Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Calcium and Salicylic Acid Modulate Oxidative Stress Through Osmoregulation in Cadmium Stressed Chickpea (Cicer Arietinum L.)
*Corresponding author: Kamal Jit Singh, Department of Botany, Panjab University, Chandigarh (160014), India.
Received: January 11, 2025; Published: January 23, 2025
DOI: 10.34297/AJBSR.2025.25.003343
Abstract
The entry of heavy metal like Cadmium (Cd) in our agroecosystem have raised the world-wide concerns. Cd being a non-essential, non-biodegradable and toxic metal has a negative impact on growth and productivity of crop plants. We aimed at lowering Cd toxicity by supplementing Calcium (Ca) and Salicylic Acid (SA) through the study of parameters related to stress physiology and growth characteristics of chickpea. Both the supplements alone or in combination improved physiological and biological characteristics of Cadmium (Cd) stressed chickpea plants. A restoration of leaf pigments like chlorophyll and carotenoid including the membrane stability with low Electrolyte Leakage (EL) enhanced stress tolerance of the genotype. The combination treatment was effective in lowering Cd toxicity by lowering Malondialdehyde (MDA) and Hydrogen Peroxide (H2O2) content accompanied by proline build-up. The latter allowed better osmoregulation and antioxidation confirmed through the enhanced content of endogenous Ascorbic Acid (AsA). Thus, our findings suggest that Ca2+ competes with Cd2+ to reduce its accumulation and acts synergistically with SA that modulates oxidative stress by detoxification, osmoregulation and membrane stability.
Keywords: Abiotic stress, Antioxidants, Drought, Legumes, Lipid peroxidation
Introduction
Legumes as members of the family Fabaceae comprising beans, peas and lentils makes a part of staple diet in most parts of the world. Chickpea (Cicer arietinum L.) an annual legume of sub-family Faboideae is the second most extensively cultivated crop after soybean. Chickpea seeds are rich in lysine and arginine and low in Sulfur (S)-containing amino acids like cysteine and methionine [1]. Averaged protein content of the chickpea seeds is higher ( ≈18% ) than lentil and field pea [2]. Naturally occurring heavy metal elements in the earth’s crust persist for a long time in the environment following their release and are non-biodegradable [3]. They are toxic to microorganisms, plants, animals and humans having capacity to alter important metabolic pathways [4]. Cadmium (Cd) is one such toxic and non-essential heavy metal [5]. It is rapidly absorbed by the plant roots as Cd2+ and accumulates in various tissues affecting growth and productivity of crops [6]. Cadmium toxicity prevents the photo-activation of PS II by disrupting transfer of electrons [7]. Its accumulation inhibits photosynthesis, enzymes of Calvin cycle, carbohydrate metabolism, and antioxidant metabolism [8]. Therefore, Cd indirectly contributes to the generation of ROS with degradation of leaf chloroplasts [9].
Calcium, an essential macro-nutrient absorbed by plant roots is transported to shoots via xylem with a positive correlation between transpiration rate and its delivery to shoots [10]. Numerous studies have also emphasized the importance of Ca2+ as a secondary messenger in the transmission of abiotic and biotic signals in plants [11]. Due to physiochemical similarities, Cd2+ replaces Ca2+ in some Ca+2-binding proteins thus disrupting Ca-mediated signalling pathways [12]. Exogenous application of Ca+2 acts against abiotic stress by improving plasma membrane integrity and re-establishing calcium signal transduction system in plants [13].
Phytohormone Salicylic Acid (SA), the ubiquitous phenolic compound regulates the development and defence of higher plants through phytochelatins and/or inactivating Cd by combining with other molecules [14]. Exogenous application of SA at the lower concentrations enhances photosynthesis, growth, physiological and biochemical processes but higher doses may cause plant stress [15]. SA protects the membrane integrity with improved photosynthetic capacity by scavenging ROS and enhanced defence against Cd caused oxidative stress [16]. Based on the present information, we hypothesized that SA and Ca play a key role in crop improvement and tolerance against Cd induced stress. It was investigated through various physiological and biochemical parameters focusing on their underlying mechanisms.
