intake recommendations for CKD patients are dependent on the stage of the disease, which is determined by declining GFR function [2]. Leads to increase pressure and glomerular morphologic changes, resulting in renal dysfunction [10]. Glomerular hyperfiltration is defined as modifying renal hemodynamics through glomerular capillary hyperemia and increasing intraglomerular pressure [10]. HPDs induce glomerular hyperfiltration, hyperemia, and increased hydraulic pressure, resulting in vasodilation of the afferent arteriole. HPDs contribute to progressive glomerular damage, which, combined with the renal deterioration from diseased kidneys may contribute to CKD progression [10].
(Figure 1)
In other hand, Low protein in dietary intake decrease the over load in kidney and contribute to kidney stay in stable phase, so the Low-protein diets (LPDs) have been shown to improve hyperfiltration, reduce nitrogenous waste, and ease the renal workload by decreasing glomerular pressure [10]. Proteinuria declined by 20-50% in CKD patients who adhered to a LPD [10]. Although LPDs provide direct benefits to CKD patients, healthcare professionals are concerned about Protein-energy malnutrition and protein-energy wasting (PEW) in CKD patients due to Inadequate energy intake [10]. When determining the estimated energy requirements For CKD patients, 25-35 kcal/kg/body weight/d is recommended to maintain energy and Nitrogen balance and avoid risk for malnutrition [10].
Dietitian Benefits and Dietary Management
Dietary education and patient counseling provided by a registered dietitian (RD) is essential for preventing and managing CKD. And Careful and detailed dietary planning, frequent assessment of nutritional status, and dietary monitoring compliance are critical for successful dietary management. The progressive decline in GFR is a risk factor for the development of metabolic acidosis. The main goal of therapy is to prevent or correct this metabolic acidosis, which has been shown to slow down the progression of CKD to ESRD [10]. The biggest contributor to this acid pool is the consumption of a diet higher in animal proteins [10]. The simplest treatment for this metabolic acidosis includes dietary management by reducing the protein in the diet or switching the diet to an increase in plant-based proteins [42]. It has been shown that dietary intervention of lowering protein intake or switching to plant-based protein reduces metabolic acidosis in stage 3-4 CKD patients [10].
A dietary intervention is recommended in patients with excess weight and high fat concentration in order to reduce the weight which should improve renal hyperfiltration and reduce the progression of CKD [30]. High diet with fat molecules and Increased serum lipid may lead to thrombosis, In recent study was found that the predominant profile is malnutrition due to excess weight in almost 70% of patients with and a percentage of fat mass greater than others. Regarding fat distribution, android obesity predominates, taking as reference of CVD risk an abdominal circumference [3]. In contrast, decrease of lipid concentration is considered a malnutrition, which is important compound in body for hormones and fat soluble vitamin as vitamin D synthesis. WFPB diets do not restrict fat intake; however, the foods promoted are made up of monounsaturated and polyunsaturated fats and limit processed oils and saturated fat [30]. Previous studies show a daily caloric intake of total fat to be less than 15% in WFPB diets, which is protective against CVD [31]. It is well established that omega-3 fatty acids reduce inflammation [10], blood pressure, and increase HDL cholesterol [10]. The predimed trial demonstrated that a high-fat Mediterranean diet supplemented with virgin olive oil or nuts, implemented in a primary cardiovascular prevention setting, resulted in a 30% reduction in clinical events of CVD disease; it was also found a significant protection of the Mediterranean diet against diabetes.
The WHO recommends a lifestyle includes a physical activity (exercise) based on the Mediterranean diet and a low-salt diet in the general population, and especially in patients with CVD risk, obesity and overweight. In general, many studies report that a whole food plant-based (WFPB) diet reduces the risk for CVD in CKD patients, in addition, WFPB diets provide about 75% of carbohydrates (CHO), emphasizing dietary fiber [10]. However, Fiber intake of about 27 g/d reduces serum urea and creatine in CKD.
