Minireview 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Neuron Bombardment and Cycling Neurotransmission: The Downstream Impact of Persistent Sympathetic Activation
*Corresponding author: Peter Behel is a long time Provider for Kaiser Permanente, and former President of the Biofeedback Society of California. His clinical research has been showcased in the former #1New York Times Bestseller, 8 Weeks to Optimum Health.
Received: August 24, 2024; Published: September 19, 2024
DOI: 10.34297/AJBSR.2024.24.003165
Abstract
Neuron bombardment is a defining feature of the condition known as central sensitization. Central sensitization is a phenomenon that is characterized by increased neuronal excitation that elevates pain signaling, and initiates pain hypersensitivity [1]. Central nervous system hyperresponsivity is associated with autonomic dysfunction, and elevated sympathetic nervous system activation [2]. Remarkably, the same synaptic plasticity underlying the development of central sensitization also provides the means for system-wide neuronal excitability to be down- regulated, and potentially reversed [3].
Introduction
Nociception refers to the process of transferring pain signals from the periphery to the central nervous system. This process is said to be mediated by the sympathetic nervous system [4]. Central sensitization is a condition that results in amplification of neural signaling [5]. Intense or sustained nociceptor activation is said to induce central sensitization [6]. To the extent that nociceptor activation is reported to be facilitated by sympathetic activation, a case can be made for the application of applied sympathetic modulation in both nociception and central sensitization as a means of moderating both chronic pain and centralized sensitivity [7].
Sympathetically Mediated Pain
Pain-autonomic interactions are utilized as an objective means of evaluating sensitized neuronal states by researchers who investigate neuronal hyperexcitability [8]. As an example, increased sympathetic activation has been reported to modulate referred pain, fibromyalgia and trapezius myalgia [9]. Yet even though sympathetically maintained activity in nociceptive neurons has been linked with central sensitization [10], general recognition of the role that autonomic dysregulation plays in pain generation has been slow to expand beyond the role assigned to Sympathetically Maintained Pain (SMP), a category that includes causalgia, Reflex Sympathetic Dystrophy (RSD) and Complex Regional Pain Syndrome (CRPS) [11]. Interestingly, some RSD investigators have depicted chronic pain as emerging from central sensitization, with autonomic dysregulation resulting from centralized nervous system dysfunction [12].
Other researchers have underscored the role that the centralized pain syndrome plays in the process responsible for the transition from acute pain to chronic pain [13]. To the extent that the sympathetic nervous system is responsible for facilitating and potentiating chronic pain, autonomic dysfunction interacts at multiple levels of the nociceptive neuraxis [14].
For example, the neuronal excitation that leads to amplified neural signaling is associated with the influence of adrenergic receptors on nociceptive neurons. This placement facilitates sympathetic and sensory neuron coupling, leading to sensitization of peripheral nociceptors and inducing neuronal sensitization within the dorsal horn of the spinal cord [15]. To the extent that elevated sympathetic nervous system activity can be described as pathological when coupled with nociceptive activation [16], it does not seem to be unreasonable to conceive of chronic pain occurring as the result of autonomic dysregulation.
Chronic pain is associated with elevated peripheral nociceptor activation, increased sympathetic activity in the autonomic nervous system, vagal nerve dysfunction and disorders that include urinary dysfunction, hyperhidrosis, orthostatic hypertension, gastrointestinal symptoms and sexual dysfunction [17]. Yet in true ouroboric fashion, if sympathetic nervous system dysregulation is responsible for inducing chronic pain, and chronic pain in turn produces increased sympathetic activation, one is left wondering where one layer leaves off and the next level begins. At the very least, these overlapping responses seem configured to cycle, with each leading back to the other.
Even as pain / sympathetic interaction results in elevated nociceptive neuron activation, this is not the only pain-related mechanism that seems designed to cycle. As circumstances might have it, the collective cycling of multiple pain processing functions resembles the interconnected gears inside of a watch, with the sum total of these influences ultimately contributing to pain’s chronicity, or the process that makes chronic pain chronic.
Substance P Activation
Pain signaling initiates with the release of substance P, a neurotransmitter utilized to relay pain messages from one neuron to another. This stimulates an ensuing chain response that encompasses the release of an assortment of neuropeptides, including bradykinin, neurokinin A and calcitonin gene-related peptide, or CGRP. This core cluster not only combines to conduct pain signaling, but also interacts to manage inflammatory responses, a key component of the body’s immune system functioning.
