Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
The Effect of Sprouting on the Nutritional Composition of Legumes and Its Impact on Human Health: A Comparative Study
*Corresponding author: Chioma S Udeh, Institute of public health, Enugu State University of Science and technology, Nigeria.
Received: September 30, 2024; Published: October 03, 2024
DOI: 10.34297/AJBSR.2024.24.003179
Abstract
Pigeon pea (Cajanus cajan) is a leguminous crop of significant nutritional and medicinal value, particularly in developing countries.
This study aimed to determine the ascorbic acid, phosphate, and protein content in pigeon pea (Cajanus cajan) during sprouting over a 5-day period. Pigeon pea seeds were sprouted, and samples were collected daily from day 0 to day 5. The ascorbic acid content was determined by titration with iodine solution, protein content was measured using the Kjeldahl method, and phosphate content was analysed spectrophotometrically.
The results showed that ascorbic acid content increased from 0.088 mg/100g on day 0 to 0.152 mg/100g on day 5 as the seeds sprouted. Protein content also increased, ranging from 0.63 mg/100g on day 0 to 1.66 mg/100g on day 3, before decreasing to 0.79 mg/100g on day 5. Phosphate content varied, starting at 0.275 mg/100g on day 0, peaking at 0.718 mg/100g on day 3, and then declining to 0.556 mg/100g on day 5.
This study demonstrates that sprouting significantly enhances the nutritional profile of pigeon pea, with increases in ascorbic acid and protein content. However, phosphate levels fluctuated during the sprouting process. These findings suggest that sprouting can be a useful method to improve the nutritional value of pigeon pea for food and feed applications.
Keywords: Pigeon pea, Cajanus cajan, Sprouting, Ascorbic acid, Protein, Phosphate, Nutritional value
Introduction
Ascorbic acid (Vitamin C)
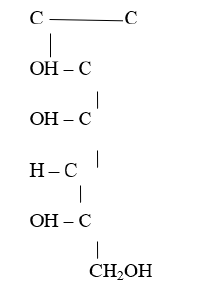
Ascorbic acid is a crystalline water-soluble substance that has a molecular weight of 176.13 datton and its molecular formula is C6H506 and it does not contain a free carboxyl group in its molecule. It has a sharp taste usually associated with acid and will form salt. It can be easily destroyed during extraction and oxidation 13 catalyzed by oxidase found in the cells of the food stuffs. They can also be set free on cutting, chopping or crushing [26] (Figure 1,2).
One important function of vitamin C is the formulation and maintenance of collagen the basis of connective tissue which are found in sun, ligament, cartilage, vertebral discs, joint, capillary walls, the bones and teeth [24].
Protein
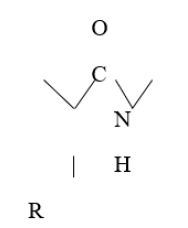
Proteins are organic compound made of amino acids arranged in a linear chain and folded into a globular form. The amino acid in a polymer is joined together by the peptide bond between the carboxyl and amino group of adjacent amino acid residues [19] (Figure 3).
The sequence of amino acids in a protein is defined by the sequence of a gene which is encoded by in the genetic code. In general the genetic code specifies 20 standard amino acids; however in certain organism the genetic code can include selenocystaine. Shortly after or even during synthesis, the residues in a protein are often chemically modified by post-translational modification which alters the stability, activity and ultimately, the function the proteins. . Proteins can also work together to achieve a particular function and they often associate to form stable complexes. Like other biological macromolecules such as polysaccharides and nucleic acid, protein is essential part of organisms and participates in virtually every process within cells [22]. Many proteins are enzymes that catalyze biochemical reactions and are vital to metabolism. Protein also has structural or mechanical factions such as action and myosin in muscles and the protein in the cystoskeletion, which maintains cell shape. Proteins are import in animal’s diet since animals cannot synthesis all the amino acid they need and must obtained essential amino acid from food. Through the process of digestion animals’ breakdown ingested proteins into free amino acid that are then used in metabolism [16].
