Case Report 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Solitary Leptomeningeal Malignant Melanoma with Neurocutaneous Melanosis: A Rare Aetiology of Headache in a Black Ethnicity
*Corresponding author: Nazmin Ahmed, Department of Neurosurgery, Ibrahim Cardiac Hospital & Research Institute, Shahbag, Dhaka, Bangladesh.
Received: November 13, 2024; Published: November 20, 2024
DOI: 10.34297/AJBSR.2024.24.003252
Abstract
Background: Primary melanocytic tumors of the central nervous system account for approximately 1% of the total number of melanomas diagnosed. These neoplasms originate from leptomeningeal melanocytes and show variable degrees of aggressiveness. An increased proliferation of these melanocytes in the skin as well as nervous system may suggest the presence of neurocutaneous melanosis, an illness characterized by the concurrence of a malignant melanoma with a giant or multiple intradermal nevus. Once regarded as a disease of childhood, adult onset is exceedingly rare and infrequently reported in the literature.
Case Description: We present a case of a 42-year-old female with a large congenital melanocytic nevus in the right cheek who presented with complaints of headache for 3 months. The patient was thoroughly evaluated both clinically and surgically leading to a rare diagnosis of primary leptomeningeal melanoma of the right temporal lobe. The patient subsequently underwent a neuronavigational guided right temporo-parietal craniotomy followed by gross total resection of the tumor with uneventful postoperative period. She was referred to the oncologist for adjuvant chemoradiation but refused. However, there was no new neurological deficit at 3 months follow up.
Conclusion: Primary leptomeningeal malignant melanoma in the setting of NCM is infrequently reported in the literature and even more rarely in a Black population. To date, our case represents the eleventh reported case in this demography for this age group.
Keywords: Neurocutaneous melanosis, Headache, Primary malignant melanoma
Introduction
Primary CNS melanomas are malignant melanocytic lesions that are highly metastatic. They are extremely rare, making up about 0.07% of total brain tumors [1,2]. They typically arise from the melanocytes within the CNS, which are the derivatives of neural crest cell [3]. During embryogenesis, these neural crest cells migrate and become integrated into various tissues such as the skin, uvea, cerebral parenchyma, leptomeninges, and mucous membranes [4]. Primary CNS melanomas have different clinical and histological features than those of the skin and retina, with a generally benign clinical course [5]. Lesions such as these originate solely from melanocytes within the leptomeninges. Beside this, Metastatic melanoma of central nervous system is usually characterized by short clinical history, multiple lesions, poor prognosis, usually seen in older population [6]. Radiologically, CNS melanocytic lesions can be of diffuse type, in which infiltration into the subarachnoid space is observed, and a focal type, that appears as a well-defined tumor [4]. Besides, malignant melanoma is usually related with congenital intradermal nevi.
The association between them has been described in 40-60% of cases of Neurocutaneous Melanosis (NCM) [7,8]. The diagnostic criteria of this rare phakomatoses were done by Fox and later refined by Kadonaga and Frieden [9]. However, the disease primarily affects children, mostly by the age of two years. [9] Usually they present with the features of hydrocephalus, cranial nerve palsies, hemiparesis, developmental delays, and seizures. [9,10]. Adult onset NCM is rarely reported in the literature which possesses a different clinical and radiological presentation. [11-13,2,14-19]. Patients having giant congenital nevi/ satellite nevi shows an increased tendency to develop malignant melanoma [15]. Due to its aggressive clinical course, patients exhibit poor neurological outcome despite surgery and adjuvant chemo-radiation. However, in the era of cytogenetics, targeted therapy can be a new hope for increased progression free survival and overall survival.
Case Presentation
History and Physical Examination
A 42-year-old female presented to the outpatient department for the evaluation of new onset headache for 3 months. The patient had no previous history of convulsion, visual disturbance, trauma, or any other neurological deficits. A detailed physical examination revealed a large congenital melanocytic nevus of about 5cm, distributed unilaterally across the right side of the patient’s face along V1 and V2 distribution, which she had since birth (Figure 1). Neurologically, she had features of papilledema and right sided superior temporal quadrantanopia. Other systemic examination revealed no abnormalities.