Water and Electrolytes are very important in kidney biochemical metabolism and function completion, regarding water intake, on many occasions it recommend a fluid restriction, despite the fact that many times diuresis of the patient would probably allow a higher intake. In other study, it was found the heterogeneity of the association between dietary mineral intake and CKD by hypertension status. Hypertension is the second leading cause of ESRD, and contributes to the progression of CKD [32]. In other hand, it is observed that low mineral intake (phosphorus, potassium, iron, and zinc) is related to early CKD, as well as advanced CKD, so not all of the results are caused by reverse causation due to food restriction [5]. The recommendations of the World Health Organization (WHO) are for a low-salt Mediterranean diet pattern, but in routine practice, nephrologists do not recommend plantbased diets because of fear to hyperkaliemia and that they have low nutritional content. It is likely that the nutritional advice that we have usually offered could not guarantee adequate caloric and mineral support, furthermore it may interfere with the quality of life of our patients [3].
In Renal anemic, the iron supplementation is intended to assure adequate iron stores for erythropoiesis, correct iron deficiency, and, in patients receiving ESA treatment, prevent iron deficiency from developing. Iron deficiency is common, and it is estimated that patients on hemodialysis have an iron loss of around 1 to 3 grams per year [22]. Even in patients not receiving dialysis, low levels of iron are often found [33]. Iron supplementation, particularly with intravenous iron, can enhance erythropoiesis and raise Hb levels in CKD patients with anemia even when TSAT and ferritin levels are not indicative of absolute iron deficiency, and even when bone marrow studies reveal adequate iron stores. Iron treatment, particularly when administered intravenously, has also been consistently demonstrated to improve the erythropoietic response to ESA treatment [20]. Iron supply is considered the essential corner stone in renal anemic cases and during Erthroepotein hormone treatment. So, for iron supplementation with ESA therapy, the KDIGO guidelines recommended that the supplemental iron should be administered to maintain serum ferritin levels >200 ng/mL. in ESRD cases treated with hemodialysis and >100 ng/mL in those with ESRD treated with peritoneal dialysis with TSAT of >20% in all individuals with CKD. In contrast, KDIGO guidelines do not recommend routine use of iron supplementation in patients with TSAT >30% or serum ferritin >500 ng/mL (>500 mg/L) [2]. Since this 2012 guidelines, there has been recognition that many individuals who are in an inflamed state and exhibit a hepcidin-induced blockade to iron may have altered iron metabolism by prevent iron renal excretion and store iron in bone marrow. Therefore, the experts that identifying these parameters is a high priority for future research [2].
There have been few studies directly investigating the relationship between dietary iron intake and CKD [5]. Previous studies have suggested that anemia may aggravate CKD [5], sufficient iron intake may prevent the development or progression of CKD by correcting anemia [5]. Although the loss of iron observed in other study (1.45 mg/day) is lower than the value reported for the US (5.3 g/day), this loss remains a public concern given that iron deficiency and anemia are highly prevalent in the country [34,35]. It was showed that the loss of calcium was estimated at 165 mg/d and that this loss was mostly due to dairy wastage (61.7 %) [36]. have shown that 72 % of calcium loss in the US was due to the waste of dairy products. Previous studies conducted in Lebanon have reported low dietary intake of calcium, particularly among children and women of reproductive age [36,37].
The current KDOQI guidelines for CKD nutrition state ergocalciferol or cholecalciferol effectively treats VD deficiency/inefficiency; however, specific dosing should be individualized and derived through a step-by-step approach [10]. This step-by-step approach includes monitoring 25(OH)D serum levels and serum calcium and serum phosphorus, which helps the healthcare team recommend specific dosing veered to the patient’s individual requirements [29]. Calcium balance is regulated by intestinal calcium absorption, kidney reabsorption, and calciotropic hormones that activate calcium exchange from the bone when serum calcium levels are low [41]. Insufficient calcium absorption and chronic calcium deficiency result in increased risk for hyperthyroidism [38]. However, excessive calcium poses an increased risk for calcification, resulting in comorbidity and higher mortality [10]. Alterations in calcium metabolism are multifactorial and include the use of active vitamin D analogues. Research shows that ingesting about 800-1000 mg/d of calcium may be sufficient to maintain calcium balance for patients with CKD 3-4 in the absence of vitamin D analogues [39].