Phosphate
Phosphate is an essential element in the body. The total body phosphate is about 25mol more than 85% of which is found in the bone, 15% in soft tissues and 1% is found in other part of the body. Phosphate uptake is sodium dependent about 85% of filtered Po4 is reabsorbed by the proximal tubules. The free energy produced by metabolic reaction may be stored as high phosphate ATP and creatine phosphate [11].
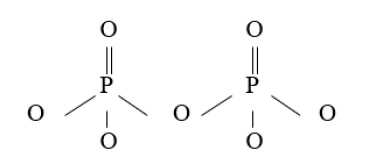
A phosphate san a inorganic chemicals, is a salt r prosperous acid. In organic chemistry, a phosphate or organophosphate is an ester of phosphoric acid. Organic phosphates are important in biochemistry and ecology. Inorganic phosphates ere mined to obtain phosphorus for use in agriculture and industry. At elevated temperature in the solid state, phosphate can condense to form pyrophosphate [3] (Figure 4).
The phosphate ion is a polyatornic ion with the molar mass of 94.737 g/mol. It consists of central phosphorus atom surrounded by four identical oxygen atoms in a tetrahedral arrangement. The phosphate ion car.ries a negative three formal charge and is the conjugate base of the hydrogen phosphate ion. Many phosphates are not soluble in water at standard temperature and pressure [18].
Pigeon pea (cajanus cajan)
The pigeon pea is a perennial member of the family fabaceae. It belongs to the genus known as Caianus ana its binomial name is Cajanus cajan. Pigeon pea is an important grain legume crop of rainfed agriculture in the semi- arid tropics. The Indian sub-continent, Eastern Africa and Central American in order are the world’s three main pigeon pea producing regions. It could be cultivated as sole crop or intermixed with such cereals as sorghum, pearl millet or maize. Being a legume, pigeon pea enriches soil through symbiotic nitrogen fixation [10].
Pigeon pea is both a food crop and a forage crop or cover crop. The dried pears may be sprouted briefly, and then cooked for a favour different from the green or dried peas. Sprouting also enhances the digestibility of dried pigeon pea [6]. Food is the primary necessity of life, it supplies energy, and it is utilized in building and maintaining the cells, tissue and in regulating the body processes. Legumes are beneficial for the overall health and wellness of individuals they are rich dietary source of good quality proteins, carbohydrate, minerals and vitamins.
This work is carried out to know the nutritional content and minerals, present in sprouting of pigeon pea.
Literature Review
Pigeon Pea (Cajanus Cajan)
Pigeon pea is one of the oldest food crops and rank fifth in important among edible legumes of the world [12]. It is a popular food in developing countries because it is rich in vitamin C another micronutrient, its green seeds are sources of flour, it can be used in split form to prepare soups or eaten with rice. In Thailand and Bengal, it serves as host for the scale insect that produce lac. In India and Java, pigeon pea young leaves are applied to sores. In Argentina, the leaf is used in treatment of genital and other skin bronchitis, cough, pneumonia, etc. The leaves are used for toothache, dysentery [4].
Seed and Seedling Description of Cajonus Cajan
Seeds range in colour from white, cream, brown, purplish to almost back and are plain or mottled, they are ellipsoid or squarish in shape. The seedling emerges 2 to 3 weeks after sowing, vegetative growth begins slowing but accelerate at 2 to 3 months. The seeding depth is always 2.5 to 10cm during its cultivation.
Matured Plant Description of Pigeon Pea
Pigeon pea is an erect shrub or short lived. (1-5 years) perennial legume often grown as an annual crop; 1 to 4 meters high. The leaves have three leaflets, the leaflets are elliptic to lonceolate green and pubescent above and silvery grayish green with longer hairs below, 2.5-10cm long and up to 3.5cm wide. The flowers are yellow with red/reddish brown lines or a red outside, borne in terminal racemes and measures 12-1 7cm in diameter.
The pods are straight to sickle shaped 5-10cm long and 0.5- 1.5cm wide [12].