In keeping with its function as inflammatory mediator, bradykinin interacts with fellow vasodilator substance P to elevate vascular permeability and regulate blood pressure. This produces vascular dilation in various arteries and veins [18]. Neurokinin A likewise assumes an assortment of roles associated with smooth muscle stimulation and vasodilation, in addition to its involvement in pain signal transmission [19]. Both substance P and CGRP are classified as potent vasodilators, with CGRP inducing a longer lasting increase in peripheral circulation, and substance P credited for inducing dilation in briefer increments [20]. CGRP in fact is said to potentiate the effect of substance P, both quantitatively and temporally [21].
Both substance P and neurokinin A are members of the tachykinin family of neuropeptide neurotransmitters. Aside from bradykinin, neurogenic release of the other three peptides present in the nociceptive C fibers are reported to induce myofascial pain in skeletal muscles [22]. Yet even as this core cluster of neurotransmitters is recognized for its influence on pain signal transmission, the neuronal activation characteristic of nociception is also implicated as the defining event that initiates inflammatory responses [23]. As circumstances would have it, substance P activation is understood to release a broad range of pro-inflammatory cytokines and chemokines in its wake [24].
The cascade of inflammatory mediators released as a result of substance P activation includes serotonin, histamine and prostaglandins, in addition to cytokines IL-1B, Tumor Necrosis Factor Alpha (TNF-a) and IL-6, which collectively are referred to as “inflammatory soup”. This inflammatory response then activates and sensitizes the nociceptive fibers involved in the conduction of chronic pain impulses. The release of substance P resulting from nociceptor C fiber activation through the induction of inflammatory soup represents a prime example of cycling neurotransmission, in that C fiber activation serves to then stimulate additional substance P release. Over time, the inflammatory mediator releases sensitize the same nociceptive neuron fibers responsible for their secretion, leading to peripheral sensitization.
As it turns out, this process is responsible for producing the maladaptive neuronal plasticity that results in persistent pain [25]. Essentially, the release of chemical mediators during an inflammatory response causes the nociceptive fibers to become hyperexcitable, spontaneously active, and hyperresponsive to lower threshold input [26]. The hypersensitivity that induces the amplification of neuronal signaling within the CNS represents the defining process associated with central sensitization [27].
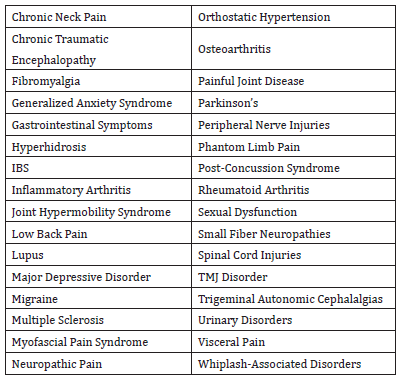
This enhanced responsivity reflects the central nervous system’s ability to adapt over time to increased levels of neural stimulation [28]. The ensuing sensitivity that emerges has recently gained the designation of nociplastic pain and has become recognized as a pain phenotype in its own right [29]. The expanded designation helps account for some of the various features associated with the long-term presence of cycling substance P, including mood affective disorders such as fear and anxiety [30], as well as PTSD and major depressive disorders [31] (Table 1).
This extended profile associated with centralized pain is reported to encompass global sensory hyperresponsivity in varying measures, including sensitivity to light, noises, food, smells and medications. Elevated responsiveness also overlaps into areas such as sleep disorders, restless leg, fatigue, interstitial cystitis, IBS, TMJ and other conditions [32]. Central nervous system hyperexcitability is also considered to be a factor in the development of osteoarthritis, where amplified neural signaling, wind-up synaptic activation and long-term potentiation can increase pain [33]. As it turns out, the constellation of conditions reported to be either directly impacted or generated by the centralized pain syndrome appears to be growing.
Discussion
Central sensitization is induced by the sympathetic facilitation of sustained discharges of polymodal nociceptors [34]. This persistent nociceptive activation results in the development of neurogenic inflammation [35]. To the extent that neuropeptide release is generated through nociceptor excitability, it is relevant to note that autonomic nervous system dysregulation exerts a stimulating effect on peptide release [36].
Dysregulated sympathetic reflexes are in fact associated with hyperexcitable peptidergic nociceptors and dysregulated nociceptive function in the generation of neuropathic pain; to the degree that targeting hyperactivity in C-nociceptors has been recommended for treating chronic pain and autonomic dysreflexia following spinal cord injury [37]. This tends to support the recognition that all pain is sympathetically mediated. As such, targeted autonomic modulation would seem to represent the most direct means of moderating chronic pain where it is generated.