Phosphorus plays a critical role in bone formation, acid-base balance, and energy production [10]. The body’s ability to maintain phosphate balance is achieved by excreting excess phosphate in the urine. As CKD progresses, declining renal function prevents the kidneys from excreting enough phosphorus needed for phosphorus homeostasis [38]. The 2020 NKF guidelines recommended CKD 1-5 and HD patients receive an intake of phosphorus that keeps serum phosphorus levels within normal ranges (3.4-4.5 mg/dL.) and to restrict dietary phosphate in the case of hyperphosphatemia [38]. In the result of program analysis, the correction of iron deficiency with oral or intravenous iron supplementation can reduce the severity of anemia in patients with CKD. Untreated iron deficiency is an important cause of hyporesponsiveness to ESA treatment. It is important to diagnose iron deficiency because treatment can readily correct the associated anemia and investigation for the cause of iron deficiency, which should follow its detection, can lead to important diagnoses [20]. It is important to diagnose iron deficiency in individuals with CKD because treatment can readily correct the associated anemia. Iron supplementation (either oral or intravenous) and/or ESA therapy are generally accepted as the standard of care for IDA in CKD (correctable causes of anemia, such as iron deficiency, should be addressed before ESA initiation) [2,3].
Assessment of Biochemical Nutrient Constituents
The chemical constituents data of food and drinking intake should be assessment by collected and analyzed in special computer analyses program agree for energy and biochemical nutrition assessment. A dietary analysis software, Nutritionist Pro™ Nutrition Analysis Software (First Data Bank, USA, 2003)*, was used for energy and nutrient analysis. This software contains several food databases including the USDA Food Database, Canadian Food Database, Mexico Food Database and the Malaysian Food Composition Tables along with other hundreds of international food items. The information will be entered for analyses. These micronutrients (iron, calcium and vitamin D) have been repetitively flagged as a public health concern in Lebanon [45,46], given their suboptimal consumption levels, in comparison with the dietary reference intakes (7-18 mg/day for iron, 700-1300 mg/day for calcium, and 15-20 μg/day for vitamin D) [40].
Conclusion
This study has provided the first summarized the essential macromolecules and micromolecules with optimum healthy limit. According on many previous studies, we recommended that the renal anemia of CKD patients to limit their protein intake as soon as with adequate protein intake in an effort to slow the decline in kidney function. Research has found that following popular high-protein diets for a prolonged period may harm the kidneys and may also benefit from eating less protein. As soon as the simplest treatment for metabolic acidosis includes dietary management by reducing the protein in the diet or switching the diet to an increase in plant-based proteins. Patient dietary intake program should be included with virgin olive oil or nuts for control of high level of fat. Practice physical exercise including strength training as possible for each patient with following suitable and fit nutritional program to improve the nutrition of our patients. Overweight and obese adults to lose 10% of their initial weight, and the primary treatment should be a lifestyle intervention. In addition, such intervention to lose weight should include a hypocaloric diet, with suitable physical activity and behavioral therapy. We also, recommend determining the initial ESA dose using the patient’s Hb concentration, body weight, and clinical circumstances, before start ESAs treatment course.
References
- Mikhail A, Brown C, Williams JA, Mathrani V, Shrivastava R, et al. (2017) Renal association clinical practice guideline on Anaemia of Chronic Kidney Disease. BMC Nephrol 18(1): 345.
- Farag HA, Baqi HR, Hussein YT, Shareef OH, Qadir SA, et al. (2020) The Role of Nutrients in Supporting The Immune System Against Viral Infection. Newly Emerged Coronavirus (COVID19): A Narrative Review. Kurdistan Journal of Applied Research pp. 84-96.