Water and Nutrient Content
Optimum rainfall for pigeon pea is between 600-1000mm per year. It does not tolerate water logging well. It does have some drought tolerance. Pigeon pea can grow on infertile as well as fertile soil. It has a relatively low response to fertilizer. Bogdan reports pigeon pea responding well to P and modestly to K, N application usually reduce yields.
Soil PH, Type and Tolerance of Pigeon Pea
Pigeon pea grows best at a PH between 5.0-7.0. It will grow on a wide range soils from coarse to fine textured. It will not do well on waterlogged days, it has some salt tolerance, it is sensitive to salt spray. FAQ reports that pigeon pea is tolerant of herbicides [27].
Life Cycle of Pigeon Pea
Vegetative growth is slow initially, seeding emerge 2- 3 weeks after sowing, growth accelerates as about 2- 3 months. Half of the plants begin to flower with 56-210 days after sowing, maturity ranges from 95 to 256 days. short days will accelerate flowering and reduce lengths growth [8].
Seeding Methods and Flowering Date
FAQ, reports that the seedling method, it can be drilled with a maize drill. Year round at elevations between 0.3000 fit.
Flowering of half the plants begins 56 to 210 days after sowing maturity ranges from 95 to 256 days. Pigeon pea is a perennial shurb which grows to 4 meters high. It is woody at the base. It grows a maximum height of 4 meters.
Root System and Establishment
Pigeon pea has an extremely deep rooting top root. Van de Maesan, et al., [25] describes pigeon pea root as thin and up to 2m deep. It is establishment is by seed, weed control is needed during establishment.
Harvesting of Pigeon Pea
Pigeon pea is generally hand harvested in the tropics; ripe pods can be harvested with harvesters from cultivars which mature uniformly with pods at a uniform level above the ground. Pigeon pea is cut for forage at the preflowering state or when first pod ripens [21].
Uses of Pigeon Pea
It is used primary as a vegetable food crop in southeast Asia, it is recommended in Hawai for green manure for vegetable crops. intercropped with cereal used a forage or hay but not grazed. Branches and stons can be used for basket and fuel. It is also, used as a shade crop and wind break, cover crops or support. It is used to improve soil quality. It also has traditional medicinal uses [10].
The FAQ reports that pigeon pea grows well interplanted with forage grasses Rhodes, grass and molasses with pongola grass. After inter crop is harvested, pigeon pea continues to grow, produce and protect the soil.
Biotic Factors of Cajanus Cajan
FAQ reports that pigeon pea in Hawari is being attached by the same scale insects, a stem borer, a pod borer and leaf- eating caterpillars. The pest effects are on nematodes.
Nutritional Values of Pigeon Pea
Pigeon pea is a good source of proteins, carbohydrate and dietary minerals, such as calcium, phosphorus, magnesium, iron, sulphur and potassium. It is also good source of thiamin, riboflavin, niacin and grown pigeon pea cultivars ranges between 17.9 and 24.3g/100g [17]. Wide species of pigeon pea have high content of protein and several high protein genotype of about 32.5% (Singh, et al., 1990) (Table 1,2).
Nutritional Evaluation of Pigeon Pea and Its Cooking Characteristics
Pigeon pea which has a high source of lysine, and the other essential amino acid, carbohydrate and mineral can be used as supplement with cereal proteins [27].
The nutritional quality of the three cultivars of pigeon pea on which includes ICPL 313, ICPL 314 and desi cultivars was studied by determining the chemical composition, mineral element composition, amino acid composition, chemical score and biological evaluation. The protein contents of whole seed of the. three cultivars ranged from 20.78 to 21.5%, Fat from 1.53 to 1.89%, crude fiber from 8.29 to 8.63% and ash content of the whole seed where 6.22%, O.55%, 4.43% and 1.72% respectively.
Also, the protein, fat crude fiber and ash of the cooked dhals were 6.69%, 0.14%, 1.91% and 1.379% respectively.