Imaging
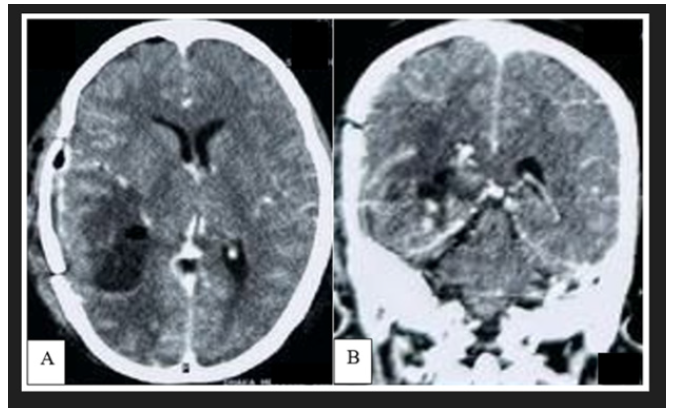
CT scan of brain demonstrated a well circumscribed hyperdense extra-axial lesion in posterior part of right temporal lobe, having attachment with part of tentorium cerebelli. The mass was surrounded by moderate vasogenic edema with no features of calcification (Figure 2). Computed tomography of the chest, abdomen, and pelvis was performed to look for any systemic involvement and was unremarkable. Brain Magnetic Resonance Imaging (MRI) showed a lobulated lesion of about 5x 4.7x 4cm diameter which was predominantly hyperintense in T1WI, intensely hypointense with a central isointese signal change in T2WI (Figure 3A,3B). After administration of gadolinium, there was intense heterogenous contrast enhancement owing to its high vascularity (Figure 3C). The lesion also exhibited similar hypointense signal change in FLAIR sequence with no restricted diffusion in DWI. Mass effect was evidenced by flattening of surrounding sulci and gyri with mild midline shifting. However, there were no features of hydrocephalus and similar lesion elsewhere in the brain (Figure 3D-3F) (Figure 3).

Figure 1: Photograph of the patient depicting cutaneous melanoma, along the V1 and V2 distribution of right trigeminal nerve, presenting since birth.

Figure 2: Preoperative non contrast CT scan of brain demonstrated a well-defined hyperdense lesion in right temporo-parietal region, surrounded by moderate perilesional edema with mass effect.

Figure 3: Preoperative MRI of brain, T1WI axial section (A) demonstrated a well delineated, lobulated, heterogeneously hyperdense lesion, located in posterior part of right temporal lobe. The mass become intensely hypointense in T2WI with evidence of moderate perilesional edema. Mass effect is evidenced by obliteration of surrounding sulci and gyri. However, no intrinsic or marginal flow voids observed (B). After administration of gadolinium, there is heterogeneous contrast uptake with presence of dural tail (marked by red arrow) (C). The lesion was heterogeneously hypointense in FLAIR sequence (D) with no evidence of restricted diffusion on DWI (E) or ADC map (F).
Surgery
Patient underwent neuron avigation guided temporo-parietal craniotomy and Gross Total Removal (GTR) of the tumor. After craniotomy, dark black pigmented dura matter with patchy islands of arachnoid and pial pigmentation observed (Figure 4). The tumor was recognized as a darkly pigmented mass in the posteromedial aspect of the right temporal lobe, which was firm, highly vascular and contained hemorrhage of different ages. After GTR, hemostasis ensured and layered closure done respecting the anatomical plane. The patient had an uneventful post-operative recovery. There was no new neurological deficit, and she was discharged on the 3rd postoperative day. Post-operative CT scan demonstrated complete resection of tumor with no evidence of tumor bed hematoma (Figure 5). On histopathology, the tissue was showing a malignant neoplasm composed of large round to polygonal as well as spindle cells having prominent nucleoli and scanty cytoplasm containing melanin pigment. Moreover, mitotic activity noted which was consistent with malignant melanoma. She referred to an oncologist for adjuvant therapy. After 3 months follow up, she had no features of recurrence.