- Ketteler M, Block GA, Evenepoel P, Fukagawa M, Herzog CA, et al. (2017) Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline Update: what’s changed and why it matters. Kidney Int 92(1): 26-36.
- Shehada AK, Albelbeisi AH, Albelbeisi A, El Bilbeisi AH, El Afifi A (2021) The fear of COVID-19 outbreak among health care professionals in Gaza Strip, Palestine. SAGE Open Med 9: 20503121211022987.
- El Bilbeisi AH, Hosseini S, Djafarian K (2017) Association of dietary patterns with diabetes complications among type 2 diabetes patients in Gaza Strip, Palestine: a cross sectional study. J Health Popul Nutr 36(1): 37
- Foundation NK II (2006) Clinical practice guidelines and clinical practice recommendations for Anemia in Chronic Kidney disease in adults. American journal of kidney diseases: Am J Kidney Dis 47(5 Suppl 3): S11-145.
- El Bilbeisi AH, Hosseini S, Djafarian K (2017) The association between physical activity and the metabolic syndrome among type 2 diabetes patients in Gaza strip, Palestine. Ethiop J Health Sci 27(3): 273-282.
- El Bilbeisi AH, Shab Bidar S, Jackson D, Djafarian (2017) The prevalence of metabolic syndrome and its related factors among adults in Palestine: a meta-analysis. Ethiop J Health Sci 27(1): 77-84.
- Baqi HR, Farag HA, El Bilbeisi AH, Askandar RH, El Afifi AM (2020) Oxidative stress and its association with COVID-19: a narrative review. Kurdistan Journal of Applied Research 1: 97-105.
- Farag HA, Hosseinzadeh Attar MJ, Muhammad BA, Esmaillzadeh A, El Bilbeisi AH (2019) Effects of vitamin D supplementation along with endurance physical activity on lipid profile in metabolic syndrome patients: A randomized controlled trial. Diabetes Metab Syndr 13(2): 1093-1098.
- El Bilbeisi AH, Hosseini S, Djafarian K (2017) Dietary patterns and metabolic syndrome among type 2 diabetes patients in Gaza Strip, Palestine. Ethiop J Health Sci 27(3): 227-238.
- Albelbeisi AH, Albelbeisi A, El Bilbeisi AH, Taleb M, Takian, A et al (2021) Public sector capacity to prevent and control of noncommunicable diseases in twelve low-and middle-income countries based on WHO-PEN standards: a systematic review. Health Serv Insights 14: 1178632920986233.
- Farag HA, Hosseinzadeh Attar MJ, Muhammad BA, Esmaillzadeh A, Bilbeisi AH (2018) Comparative effects of vitamin D and vitamin C supplementations with and without endurance physical activity on metabolic syndrome patients: a randomized controlled trial. Diabetol Metab Syndr 10: 80.
- Farag HA, Hosseinzadeh-Attar MJ, Muhammad BA, Esmaillzadeh A, El Bilbeisi AH (2019) Effects of vitamin C supplementation with and without endurance physical activity on components of metabolic syndrome: A randomized, double-blind, placebo-controlled clinical trial. Clinical Nutrition Experimental.26:23-33.
- El Bilbeisi AH, Albelbeisi A, Hosseini S, Djafarian K (2019) Dietary pattern and their association with level of asthma control among patients with asthma at Al-Shifa medical complex in Gaza strip, Palestine. Nutr Metab Insights 12:1178638819841394.
- El Bilbeisi AH, Al Jawaldeh A, Albelbeisi A, Abuzerr S, Elmadfa I, et al. (2022) Households' food insecurity and their association with dietary intakes, nutrition-related knowledge, attitudes and practices among under-five children in Gaza strip, Palestine. Front Public Health 10: 808700.
- Hwalla N, Adra N, JACKSON RT (2004) Iron deficiency is an important contributor to anemia among reproductive age women in Lebanon. Ecology of food and nutrition 43(1-2): 77-92.