Amino Acid Compositions of Pigeon Pea
(Table 3) Essential and non-essential amino acid were determined in the raw and cooked whole seed and their dhals. The proportion of glutanic acid was the highest followed by aspartic acid, pheylalnine and lysine, threonine, valine, leucine and isoleucine decreased in the whole seed of both local varieties and imported varieties on cooking. There was drastic reduction in the trytophan contents of the whole seed of all the three cultivars due to cooking. The effect of cooking on amino acid, content was greater in the dhal. Uncooked samples of the whole seed of cultivars ICPL 313 have the highest chemical score 53 and ICPL 314 had the lowest score of 48 in each .cultivar.
Methionihe was the first limiting amino acid and ICPL 314 tryptophan the second limiting amino acid. In the cooked samples of the whole seed of the three cultivars tryptohan was the first and methionine, the second limiting amino acid [9].
Minerals Composition of Pigeon Pea
Nine minerals’ elements, sodium, potassium, phosphorous, calcium, copper, zinc, iron and mangnese were determined in the raw cultivars and cooked whole and dhal of the three cultivars of pigeon pea, cooking cause significant decrease in the macro mineral element in whole seed and dlhal of the pigeon pea [25].
Biological Evaluation of Pigeon Pea
The biological values of the raw whole seed of the three-genotype ranged between 61 .6 to 69.8% Sand that of their dhal were between 62.4 to 76.6% these are no significant increase in biological values on cooking whole seed or dhal [22].
Materials
Chemical and Reagent for Ascorbic Acid, Protein and Phosphate
Acid washed sand
Sulphuric acid
Distilled water
Iodine solution
Starch indicator
Zinc metal
Screen methyl red indicator
Hydrochloric acid
40% sodium hydroxide
Jik
Equipment and Glassware
Testube, pipettes, capillary tube, measuring cylinder, retort stand, metal spatular, beaker, glass burette, foil paper, absorbent cloth, round bottom flask, plastic funnel, volumetric flask, ceramic mortar and pestle, weighing balance, kjeldahl flask, mantle heater, electric shaker, distillation column, spectrophotorneter.
Sample Collection
Seed of pigeon pea used for this research purchased from the open market of in Enugu State. These selected were the harvested from immediate past planting season and were also made sure that they were not .infested with weevils to ensure their viability and germinability.
Sprouting.
Spouting is the practice of soaking, drawing and then rising seed at regular intervals until they germinate or sprouts. This can be a semi-automated or fully automated process when done on a large scale of commercial use.
Procedure for Sprouting
The pigeon pea seed was spread on a plastic tray, covered with cotton wool. A solution was prepared using Jik and water at the ratio of 1:4 respectively, the solution was sprinkled on top of the cotton wool to prevent fungal growth on the seed during germination. The tray was finally covered with aluminum foil.
3.4.0 Sample preparation for determination of vitamin C. Procedure.
10g of the sample of pigeon pea and 5g of acid washed sand was weighed and emptied into a mortar, the two samples were mixed and grinded thoroughly. 100ml of distilled H2O was measured into a round bottom flask and mixed with the grinded sample. The solution was allowed to shake for 2hours using the electric shaker. The sample was filtered using a filtering paper and the volume was measure, 5mI of the sample was pipette and 2.5m1 of 1m H2 SO4 was added into a conical flask, 1 ml of starch indicator was added stired, finally the sample was titerated using 0.05m of iodine solution.