Discussion
NCM is a rare phakomatoses of non-familial inheritance, originally described by Rokitansky [20]. Fox proposed the diagnostic criteria of NCM in 1972, [8] which later on revised by Kadonaga and Frieden [9] as 1. presence of large (20cm in adults) or multiple (≥3) congenital melanocytic nevi associated with meningeal melanosis or melanoma, 2. No evidence of cutaneous melanoma, except in patients in whom the examined areas of the meningeal lesions are histologically benign. 3. No evidence of meningeal melanoma, except in patients in whom the examined areas of the cutaneous lesions are histologically benign, after extensive review of 39 cases. They emphasized to rule out any evidence of primary cutaneous melanoma to distinguish this syndrome from a possible occurrence of primary melanoma with concurrent presence of brain metastasis. [9] Our case presented a large and multiple congenital nevus involving V1 and V2 distribution of right trigeminal nerve since her birth and histopathologic ally proven primary intracranial malignant melanoma (Figure 1). Simultaneously, there was no evidence of metastasis from other parts of the body. Based on the above-mentioned features, the patient satisfied the diagnostic criteria for NCM.
The pathogenesis of NCM is thought to be related with congenital dysmorphogenetic of neural crest–derived pluripotent precursor cells that later on affect the skin, meninges and eye and can manifest as Nevus of Ota [21,22]. In NCM, there is no involvement of pigment containing cell in the eye, whereas in Nevus of Ota there should be classical involvement of all three organs. Once thought to be a disease of childhood and early teenage years, NCM is now reported in adult population in last three decades (Table 1). According to a recent review of 74 patients of NCM, mean age of the reported cases was 16.6 years with a predilection to male sex. Dandy Walker Malformation was the most commonly reported syndromic association [10]. According to the embryogenesis, leptomeningeal abnormalities in MCM might affect the development of the fourth ventricle and the cerebellum, resulting in an increased association of DWM with NCM [19]. After diagnosis of a patient as a case of NCM, the incidence of developing malignant melanoma is exceedingly rare. In a review of 14 patients with congenital melanocytic nevi of all sizes, Krengel et al. followed this cohort for 3.4–23.7 years and observed 0.7% risk of developing malignant melanoma [23]. Since the description of Rokitansky in 1961, about 200 cases of NCM reported in English literature [15]. Only 10 cases of NCM with primary intracranial malignant melanoma which have both focal and diffuse component, have been reported in adult population and our case is the 11th instances for this exceedingly rare entity (Table 1).

Table 1: Reported cases of Malignant leptomeningeal melanoma with Neuro Cutaneous Melanosis (NCM) in adult patient.
Note*: M: Male, F: Female, HA: Headache, HCP: Hydrocephalus, DWM: Dandy Walker Malformation, ICH: Intracerebral Haemorrhage, GTR: Gross Total Resection, NTR: Near Total Resection, PR: Partial Resection, CT: Chemotherapy, RT: Radiotherapy, IFN β: Interferon β, OS: Overall survival, AWD: Alive with Disease, AWOD: Alive Without Disease.
The symptoms of NCM usually begin in the first two years of life and may present with signs of raised intracranial pressure, hydrocephalus, cranial nerve palsies, hemiparesis, developmental delays, and seizures [10,19]. Reported syndromic associations are Sturge Weber Syndrome, Dandy Walker Malformation, Syringomyelia, Intraspinal lipoma and Pial telangiectasis [24,9]. Other conditions, like Meckel’s diverticulum, Ureteral malformation, renal pelvis, renal cyst and rhabdomyosarcoma can be associated [14]. Those associated abnormalities are mostly noted in pediatric population whereas in adult it is rare. By contrast, our patient had only new onset headache at the age of 42 years, no syndromic association or any other clinical entities; thus late-onset NCM is extremely rare with minimal symptoms.