- Albelbeisi AH, Albelbeisi A, El Bilbeisi AH, Takian A, Akbari Sari A (2020) Capacity of palestinian primary health care system to prevent and control of non‐communicable diseases in Gaza Strip, Palestine: A capacity assessment analysis based on adapted WHO‐PEN tool. The International Journal of Health Planning and Management 35(6): 1412-1425.
- Farag HA, Baqi HR, Qadir SA, El Bilbeisi AH, Hamafarj KK, et al. (2020) Effects of Ramadan fasting on anthropometric measures, blood pressure, and lipid profile among hypertensive patients in the Kurdistan region of Iraq. SAGE open medicine 8: 2050312120965780.
- El Bilbeisi AH, Al Jawaldeh A, Albelbeisi A, Abuzerr S, Elmadfa I, et al. (2022) Households’ food insecurity and its association with demographic and socioeconomic factors in Gaza Strip, Palestine: a cross-sectional study. Ethiop J Health Sci 32(2): 369-380.
- Abuzerr S, Zinszer K, Shaheen A, El Bilbeisi AH, Al Haj Daoud A, et al. (2021) Impact of the coronavirus disease 2019 pandemic on the Palestinian family: A cross-sectional study. SAGE Open Med 9: 20503121211001137.
- Albelbeisi AH, Albelbeisi A, El Bilbeisi AH, Taleb M, Takian A, et al. (2021) Barriers toward the practice of healthy behaviors among patients with non-communicable diseases in Gaza Strip, Palestine. SAGE Open Medicine 9: 20503121211029179.
- Albelbeisi AH, Albelbeisi A, El Bilbeisi AH, Taleb M, Takian A, et al (2021) Barriers of adherence among Palestinian healthcare professionals towards the protocol of health education and counselling on healthy behaviours for non-communicable diseases. Ethiop J Health Sci 31(1): 73-84
- El Bilbeisi AH, Hosseini S, Djafarian K (2018) Prevalence of metabolic syndrome and its components using two proposed criteria among patients with type 2 diabetes in Gaza Strip, Palestine. BAOJ Nutrition 4: 054.
- El Bilbeisi AH, Al Jawaldeh A, Albelbeisi A, Abuzerr S, Elmadfa I, et al. (2022) Association of household food insecurity with dietary intakes and nutrition-related knowledge, attitudes, and practices among parents, aged≥ 18 years in Gaza strip, Palestine: a descriptive study. Heliyon 8(6): e09582.
- El Bilbeisi AH, El Afifi A, Djafarian K (2019) Association of healthy eating index with metabolic syndrome and its components among type 2 diabetes patients in Gaza Strip, Palestine: A cross sectional study. Integr Food Nutr Metab 6: 1e7.
- El Bilbeisi AH, El Afifi A, Baloushah S, Alblbeisi A, Albelbeisi AH et al. (2020) Maternal dietary patterns during early pregnancy and their association with pregnancy outcome among obese women in Gaza Strip, Palestine: a prospective cohort study. Austin J Nutri Food Sci 8(1): 1138.
- el Bilbeisi AH, Srour M, el Afifi A, Farag HA, Djafarian K (2019) Dietary patterns and their association with depression among type 2 diabetes patients in Gaza Strip, Palestine. Food and Nutrition Sciences 10(5): 533-550.
- El Bilbeisi AH, Hosseini S, Djafarian K (2018) Dietary patterns and their association with blood pressure control among hypertensive patients in Gaza Strip, Palestine. Journal of Family Medicine and Health Care 4(2): 5-12.
- Djafarian K, Hosseini S, Bilbeisi E, Hamid A (2017) The prevalence of malnutrition and associated factors among hemodialysis patients at al-shifa medical complex in Gaza Strip, Palestine. International Journal of Hospital Research 6(1): 36-44.