Result
Ascorbic Acid (Vitamin C)
The result of ascorbic acid in the sprouting seeds from zero to day five
Table 4
Using 5g of acid washed sand and 100ml of distilled water for ascorbic acid extraction. Zero-day ascorbic acid in mg/g
Volume of extract = 50ml
Weight of sample = 10g
Volume of extracted sample used = 5mg
Initial value = 16.30
Final value = 16.40
Total value = 0.1
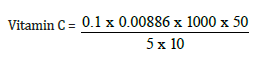
= 0.88mg/kg converting to grams 0.88/10 = 0.088mg/100g
DAY 1 ascorbic acid in mg/100g
Volume of extract = 52ml
Weight of sample = 10g
Initial value = 16.00
Final value = 16.10
Total value = 0.1
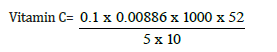
Vitamin C = 0.92mg/kg, converting to grams
0.92/10 = 0.092mg/100g
DAY 2
Volume of extract = 60ml
Weight of sample = 10g
Initial value = 24.00
Final value = 24.10
Total value = 0.1
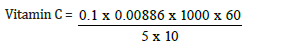
Vitamin C = 1.06mg/kg, converting to gram
1.06/10 = 0.106mg/100g
DAY 3
Volume of extract = 69ml
Weight of sample = 10g
Initial value = 25.00
Final value = 25.10
Total value = 0.1
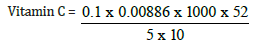
Vitamin C = 1.22mg/kg, converting to gram
0.122/10 = 0.122mg/100g
DAY 4
Volume of extract = 80ml
Weight of sample = 10g
Initial value = 28.00
Final value = 28.10
Total value = 0.1
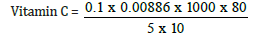
Vitamin C = 1.42mg/kg, converting to gram
1.42/10 = 0.142mg/100g
DAY 5
Volume of extract = 86ml
Weight of sample = 10g
Initial value = 42.00
Final value = 42.10
Total value = 0.1
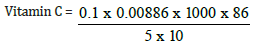
Vitamin C = 1.52mg/kg, converting to gram
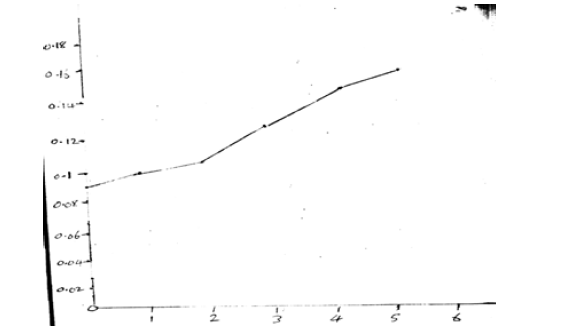
1.52/10 = 0.152mg/100g (Figure 5)
Protein
The result of protein in the sprouting seeds of pigeon pea from zero day to five
Table 5
DAY ZERO
Quantity of sample used = 0.5g
Initial value = 15.00
Final value = 18.60
Total value = 4.30

6.3/10 = 0.62
Protein = 0.63mg/100g
DAY 1
Quantity of sample used = 0.5g
Initial value = 15.00
Final value = 18.60
Total value = 3.6

6.3/10 = 0.62
Protein = 0.75mg/100g
DAY 2
Quantity of sample used = 0.5g
Initial value = 10.00
Final value = 17.7
Total value = 7.70
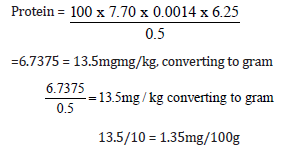
Protein = 1.35mg/100g
DAY 3
Quantity of sample used = 0.5g
Initial value = 0.00
Final value = 9.50
Total value = 9.50
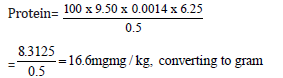
16.6/10 = 1.66mg/100g
Protein = 1.66mg/kg
DAY 4
Quantity of sample used = 0.5g
Initial value = 4.40
Final value = 9.50
Total value = 5.10
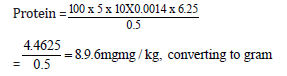
8.9/10 = 0.89mg/kg
Protein = 0.89mg/100g
DAY 5
Quantity of sample used = 0.5g
Initial value = 6.00
Final value = 10.50
Total value = 4.50
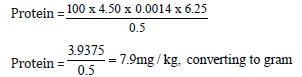
7.9/10 = 0.79mg/kg
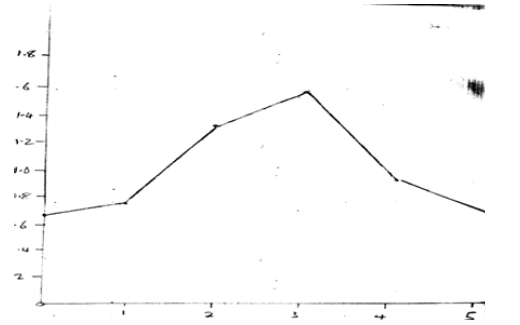
Protein = 0.79mg/100g (Figure 6)
Phosphate
The result of phosphate content in the sprouting of seed of pigeon pea from zero to day five
Table 6
ZERO
Weight of sample = 0.2g
Zero-day concentration = 0.088