The apparatus of nociceptor priming and sustained neuropeptide release that leads to central nervous system hyperactivity and persistent neuroinflammation is potentiated by sustained sympathetic activation. This recognition carries a couple of key implications. It suggests that without dysregulated sympathetic output, there might not be a chronic pain syndrome, and that persistent pain itself constitutes the expression of autonomic nervous system dysregulation. If sustained neuropeptide release represents the operant mechanism at hand, then persistent sympathetic activation provides the throttle. Like a valve stuck open, this model suggests the proposition that chronic pain itself by definition represents the veritable expression of autonomic dysregulation.
Interestingly, a generalized survey of popular stress mechanisms often terminates around the respective roles that the HPA axis and sympathoadrenal response mechanisms (cortisol and adrenaline) provide, without necessarily reckoning with the cellular and molecular influences that sympathetic dysregulation also interacts with. The extent to which nociceptive reflexes by definition are predicated on actual or potential tissue damage however strongly point to neurogenic inflammation as essentially constituting a stress response, albeit one that is cloaked and concealed within a nociceptive reflex. Intriguingly, functional HPA axis alterations have been included among structural and neurochemical changes reported to be influenced by central sensitization, which additionally includes mention of underlying pathophysiologic features observed in post-COVID-19 syndrome [32].
Chronic neuron bombardment that results from lowered firing thresholds and / or spontaneous firing is associated with centralized sensitization [38], and the neuronal excitability said to potentiate pain wind-up is reported to be the outcome of the repetitive activation of the C fibers [39]. Moderating C fiber nociceptor activation through autonomic modulation represents a functionally based chronic pain treatment aimed at mediating pain by down regulating neuronal hyperexcitability.
Acknowledgement
None.
Conflict of Interest
None.
References
- Shah J (2006) New frontiers in the pathophysiology of neuromusculoskeletal pain. Neuromuscular Disorders and Techniques: Novel Observations and Fresh Looks 9.
- Hazra S, Venkataraman S, Handa G, Yadav SL, Wadhwa S, et al. (2020) A cross-sectional study on central sensitization and autonomic changes in fibromyalgia. Frontiers in Neuroscience 14: 788.
- Woolf C (2011) Central sensitization: Implications for the diagnosis and treatment of pain. Pain 152(3): S2-S15.
- Kendroud S, Fitzgerald L, Murray I, Hanna A (2022) Physiology, Nociceptive Pathways. In: StatPearls. Treasure Island (FL): StatPearls Publishing.
- Dydek A, Givler A (2023) Central pain syndrome. In StatPearls. Treasure Island (FL): StatPearls Publishing.
- van den Broeke E, Lambert J, Huang G, Mouraux A (2016) Central sensitization of mechanical nociceptive pathways is associated with a long-lasting increase of pinprick-evoked brain potentials. Frontiers in Human Neuroscience 10(531): 1-10.
- Campbell J, Meyer R, Raja S (1992) Is nociceptor activation by alpha 1 adrenal receptor the culprit in sympathetically maintained pain? APS Journal 1(1): 3-11.
- Lutolf R, Rosner J, Curt A, Hubli M (2021) Indicators of central sensitization in chronic neuropathic pain after spinal cord injury. European Journal of Pain 26: 2062-2075.
- Domenech Garcia V, Peiroten A, Imaz M, Palsson T, Herrero P, et al. (2022) Not just sensitization: Sympathetic mechanisms contribute to expand experimental referred pain. Korean J Pain 35(3): 240-249.
- Janig W (2004) Sympathetic nervous system and pain. Primer on the Autonomic Nervous System: 374-376.
- Kruglov D, McGuckin D (2023) The Role of Autonomic Nervous System in Pain Chronicity. IntechOpen.
- Schwartzman R, Popescu A (2002) Reflex sympathetic dystrophy. Current Rheumatology Reports 4(2): 165-169.
- Toth C (2013) Peripheral and central sensitization. Cambridge University Press: 51-64.
- Arslan D, Ünal Çevik I (2022) Interactions between the painful disorders and the autonomic nervous system. Agri 34(3): 155-165.
- McMahon SB (1991) Mechanisms of sympathetic pain. Br Med Bull 47(3): 584-600.
- Dusi V (2020) Nociception, Sympathetic Nervous System, and Inflammation. In: Govoni, S., Politi, P., Vanoli, E. (eds) Brain and Heart Dynamics. Springer, Cham.