The diagnostic value of CT in tumors presenting as isodense or hyperdense lesions is poor [4]. Our reported case showed a well-defined hyperdense lesion in the right temporal lobe and this hyper density was due to the presence of intra-tumoral hemorrhage (Figure 2). Melanocytes are paramagnetic; because of this, both T1 and T2 relaxation times are shortened and thus hyperintense on T1-weighted images and hypointense on T2-weighted images [25,6]. The diamagnetic feature (calcification) and paramagnetic features (hemosiderin) would produce signal dropout and blooming, respectively, on susceptibility-weighted images. Phase-filtered images may also help differentiate hemosiderin-containing calcifications in meningioma versus microhemorrhages in melanoma, as calcifications will appear hyperintense from a negative phase shift, while hemosiderin will show loss of signal from a positive phase shift [26]. Rarely, melanoma might have both solid and cystic component where they can demonstrate heterogenous signal intensity in all sequences [14,15]. Beside this, intratumoral hemorrhage of different ages can exhibit heterogenous signal change too which can bias the radiologist with another hemorrhagic intracranial tumor [27]. Our reported case demonstrated similar features with an additional presence of dural tail which mimics the diagnosis of tentorial meningioma (Figure 3). Besides this, multiple linear high-intensity regions can be observed on the surface, within cerebral fissures or sulci which indicates leptomeningeal spreading. However, this pattern of enhancement is not pathognomonic for leptomeningeal melanosis and can be observed in infectious, inflammatory, and leptomeningeal carcinomatosis of the central nervous system. [12].
CNS melanomas have heterogeneous histological features and are classified as four subtypes, the most common of which is epithelioid; other subtypes are pleomorphic, spindle-shaped, and mixed cell types [28, 5,29]. Ge, et al. observed alveolar clusters and sheets of polygonal and elongated cells with irregularly contoured nuclei and prominent nucleoli and eosinophilic cytoplasm, containing fine brown granular pigments in his reported case. Primary leptomeningeal melanomas also demonstrate cellular pleomorphism, mitotic activity, and necrosis with hemorrhage [12]. Immunohistochemistry confirms the diagnosis of these rare neoplasms. Although S-100 represents a nonspecific marker for melanomas, as it also reacts with gliomas and meningiomas, HMB-45 has a far greater specificity for melanomas [30]. Notably, unlike meningiomas, CNS melanocytic lesions are generally negative for epithelial membrane antigen [1].
Treatment options for primary CNS melanoma mainly involve surgical excision, usually with good results, while radiotherapy, chemotherapy and immunotherapy may be given as adjuvant therapies. [21,10,31] Irradiation can be given as whole-brain radiotherapy or stereotactic radiation therapy directed to the site of the tumor. One of the reported patient received whole brain irradiation and intrathecal chemotherapy 15 mg methotrexate-MTX, 5mg dexamethasone for 8 weeks with complete remission.
However, he ended up receiving monthly intrathecal chemotherapy and due to noncompliance, he succumbed [4]. Other chemotherapy agents that can be used are MTX, dacarbazine, vincristine, temozolomide, and interferon. However, there is no established treatment protocol due to the rarity of these tumors [32]. Once symptomatic, patients with NCM have a poor prognosis. In a series of 71 cases, mortality occurred in 55 patients (77%), with a median survival time of 6.5 months after symptom onset and a median age at death of 4.5 years [31]. Beside this, leptomeningeal lesions associated with NCM usually undergo malignant transformation and have very poor prognosis. In a study (n=160) of patients with large cutaneous melanocytic nevi (LCMN) who were followed prospectively for an average of 5.5 years in the New York University (NYU)-LCMN Registry, Bittencourt et al. found that the 5-year cumulative life-table risk for developing melanoma was 2.3% [33]. This observation was similar with the case, reported by Ma and his colleagues who concluded that the congenital giant nevus involving the head, neck, back and buttocks where melanin cell "satellite nevi " appear, the development of malignant intracranial melanoma is higher [15].
Learning point from our case report is the presence of primary intracranial malignant melanoma with diffuse leptomeningeal seeding in association with congenital nevi, is rarely seen in an older patient, especially in black ethnicity. Considering the age, sex and radiological presentation, this case mimicked tentorial meningioma. Early detection and timely surgical intervention plays pivotal role for progression free survival and overall survival [34,35].