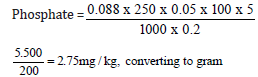
= 2.75/10
=0.275mg/100g
Phosphate=0.275mg/100g
DAY 1
Weight of sample = 0.29g
Day one concentration = 0.178
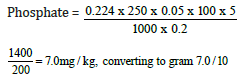
=0.70mg/100g
Phosphate=0.70mg/100g
DAY 2
Weight of sample = 0.2g
Day two concentration = 0.421
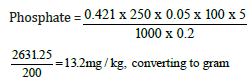
=13.2/10 = 1.32mg/100g
Phosphate=1.32mg/100g
DAY 3
Weight of sample = 0.2g
Day three concentration = 0.230
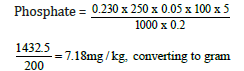
7.18/10 = 7.18mg/100g
Phosphate=0.718mg/100g
DAY 4
Weight of sample = 0.2g
Day four concentration = 0.204
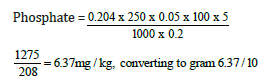
=0.63mg/100g
Phosphate = 0.637mg/100g
DAY 5
Weight of sample = 0.2g
Day four concentration = 0.178
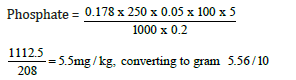
=0.556mg/100g
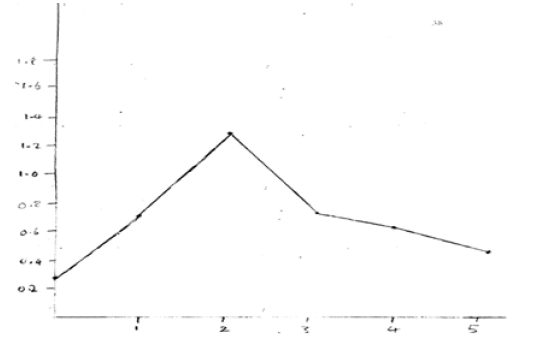
Phosphate = 0.556mg/100g (Figure 7)
Discussion
The results of this study demonstrate that sprouting significantly impacts the nutritional profile of pigeon pea (Cajanus cajan). Over the 5-day sprouting period, notable changes were observed in the concentrations of ascorbic acid, protein and phosphate.
The ascorbic acid content steadily increased from 0.088 mg/100g on day 0 to 0.152 mg/100g on day 5 as the seeds sprouted. This gradual rise in ascorbic acid can be attributed to the metabolic activation and synthesis of vitamin C during the germination process. Sprouting triggers enzymatic activities that enhance the conversion of precursor molecules into ascorbic acid. This finding is consistent with previous reports that sprouting improves the vitamin C content of various legumes and grains.
Protein content also increased during the sprouting period, rising from 0.63 mg/100g on day 0 to a peak of 1.66 mg/100g on day 3. However, protein levels then declined to 0.79 mg/100g by day 5. The initial increase in protein can be explained by the mobilization of storage proteins in the seeds to support the rapid growth and development of the sprouts. The subsequent decrease may be due to the utilization of proteins for metabolic activities and the synthesis of other biomolecules as the sprouts mature.
Phosphate content fluctuated throughout the sprouting process. It started at 0.275 mg/100g on day 0, increased to 0.718 mg/100g on day 3, and then decreased to 0.556 mg/100g by day 5. The changes in phosphate levels can be attributed to the dynamic role of phosphorus in various biochemical pathways involved in energy metabolism, cell signaling, and structural components during seed germination and seedling growth.
The observed changes in the nutritional parameters of pigeon pea during sprouting demonstrate the potential of this simple processing technique to enhance the bioavailability and quality of this important legume crop. The increases in ascorbic acid and protein content are particularly significant, as these nutrients play crucial roles in human health and nutrition.