Material and Methods
Chickpea (Cicer arietinum L. var. PBG-7) seeds were procured from Punjab Agricultural University, Ludhiana, Punjab, India. Healthy seeds were surface sterilized with 0.01% mercuric chloride followed by thorough washing with distilled water and overnight soaking in a thick slurry of rhizobium culture mixed with activated charcoal. Plants were raised in earthenware pots filled with approximately 5kg of washed river sand and were lined with perforated polythene bags. Only three healthy plants were selected after thinning in each pot. The plants were grown and maintained in dome shaped out-house under natural daylight conditions. Cadmium (CdSO4: 0.25 and 0.75 mM), Salicylic Acid (SA: 0.5 mM) and Calcium (CaCl2: 0.75 mM) treatments were given twice with a gap of 7d alone and in combination along with nutrient medium [17]. Control plants were irrigated with water and nutrient medium only. Observations were made with fresh leaf samples at the reproductive stage (150 DAS) of crop. Standardized procedures were followed to measure total content of chlorophyll and carotenoid [18]. The absorbance was read with Thermo-Scientific Evolution-201 UV-Visible Spectrophotometer at 663nm, 645nm and 480nm against 80% acetone (blank). Electrolyte leakage was measured as per [19]; malondialdehyde [20]; hydrogen peroxide [21]; proline [22] and ascorbic acid [23].
Statistical Analysis
All the values in triplicates were represented as mean ± SE (Standard Error). Data was statistically analysed using one-way ANOVA in SPSS-25 by taking the probability level of 5%. Least Significant Difference (LSD) post hoc test was used to compare the multiple comparisons of mean.
Results
Chlorophyll Content
Leaf chlorophyll content was reduced up to 44.58% (Cd0.25mM) and 64.74% (Cd0.75mM) with Cd in comparison to control. The pigment loss remained at 23.43% (Cd0.25) and 52.62% (Cd0.75mM) with added SA0.5mM; and at 25.76% (Cd0.25mM) and 53.55% (Cd0.75mM) with Ca0.75mM. Their combination (SA0.5+Ca0.75) treatment has restricted the loss of chlorophyll to 5.98% (Cd0.25mM) and 30.2% (Cd0.75mM) (Figure 1).
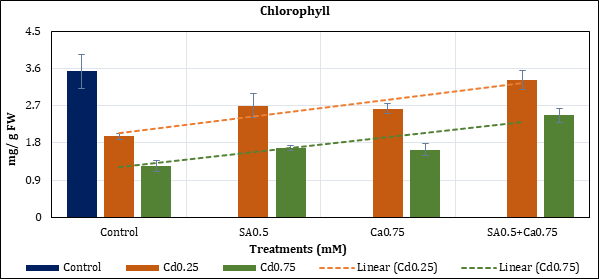
Figure 1: Effect of Cd alone and in combination with SA and Ca on total chlorophyll in chickpea plants. Each value represents the mean ± SE of three replicates. Mean difference is significant at P≤0.05 (LSD0.05=2.29).
Carotenoids
Leaf carotenoids were lowered up to 39.03% (Cd0.25mM) and 61.52% (Cd0.75mM) with Cd in comparison to control. The added supplements like SA and Ca have checked their depletion to 19.89% (Cd0.25mM) and 47.87% (Cd0.75mM) with SA0.5mM; and to 21.33% (Cd0.25mM) and 48.8% (Cd0.75mM) with Ca0.75mM. In combination treatment (SA0.5+Ca0.75mM), the green pigment loss remained at 4.41% (Cd0.25mM) and 31.45% (Cd0.75mM) (Figure 2).