Conclusion
Our case is exceptional because of the late diagnosis of NCM in a 42-year-old patient with atypical radiological and clinical features further adding to the rarity of similar reported cases in the literature. A giant nevus or multiple congenital nevus combined with malignancy of the central nervous system should raise suspicion for NCM, regardless of age.
Acknowledgement
None.
Conflict of Interest
The authors declared no conflict of interest.
Funding
This research received no grant from any source.
References
- Balakrishnan R, Porag R, Asif DS, Satter AM, Taufiq M, et al. (2015) Primary intracranial melanoma with early leptomeningeal spread: A case report and treatment options available. Case Rep Oncol Med 2015: 293802.
- Kimura H, Itoyama Y, Fujioka S, Ushio Y (1997) Neurocutaneous melanosis with intracranial malignant melanoma in an adult: a case report. No Shinkei Geka 25(9): 819-822.
- Sommer L (2011) Generation of melanocytes from neural crest cells. Pigment Cell Melanoma Res 24(3): 411-421.
- Pan Z, Yang G, Wang Y, Yuan T, Gao Y, et al. (2014) Leptomeningeal metastases from a primary central nervous system melanoma: A case report and literature review. World J Surg Oncol 12: 265.
- Jaiswal S, Vij M, Tungria A, Jaiswal AK, Srivastava AK, et al. (2011) Primary melanocytic tumors of the central nervous system: A neuroradiological and clinicopathological study of five cases and brief review of literature. Neurol India
- Wadasadawala T, Trivedi S, Gupta T, Epari S, Jalali R, et al. (2010) The diagnostic dilemma of primary central nervous system melanoma. J Clin Neurosci 17(8): 1014-1017.
- Smith AB, Rushing EJ, Smirniotopoulos JG (2009) Pigmented lesions of the central nervous system: Radiologic-pathologic correlation. Radiographics 29(5): 1503-1524.
- Fox H (1972) Neurocutaneous melanosis. The phakomatoses. Handbook of clinical neurology 14: 414-428.
- Kadonaga JN, Frieden IJ (1991) Neurocutaneous melanosis: Definition and review of the Literature. Am Acad Dermatol
- Rahman RK, Majmundar N, Ghani H, San A, Koirala M, et al. (2022) Neurosurgical management of patients with neurocutaneous melanosis: a systematic review. Neurosurgical focus 52(5): E8.
- Chute DJ, Reiber K (2008) Three Unusual Neuropathologic‐Related Causes of Sudden Death. Journal of forensic sciences 53(3): 734-738.
- Ge P, Wang H, Zhong Y, Chen B, Ling F, et al. (2010) Rare presentation in an adult patient with neurocutaneous melanosis. NCM is a rare presentation in an adult patient. International journal of dermatology 49(11): 1311-1313.
- Kiecker F, Hofmann MA, Audring H, Brenner A, Labitzke C, (2007) Large primary meningeal melanoma in an adult patient with neurocutaneous melanosis. Clinical neurology and neurosurgery 109(5): 448-451.
- Liu BC, Wang YB, Liu Z, Jiao Y, Zhang XF, et al. (2022) Neurocutaneous melanosis with an intracranial cystic-solid meningeal melanoma in an adult: A case report and review of literature. World J Clin Cases 10(15): 5025-5035.
- Ma M, Ding ZL, Cheng ZQ, Wu G, Tang XY, et al. (2018) Neurocutaneous Melanosis in an Adult Patient with Intracranial Primary Malignant Melanoma: Case Report and Review of the Literature. World Neurosurg 114: 76-83.
- Qazi SS, Shah SM, Baqai MW, Enam SA (2022) Primary leptomeningeal melanoma in association with neurocutaneous melanosis: A case report. Surgical Neurology International 13: 547.
- Shinno K, Nagahiro S, Uno M, Kannuki S, Nakaiso M, et al. (2003) Neurocutaneous Melanosis Associated with Malignant Leptomeningeal Melanoma in an Adult: Clinical Significance of 5-S-Cysteinyldopa in the Cerebrospinal Fluid—Case Report. Neurologia medico-chirurgica 43(12): 619-625.