Funding
Not Applicable.
Consent of Publication
All authors are aware of this publication
Data Availability
Not applicable.
Ethical Approval
Not Applicable.
Acknowledgement
None.
Conflict of Interest
None.
References
- Abrams DF (1979) Pigeon pea for health, longevity and economy. Provoker press: 45-48.
- Akabar G, steenbock H, Desikachar H (1995) The effect of germination, stage of maturity and variety upon the nutritive value of pigeon pea proteins. Journal on Nutrition 11(3): 66 -69.
- Babal V0, Hadley H, Hymowitz T0 (2005) Changes in carbohydrate contents of germinating pigeon pea seeds. Wikipeadia press: 41-49.
- Bleigh EG (1997) Composition of foods, Agriculture Handbook. Washington: 22-25.
- Bogdan AV (1999) Tropical pasture and folder plants, logman inc: 325-328.
- Crawfurd DE, Bedsheh V0, Sattar MV, Nizakat (1999) Effect of irradiation and other processing methods on in vitro digestibility of rapseed protein. Journal of Applied science and Environmental Management 16(5): 273-275.
- Dahati MO (2006) Effect of sprouting on in vitro digestibility of some focally consumed leguminous seeds. European Journal of Applied science 10(3): 55- 58.
- Duke JA, Uzogara GS, Anyakuju EO, onyeike EN (1997) Handbook of legumes of world economic importance, plenum press: 80-85.
- Evans DO (2001) Cover crop for orchards in Hawaii, Hawaii: 40-49.
- Kay DE (2007) Legumes crops. : 55-86.
- Jaypee MO (1999) Legumes Nutritional quality of plant foods. pudoc press wageningen Nutherlands: 39-42.
- Morton JF (1996) The pigeon pea a high protein tropical bush legumes. wikipedia press: 49.
- Muzaffar AK (2004) Pigeon pea as important food sources. Pakistan Journal on Nutrition 3(1): 5-13.
- Neil WR, Reece (2001) Micro environmental controls on biornineralization, Collins professional and Technical Books: 685-698.
- Nelson OM, Wilfred M, Kingo V0 (2002) Principal of AgrGnomy 4°’ edition, W.H freeman and company: 130-180.
- Ogbuanude KL (2002) Introduction to plant physiology NIPASS publishers: 57-62.
- Onyenuga VA, Akapunam M, Ifechi (1995) Nigeria’s foods and feeding- stiffs. University of Ibadan: 19-22.
- Pambell V0, Jelfery W (2003) Principles of propagation by seeds. Prentice Hall of India: 50- 57.
- Rubins 0V (2006) Pulses and beans in human nutrition. American Journal on Nutrition 8(6): 91-97.
- Shipard I (2005) “Nutrition facts and analysis for pigeon pea, mature seeds, Journal of Nutrition data on legumes and legume product 12(9): 111-125.
- Sinha SK, Concon JM, Everson G (1997) Food legumes distribution, adaptability and biology of yield. FAQ production and protection paper: 1-17.
- Smith 0, Jully S (1993) Rapid micro-kjeldahl digestion of cereal grains and other biological materials. CRC press: 39-81.
- Tony F, Cliff R (1996) Sprouting of seeds and nutritional composition of seeds and sprouts. Journal of food science 12(3): 102-111.
- Tylor MY, Dick TH (1997) Proteins and protein concentration. Journal of food science 49(20): 491-497.
- Van der Maesen (1995) Cajanus Dc and Atylosia (Leguminosae). Wageningen, the Netherlands: 225-234.
- Waugh VN, Briggs MN, Enstrom EJ (1998) The function and metabolism of vitamirr C in man. luma press: 41-49.
- Willians MK, Dale BF, Vignouna AL (1998) Nutritional evaluation of pigeon pea, American Journal of food science and Nutrition 27(9): 93-101.

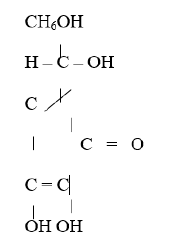




 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.