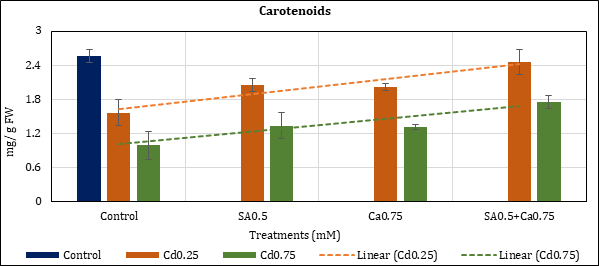
Figure 2: Effect of Cd alone and in combination with SA and Ca on carotenoids in chickpea plants. Each value represents the mean ± SE of three replicates. Mean difference is significant at P≤0.05 (LSD0.05=0.58).
Electrolyte Leakage
Cadmium treatments have enhanced the leakage of ions up to 23.34% (Cd0.25mM) and 32.79% (Cd0.75mM) in comparison to control. This leakage in SA and Ca added Cd treatments was 13.3% (Cd0.25+- SA0.5mM) and 26.29% (Cd0.75+SA0.5mM) to 16.34% (Cd0.25+Ca0.75mM) and 28.5% (Cd0.75+Ca0.75mM). The combination of SA+Ca has checked the Cd induced leakage of ions to 3.54% (Cd0.25mM) and 18.52% (Cd0.75mM) (Figure 3).

Figure 3: Effect of Cd alone and in combination with SA and Ca on electrolyte leakage in chickpea plants. Each value represents the mean ± SE of three replicates. Mean difference is significant at P≤0.05 (LSD0.05=2.83).
MDA Content
Malondialdehyde content of Cd stressed chickpea leaves has increased up to 29.02% (Cd0.25mM) and 65.66% (Cd0.75mM) in comparison to control. Its level remained higher than of control i.e., up to 17.09% (Cd0.25) and 51.14% (Cd0.75) with added SA0.5mM; and up to 23.41% (Cd0.25) and 56.17% (Cd0.75) with added Ca0.75mM. In their combined (SA+Ca) application the content increase was 3.44% (Cd0.25) and 33.04% (Cd0.75) (Figure 4).
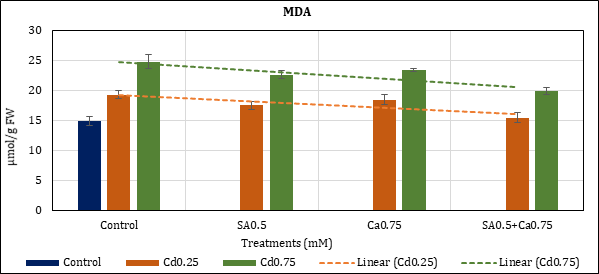
Figure 4: Effect of Cd alone and in combination with SA and Ca on MDA content in chickpea plants. Each value represents the mean ± SE of three replicates. Mean difference is significant at P≤0.05 (LSD0.05=2.57).
Hydrogen Peroxide
H2O2 accumulation in Cd stressed chickpea leaves went up to 45.78% (Cd0.25mM) and 69.74% (Cd0.75mM) in comparison to control (10.04 μmol). Added SA and Ca treatments to Cd checked its rise to 29.41% (Cd0.25+SA0.5mM) and 50.16% (Cd0.75+SA0.5mM); and to 36.29% (Cd0.25+Ca0.75mM) and 59.78% (Cd0.75+Ca0.75mM). In the combination treatment, rise of H2O2 was restricted to 7.71% (Cd0.25+SA0.5+- Ca0.75mM) and 27.98% (Cd0.75+SA0.5+Ca0.75mM) (Figure 5).
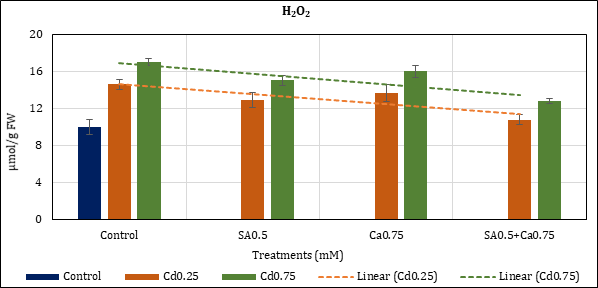
Figure 5: Effect of Cd alone and in combination with SA and Ca on H2O2 content in chickpea plants. Each value represents the mean ± SE of three replicates. Mean difference is significant at P≤0.05 (LSD0.05=2.21).