- Vadoud Seyedi R, Heenen M (1994) Neurocutaneous melanosis. Dermatology 188(1): 62-65.
- Walbert T, Sloan AE, Cohen ML, Koubeissi MZ (2009) Symptomatic neurocutaneous melanosis and Dandy-Walker malformation in an adult. Journal of clinical oncology 27(17): 2886-2887.
- Rokitansky J (1861) Ein Ausgezeichneter Fall Von Pigment-Malmit Ausgebreiteter Pigmentierung der Inneren Hirn-Und Ruchenmarkshaute. Allg Wien Med Z 6: 113-1136.
- Gupta A, Chacko G, Chacko AG (2021) Prevalence and pattern of leptomeningeal pigmentation in the human brain and its role in the safe surgical excision of extra-axial brain tumors. Neurol India 69(5): 1204-1209.
- Quillo Olvera J, Uribe Olalde JS, Alcántara Gómez LA, Rejón Pérez JD, Palomera Gómez HG (2015) Primary malignant melanoma of the central nervous system: A diagnostic challenge. Cir Cir 83(2): 129-134.
- Krengel S, Hauschild A, Schafer T (2006) Melanoma risk in congenital melanocytic naevi: a systematic review. Br J Dermatol 155(1): 1-8.
- De la Fouchardière A, Cabaret O, Pètre J, Aydin S, Leroy A, et al. (2015) Primary leptomeningeal melanoma is part of the BAP1-related cancer syndrome. Acta Neuropathol 12996): 921-923.
- Chen L, Zhai L, Al Kzayer LF, Sarsam SN, Liu T, et al. (2019) Neurocutaneous melanosis in association with large congenital melanocytic nevi in children: A report of 2 cases with clinical, radiological, and pathogenetic evaluation. Front Neurol 10: 79.
- Adams LC, Böker SM, Bender YY, Fallenberg EM, Wagner M, et al. (2017) Assessment of intracranial meningioma-associated calcifications using susceptibility-weighted MRI. J Magn Reson Imaging 46(4): 1177-1186.
- Das K, Nair A, Jaiswal S, Sahu R, Srivastava A, et al. (2017) Supratentorial intermediate grade meningeal melanocytoma with intratumoral bleed in the background of neurocutaneous melanosis: Report of an unusual case and review of literature. Asian J Neurosurg 12(1): 98-102.
- Bhandari L, Alapatt J, Govindan A, Sreekumar T (2012) Primary cerebellopontine angle melanoma: A case report and review. Turk Neurosurg 22(4): 469-474.
- Krpan AM, Rakusic Z, Herceg D (2020) Primary leptomeningeal melanomatosis successfully treated with PD-1 inhibitor pembrolizumab: A case report. Medicine 99(50): e22928.
- Mattar MA, Maher H, Zakaria WK (2020) Intracranial malignant melanoma: An Egyptian institute experience. Interdiscip Neurosurg 26: 101370.
- Suranagi VV, Maste P, Malur PR (2015) Primary intracranial malignant melanoma: A rare case with review of literature. Asian J Neurosurg 10(1): 39-41.
- Tanoue N, Ummah FC, Hanada T, Takajo T, Kamil M, et al. (2018) Incidentally found primary cerebral malignant melanoma associated with Ota nevus-wide dissemination after an initial phase of slow growth. Hiroshima J Med Sci 67(1): 21-29.
- Bittencourt FV, Marghoob AA, Kopf AW, Koenig KL, Bart RS, et al. (2000) Large congenital melanocytic nevi and the risk for development of malignant melanoma and neurocutaneous melanocytosis. Pediatrics 106(4): 736-741.
- Araújo C, Resende C, Pardal F, Brito C (2015) Giant congenital melanocytic nevi and neurocutaneous melanosis. Case Rep Med 2015: 545603.
- Mohapatra A, Choudhury P (2020) An uncommon case of primary leptomeningeal melanoma in a 66-year-old white Caucasian male. Cureus 12(10): e10793.




 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.