- Abuzerr S, Zinszer K, Shaheen A, El Bilbeisi AH, Salem A, et al. (2022) Attitudes and Satisfaction toward the Taken Procedures to Tackle COVID-19 Pandemic in Palestine. Ethiop J Health Sci 32(1): 27-36
- El Bilbeisi AH, El Afifi A, Taleb M, El Qidra R, Djafarian K (2019) Malnutrition and their association with diabetes complications among hospitalized type 2 diabetes patients in Gaza strip, Palestine. Austin J Nutri Food Sci 7(8): 1132.
- Wahedy KM, H El Bilbeisi AH, J Bakry M (2021) DIETARY PATTERNS AND THEIR ASSOCIATION WITH GLYCEMIC CONTROL AND RISK OF GESTATIONAL DIABETES MELLITUS IN GAZA STRIP, PALESTINE: A CASE-CONTROL STUDY. Bulletin of Pharmaceutical Sciences Assiut University 44(2): 537-549.
- El Bilbeisi AH, El Afifi A, Farag HA, Djafarian K (2021) Effects of vitamin D supplementation along with and without endurance physical activity on calcium and parathyroid hormone levels in metabolic syndrome patients: A randomized controlled trial. Clinical Nutrition Open Science 35: 1-1.
- Taleb MH, Elkhair EA, Timraz RA, Bilbeisi E, Hassan AH (2022) Prevalence of Antibiotics Resistance among Patients Undergoing Bronchoscopy in Chest Department at Al-Shifa medical complex in Gaza Strip, Palestine. Bulletin of Pharmaceutical Sciences Assiut University 45(2): 811-822.
- Profile NC. FAO-NUTRITION COUNTRY PROFILES.
- Akl CG (2012) Prevalence and determinants of overweight and obesity in a nationally representative sample of Lebanese children 5 to 12 years old-by Christelle Georges Akl (Doctoral dissertation).
- Zgheib RS,Farah Naja, Nahla Hwalla, Fatima Al Zahraa Chokor, Lara Nasreddine (2022) Infant and young child feeding practices in Lebanon: a national cross-sectional study (Doctoral dissertation). Public Health Nutr 26(1): 143-159.
- Kliger AS, Foley RN, Goldfarb DS, Goldstein SL, Johansen K (2013) KDOQI US commentary on the 2012 KDIGO clinical practice guideline for anemia in CKD. Am J Kidney Dis 62(5): 849-859.
- Park CS, Vogel E, Larson LM, Myers SS, Daniel M (2019) The global effect of extreme weather events on nutrient supply: a superposed epoch analysis. Lancet Planet Health 3(10): e429-438.
- Gutierrez OM (2019) Sodium and phosphorus-based food additives: persistent but surmountable hurdles in the management of nutrition in chronic kidney disease Adv Chronic Kidney Dis 20(2): 150-156.
- Kalantar Zadeh K, Joshi S, Schlueter R, Cooke J, Brown Tortorici A (2020) Plant-dominant low-protein diet for conservative management of chronic kidney disease. Nutrients 12(7): 1931.
- Sabatino A, Cuppari L, Stenvinkel P, Lindholm B, Avesani CM (2021) Sarcopenia in chronic kidney disease: what have we learned so far?. J Nephrol 34(4): 1347-1372.
- El Bilbeisi AH, Abo Khosa SM, Taleb MH, El Afifi AM (2023) Assessment of serum, dietary zinc levels, and other risk factors during the third trimester among pregnant women with and without pregnancy-induced hypertension: a case-control study. Front Nutr 10:1155529.
- Park JI, Baek H, Jung HH (2016) Prevalence of chronic kidney disease in Korea: The Korean national health and nutritional examination survey 2011-2013. J Korean Med Sci 31(6): 915-923.
- Hwalla N, Al Dhaheri AS, Radwan H, Alfawaz HA, Fouda MA (2017) The prevalence of micronutrient deficiencies and inadequacies in the Middle East and approaches to interventions. Nutrients. 9(3): 229.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.