Proline
Accumulated contents of the osmolyte went up to 17.2% (Cd0.25mM) and 41.09% (Cd0.75mM) in Cd stressed chickpea in comparison to control (45.64 μmol). Their rise in SA0.5mM added treatment was 24.48% (Cd0.25) and 53.67% (Cd0.75mM); and in Ca0.75mM 21.9% (Cd0.25) and 50.12% (Cd0.75). The corresponding rise of proline in combined (SA0.5+Ca0.75 mM) Cd treatments remained at 32.31% (Cd0.25) and 68.41% (Cd0.75mM) (Figure 6).
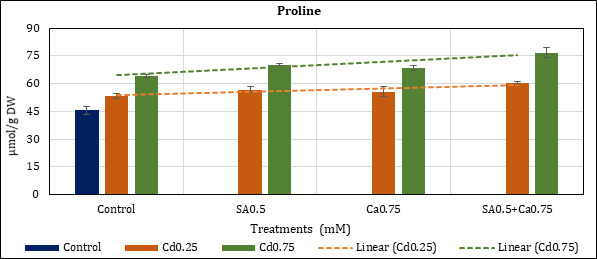
Figure 6: Effect of Cd alone and in combination with SA and Ca on proline content in chickpea plants. Each value represents the mean ± SE of three replicates. Mean difference is significant at P≤0.05 (LSD0.05=5.66).
Ascorbic Acid
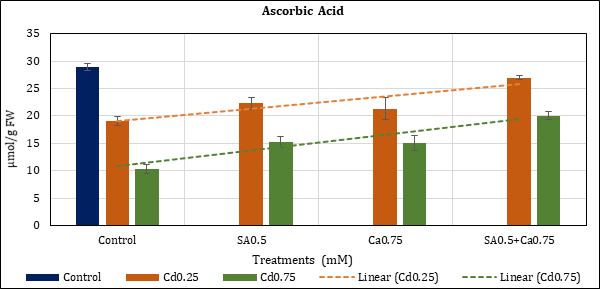
Figure 7: Effect of Cd alone and in combination with SA and Ca on ascorbic acid content in chickpea plants. Each value represents the mean ± SE of three replicates. Mean difference is significant at P≤0.05 (LSD0.05=3.67).
AsA content of the leaves dropped up to 33.85% (Cd0.25) and 64.11% (Cd0.75) with Cd in comparison to control. The loss with Cd added supplements remained at 22.86% (Cd0.25+SA0.5mM), 47.15% (Cd0.75+SA0.5mM), 26.23% (Cd0.25+Ca0.75mM), and 47.97% (Cd0.75+- Ca0.75mM). In the combined treatment (SA0.5+Ca0.75mM) loss of contents was checked to 6.74% (Cd0.25) and 30.59% (Cd0.75) (Figure 7).
Discussions
As observed the photosynthetic pigments like chlorophyll and carotenoid were negatively affected by Cd stress. Chlorophyll loss in the Cd exposed chickpea might be due to the inhibited synthesis of enzymes such as δ-aminolevulinic acid dehydratase and protochlorophyllide reductase involved in the chlorophyll biosynthesis and the impaired supply of Mg2+, Fe2+, Zn2+ [24-26]. The content restoration of both chlorophyll and carotenoids noticed with the application of Ca and SA appeared to have mitigated the effect of heavy metal stress, thus confirming the previous findings related to calcium. According to which Ca application maintained the chloroplast membrane, preventing chlorophyll decomposition and acting as a secondary messenger for cytokinin to promote chlorophyll biosynthesis [27,28]. The necessity of carotenoids for photosystem integrity was emphasized in protecting chlorophylls against photo oxidative damage with quenching of ROS [29]. SA directly involved in the cell signalling also acts as scavenger of hydroxyl radical and iron-chelating compound that inhibits their direct impact and generation [30].