- Sillevis R, Cuenca Zaldívar JN, Fernández Carnero S, García Haba B, Sánchez Romero EA, et al. (2023) Neuromodulation of the Autonomic Nervous System in Chronic Low Back Pain: A Randomized, Controlled, Crossover Clinical Trial. Biomedicines 11(6): 1551.
- Shibata M, Ohkubo T, Takahashi H, Inoki R (1986) Interaction of bradykinin with substance P on vascular permeability and pain response. Japanese Journal of Pharmacology 41(3): 427-429.
- Samsam A, Covenas R, Ahangari R, Yajeya J, Narvaez J, et al. (2000) Simultaneous depletion of neurokinin A, substance P and calcitonin gene-related peptide from the caudal trigeminal nucleus of the rat during electrical stimulation of the trigeminal ganglion. Pain 84(2-3): 389-395.
- Schlereth T, Schukraft J, Kramer Best H, Geber C, Ackermann T, et al. (2016) Interaction of calcitonin gene-related peptide (CGRP) and substance P (SP) in human skin. Neuropeptides 59: 57-62.
- Gontijo J, Smith L, Kopp U (1999) CGRP activates renal pelvic substance P receptors by retarding substance P metabolism. Hypertension 33(1): 493-498.
- Bjergaard U, Nielsen L, Jensen K, Edvinsson L, Jansen I, et al. (1991) Calcitonin gene- related peptide, neurokinin A and substance P: Effects on nociception and neurogenic inflammation in human skin. Peptides 12(2): 333-337.
- Onaga T (2014) Tachykinin: Recent developments and novel roles in health and disease. Biomolecular Concepts 5(3): 225-243.
- Green D, Limjunyawong N, Gour N, Pundir P, Dong X, et al. (2019) A mast-cell-specific receptor mediates neurogenic inflammation and pain. Neuron 101(3): 412-420.
- Ellis A, Bennett D (2013) Neuroinflammation and the generation of neuropathic pain. British Journal of Anesthesia 111(1): 26-37.
- Becerra L, Bishop J, Barmettler G, Kainz V, Burstein R, et al. (2017) Brain network alterations in the inflammatory soup animal model of migraine. Brain Res 1660: 36-46.
- Nijs J, George S, Clauw D, Fernandez de las Penas F, Kosek E, et al. (2021) Central sensitization in chronic pain conditions: latest discoveries and their potential for precision medicine. The Lancet Rheumatology 3(5): e383-e391.
- Volchek M, Graham S, Fleming K, Mohabbat A, Luedtke C, et al. (2023) Central sensitization, chronic pain, and other symptoms: better understanding, better management. Cleveland Clinic Journal of Medicine 90(4): 245-254.
- Nijs J, Malfliet A, Nishigami T (2023) Nociplastic pain and central sensitization in patients with chronic pain conditions: a terminology update for clinicians. Brazilian Journal of Physical Therapy 27(3): 100518.
- Rosenkrantz M (2007) Substance P at the nexus of mind and body in chronic inflammation and affective disorders. Psychol Bull 133(6): 1007-1037.
- Humes C, Sic A, Knezevic N (2024) Substance P’s impact on chronic pain and psychiatric conditions-a narrative review. International Journal of Molecular Sciences 25(11): 5905.
- Volchek M, Graham S, Fleming K, Mohabbat A, Luedtke C, et al. (2023) ibid.
- Lluch E, Torres R, Nijs J, Van Oosterwijck J (2014) Evidence for central sensitization in patients with osteoarthritis pain: A systematic literature review. European Journal of Pain 18(10): 1367-1375.
- Sanjue H, Jun Z (1989) Sympathetic facilitation of sustained discharges of polymodal nociceptors. Pain 38(1): 85-90.
- Matsuda M, Huh Y, Ji RR (2019) Roles of inflammation, neurogenic inflammation and neuroinflammation in pain. Journal of Anesthesiology 33(1): 131-139.
- Wils J, Duparc C, Cailleux AF, Lopez AG, Guiheneuf C, et al. (2020) The neuropeptide substance P regulates aldosterone secretion in human adrenals. Nature Communications 11(1): 2673.
- Walters E (2018) How is chronic pain related to sympathetic dysfunction and autonomic dysreflexia following spinal cord injury. Autonomic Neuroscience 209: 79-89.
- Arendt Nielsen L (2016) Increased pain sensitivity in persistent musculoskeletal pain, but not in other musculoskeletal pain states. Scandinavian Journal of Pain 13(1): 125-126.
- Li J, Simone D, Larson A (1999) Windup leads to characteristics of central sensitization. Pain 79(1): 75-82.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.