The reduced photosynthetic pigments like chlorophyll and carotenoid accompanied the enhanced leakage of ions in Cd-contaminated chickpea [31], common bean [32] and pea [33]. The electrolyte leakage was largely suppressed in our case by using the combination treatment of SA and Ca in Cd-stressed chickpea. Thus, supporting the present investigations SA assisted low electrolyte leakage in Cd stressed chickpea provide protection against oxidative damage [34] and further Ca maintained the integrity of cell membrane by controlling its structure and function [35].
The over production of MDA in Cd stressed chickpea noticed in our study was involved in cell membrane damage through free oxygen radicals and is the product of lipid peroxidation [36]. Similarly, the build-up of H2O2 in present case pointed towards the existing environmental stress as an indicator signalling molecule [37]. Moreover, a check on the accumulating levels of MDA and H2O2 as stress indicators, has confirmed the antioxidative role of SA and Ca combination treatment in Cd stressed chickpea. Both these molecules act as endogenous signal molecules inducing the antioxidant response in protecting membrane damage against oxidative stress [38]. According to Li, et al. [39], Ca protects against Cd-induced oxidative injury through the avoidance of H2O2 generation as well as reduced uptake of Cd. It binds to the membrane phospholipids thus, stabilizing lipid bilayer and providing structural integrity [40]. The low endogenous AsA content possibly due to its oxidation was largely restored with supplemented SA and Ca thus, improving the tolerance of Cd stressed chickpea. According to Noctor and Foyer [41], AsA imparts stress tolerance by detoxifying ROS in the plant cells.
As noticed, the enhanced proline accumulation under Cd stress may be due to its over production or the reduced activity of proline degrading enzymes [42]. According to Matysik, et al., [43], the antioxidant nature of proline scavenges ROS and acts as singlet oxygen quencher. Proline as osmoprotectant and antioxidant protects the plants against environmental stresses [44]. The further build-up of proline with Ca and SA application thus confirmed the involvement of calcium in signalling and proline metabolism [45]. The enhanced proline synthesis with SA and/or Ca suggested their protective role against Cd stress in plants [46]. According to La, et al. [47], SA pre-treatment allows the additional proline accumulation with enhanced expression of proline synthesis related genes as a redox control measure during drought.
Conclusions
Growth and physiological characteristics of chickpea plants declined considerably with cadmium. Exogenous application of Ca and SA allowed the better osmoregulation through proline accumulation, ensuring membrane stability and synthesis of photosynthetic pigments. The cumulative effect of the used supplements enhanced the tolerance through lower MDA and H2O2 contents of leaves in Cd stressed chickpea.
Acknowledgements
The financial support of the University Grants Commission, New Delhi, India in conducting present investigation is gratefully acknowledged.
Conflict of Interests
The authors declare that there is no competing interest.
References
- Jukanti AK, Gaur PM, Gowda CLL, Chibbar RN (2012) Nutritional quality and health benefits of chickpea (Cicer arietinum): A review. British Journal of Nutrition 108(S1): S11-S26.
- Upadhyaya HD, Bajaj D, Narnoliya L, Das S, Kumar V, et al. (2016) Genome-wide scans for delineation of candidate genes regulating seed-protein content in chickpea. Frontiers in Plant Science 23(7): 302.
- Wu G, Kang H, Zhang X, Shao H, Chu L, et al. (2010) A critical review on the bio-removal of hazardous heavy metals from contaminated soils: issues, progress, eco-environmental concerns and opportunities. Journal of Hazardous Materials 174: 1-8.
- Hossain MA, Piyatika P, da Silva JAT, Fujita M (2012) Molecular mechanism of heavy metal toxicity and tolerance in plants: Central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. Journal of Botany 1-37.
- Sanità di Toppi L, Gabbrielli R (1999) Response to cadmium in higher plants. Environmental and Experimental Botany 41(2): 105-130.
- Amani AL (2008) Cadmium induced changes in pigment content, ion uptake, proline content and phosphoenolpyruvate carboxylase activity in Triticum aestivum Australian Journal of Basic and Applied Sciences 2: 57-62.
- Farooq MA, Ali S, Hameed A, Bharwana SA, Rizwan M, et al. (2016) Cadmium stress in cotton seedlings: physiological, photosynthesis and oxidative damages alleviated by glycine betaine. South African Journal of Botany 104: 61-68.
- Shi G, Liu C, Cai Q, Liu Q, Hou C (2010) Cadmium accumulation and tolerance of two safflower cultivars in relation to photosynthesis and antioxidative enzymes. Bulletin of Environmental and Contamination Toxicology 85(3): 256-263.
- Gallego SM, Pena LB, Barcia RA, Azpilicueta CE, Iannone MF, et al. (2012) Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environmental and Experimental Botany 83: 33-46.
- White PJ (2001) The pathways of calcium movement to the xylem. Journal of Experimental Botany 52(358): 891-899.
- Volotovski ID (2011) Role of calcium ions in photosignaling processes in a plant cell. Biophysics 56(5): 778.
- Choong G, Li Y, Templeton DM (2014) Interplay of calcium and cadmium in mediating cadmium toxicity. Chemico-biological interactions 211: 54-65.
- Cramer GR, Läuchli A, Polito VS (1985) Displacement of Ca2+ by Na+ from the plasmalemma of root cells: A primary response to salt stress? Plant physiology 79(1): 207-211.
- Drazic G, Mihailovic N (2005) Modification of cadmium toxicity in soybean seedlings by salicylic acid. Plant Science 168(2): 511-517.
- Hayat Q, Hayat S, Ifran M, Ahmad A (2010) Effect of exogenous salicylic acid under changing environment: a review. Environmental and Experimental Botany 68(1): 14-25.
- Liu Z, Ding Y, Wang F, Ye Y, Zhu C (2016) Role of salicylic acid in resistance to cadmium stress in plants. Plant Cell Reports 35(4): 719-731.
- Minchin FR, Pate JS (1975) Effects of water, aeration and salt regime on nitrogen fixation in a nodulated legume: definition of an optimum root environment. Journal of Experimental Botany 26: 60-80.
- Arnon DI (1949) Copper enzyme in isolated chloroplast: Polyphenol oxidase in Beta vulgaris. Plant Physiology 24(1): 1-15.
- Lutts S, Kinet JM, Bouharmont J (1996) NaCl-induced senescence in leaves of rice (Oryza sativus L.) cultivars differing in salinity resistance. Annals of Botany 78: 389-398.
- Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplast-I: Kinetics stoichiometery of fatty peroxidation. Archives of Biochemistry and Biophysics 125: 189-198.
- Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Science 151(1): 59-66.
- Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant and Soil, 39(1): 205-207.
- Mukherji SP, Chaudhari MA (1983) Implications of water stress induced changes in the levels of endogeneous ascorbic acid and hydrogen peroxide in Vigna Plant Physiology 58: 166-170.
- Padmaja K, Prasad DDK, Prasad ARK (1990) Inhibition of chlorophyll synthesis in Phaseolus vulgaris seedlings by cadmium acetate. Photosynthetica 24: 399–405.
- Van Assche F, Clijsters H (1990) Effects of metals on enzyme activity in plants. Plant, Cell & Environment 13(3): 195-206.
- Küpper H, Küpper F, Spiller M (1996) Environmental relevance of heavy metal substituted chlorophylls using the example of water plants. Journal of Experimental Botany, 47(2): 259-266.
- Lechowski Z, Bialczyk J (1993) Calcium mediated cytokinin action on chlorophyll synthesis in isolated embryo of Scots pine. Biologia Plantarum 35(1): 53-62.
- Naeem M, Naeem MS, Ahmad R, Ihsan MZ, Ashraf MY, et al. (2018) Foliar calcium spray confers drought stress tolerance in maize via modulation of plant growth, water relations, proline content and hydrogen peroxide activity. Archives of Agronomy and Soil Science 64(1): 116-131.
- Behera RK, Mishra PC, Choudhary NK (2002) High irradiance and water stress induced alterations in pigment composition and chloroplast activities of primary wheat leaves. Journal of Plant Physiology 159(9): 967-973.
- Dinis TC, Maderia VM, Almeida LM (1994) Action of phenolic derivates (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Archives of Biochemistry and Biophysics 315(1): 161-169.
- Wani AS, Tahir I (2019) Screening of different chickpea varieties for their sensitiveness and tolerance to cadmium and/or salt stress. Journal of Advanced Plant Sciences 2: 104.
- Gallego SM, Benavides MP, Tomaro ML (1999) Effect of Cd ions on antioxidant defense system in sunflower cotyledons. Physiologia Plantarum 42: 49-55.
- Popova L, Maslenkova L, Yordanova R, Krantev A, Szalai G, et al. (2007) Salicylic acid protects photosynthesis against cadmium toxicity in pea plants. General and Applied Plant Physiology 34: 133-148.
- Agami RA, Mohamed GF (2013) Exogenous treatment with indole-3-acetic acid and salicylic acid alleviates cadmium toxicity in wheat seedlings. Ecotoxicology and Environmental Safety 94: 164-171.
- Siddiqui MH, Al Whaibi MH, Sakran AM, Basalah MO, Ali HM (2012) Effect of calcium and potassium on antioxidant system of Vicia faba under cadmium stress. International Journal of Molecular Sciences 13: 6604-6619.
- Luo H, Li H, Zhang X, Fu J (2011) Antioxidant responses and gene expression in perennial ryegrass (Lolium perenne) under cadmium stress. Ecotoxicology 20: 770-778.
- Maksymiec W (2007) Signalling responses in plants to heavy metal stress. Acta Physiologiae Plantarum 29: 177-187.
- Methenni K, Abdallah MB, Nouairi I, Smaoui A, Ammar WB, et al. (2018) Salicylic acid and calcium pre-treatments alleviate the toxic effect of salinity in the Oueslati olive variety. Scientia Horticulturae 233: 349-358.
- Li P, Zhao C, Zhang Y, Wang X, Wang J, et al. (2016) Calcium alleviates cadmium-induced inhibition on root growth by maintaining auxin homeostasis in Arabidopsis Protoplasma 253: 185-200.
- Hirschi KD (2004) The calcium conundrum. Both versatile nutrient and specific signal. Plant Physiology 136(1): 2438-2442.
- Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annual Review of Plant Physiology and Molecular Biology 49: 249-279.
- Charest C, Pan T (1990) Cold acclimation of wheat properties of enzymes involved in proline metabolism. Physiologia Plantarum 80: 159-168.
- Matysik J, Alia, Bhalu B, Mohanty P (2002) Molecular mechanism of quenching of reactive oxygen species by proline under stress in plants. Current Science 82: 525-532.
- John R, Ahmad P, Gadgil K, Sharma S (2009) Heavy metal toxicity: Effect on plant growth, biochemical parameters and metal accumulation by Brassica juncea International Journal of Plant Production 3: 65-76.
- Parre E, Ghars MA, Leprince AS, Bordenave M, Luc R, et al. (2007) Calcium signalling via phospholipase C is essential for proline accumulation upon ionic but not non-ionic hyperosmotic stresses in Arabidopsis. Plant Physiology 144(1): 503-512.
- Al Whaibi MH, Siddiqui MH, Basalah MO (2012) Salicylic acid and calcium-induced protection of wheat against salinity. Protoplasma 249: 769-778.
- La VH, Lee BR, Islam MT, Park SH, Jung H, et al. (2019) Characterization of salicylic acid-mediated modulation of the drought stress responses: Reactive oxygen species, proline and redox state in Brassica napus. Environmental and Experimental Botany 157: 1-10.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.