Case Report 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Combined Alopecia Areata and Acquired Hypertrichosis Lanuginosa in Celiac Disease- A Rarest Phenomenon
*Corresponding author: Parveen Malhotra, Department of Medical Gastroenterology and Obstetrics & Gynaecology, PGIMS, Rohtak, Haryana, India.
Received: February 12, 2025; Published: February 18, 2025
DOI: 10.34297/AJBSR.2025.25.003377
Abstract
Celiac disease, also known as gluten-sensitive enteropathy or non-tropical sprue, is immune related disorder of small bowel which is seen in genetically predisposed people and is due to permanent intolerance to wheat gliadins and other cereal prolamins. CD is often associated with extraintestinal manifestations which includes several skin diseases like Alopecia Arerata (AA) and Acquired Hypertrichosis Lanuginosa (AHL). We hereby are reporting two cases of Alopecia areata but in one of them it was associated with AHL which is very rare and can be the first case report in the literature, as we were not able to search the combination of both AA and AHL in CD.
Keywords: Celiac Disease, Dermatological diseases, Alopecia arerata, Endoscopy, Acquired hypertrichosis lanuginosa
Introduction
Celiac Disease (CD) is an immune-mediated disorder of small bowel that is seen genetically predisposed people [1]. The high content of glutamine and proline in wheat, rye, oat and barley are the major culprit for causing CD [2]. The presentation of CD with typical gastrointestinal symptoms in the past has shifted towards atypical symptoms in half of the cases [3]. CD is characterized by intestinal malabsorption and intestinal villi atrophy which improves after Gluten-Free Diet (GFD) [4]. The classic form of CD presents with diarrhea, abdominal pain, weight loss and nutritional deficiencies, particularly of iron, folate, calcium, and vitamin D [5]. The extraintestinal manifestations include anaemia [6], transaminitis [7], osteopenia [8], neurological [9], psychiatric and affective disorders [10-12], hyposplenism [13] and autoimmune diseases [4]. Now we have sufficient documented evidence of involvement of skin diseases among the extra-intestinal manifestations of CD [14,15]. Alopecia Areata (AA) is a chronic autoimmune disease characterized by nonscarring alopecia which presents histologically as perifollicular lymphocyte infiltration, hinting toward immunologic aetiology like CD, as both have organ-specific auto-antibodies [16-18], T-lymphocyte infiltration at the site of lesion [19,20], association with HLA genes [21,22] and possible etiologic importance of viral co-factors [23,24]. The general prevalence of CD in population is 1 in 305 [25] and of AA is 1 in 819 [26]. Corazza and co-workers [27] found that prevalence of CD in patients with AA was 1 in 85. The remission and recurrence can be seen in the clinical course of AA [28], and even gluten-free diet may lead to complete regrowth of scalp and other body hair and no further recurrence of AA at follow-up [29-31] due to normalization of the immune response. This study suggests that AA patients constitute a novel risk group of celiac disease, thus screening for it should be done in all cases of alopecia universalis [29]. The Acquired Hypertrichosis Lanuginosa (AHL) is a rare disease and there are less than 40 cases in the literature [32,33]. There is rapid growth of fine, down-like hair all over the body, occasionally associated with glossitis and loss of taste. A lesser degree of hypertrichosis in which lanugo hair occurs only on the face is more common. It is three times more common in females than in males and most commonly associated with malignancy like lymphomas, carcinoid tumours, and the Zollinger- Ellison syndrome [34,35]. In 1988, Corazza, et al. [34] described the only case reported in the literature of acquired hypertrichosis lanuginosa associated with CD which confirms the paraneoplastic features of AHL CD is associated with tumours and may even precede clinical presentation of the tumours.
First Case Report
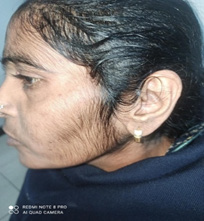
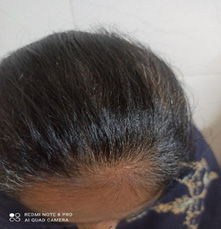
A thirty-five-year male old female presented with long duration of abdominal pain, diarrhoea for last one year and thinning of hairs on scalp & abnormal growth of hairs on face for last six months. On evaluation, she was diagnosed to be suffering from celiac disease. The serum IgATTG antibodies level was raised to the tune of 152 I.U./ ml and moderate scalloping of duodenal folds was seen on upper gastro-intestinal endoscopy and on histopathological examination, Marsh grade 3 celiac disease was diagnosed. The general physical examination showed thinning of hairs on scalp and Prescence of thin lanugo type of hairs on both sides of faces. The systemic examination including gynaecological & neurological, biochemical labs including thyroid and blood sugar, viral screen, ultrasonogram abdomen, computed tomography scan of brain & chest X-ray was normal. There was no significant drug history which can lead to AHL like oral contraceptives, steroids, phenytoin etc. Moreover, no evidence of internal malignancy or polycystic ovarian syndrome was pin-pointed in this case. She is on gluten free diet for last three months and response of same is awaited (Figures 1-4).
Second Case Report
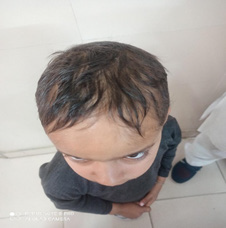
A ten-year male presented with short stature and alopecia for last two years. On evaluation, he was diagnosed as celiac disease on basis of increased serum IgATTG antibodies level of 130 I.U./ ml, severe scalloping of duodenal folds and Marsh grade 2 celiac disease on histopathological examination. The general physical examination revealed short stature and alopecia but rest systemic examination, biochemical labs including thyroid profile & blood sugar, viral screen, ultrasonogram abdomen, chest X-ray were normal. He has been put on strict gluten restricted diet for last two months and response is awaited.
Discussion
Acquired Hypertrichosis Lanuginosa (AHL) is a rare cutaneous disorder usually associated with internal malignancy that consists of the development of abnormal hair growth of the lanugo type, often confined to the skin of the face and neck, although other areas also may be involved. The cause of AHL is not clear, but it’s often associated with malignancy, metabolic and endocrine disorders and use of certain drugs. AHL can present before, with, or after the malignancy and can act as tumour spy [34]. In women, the most frequent malignancy associated with AHL is colorectal cancer, followed by lung cancer and breast cancer. In men, lung cancer is the most frequently associated malignancy, followed by colorectal cancer [36,37].
In alopecia areata hair loss can manifest in different patterns, most frequent being small annular or irregular lesion localized on the scalp, being able to progress until total hair loss occurs. AA is relatively more commonly seen than AHL in celiac diseases which is rare but combined Prescence of both of them in same patient is rarest. We tried our level best to review literature but were not able to find any case report or study which documented combined occurrence of AA and AHL in same case. Thus, it is highly probable that our can be the first case report in the world which has shown that AA and AHL can occur simultaneously in a celiac disease patient. The wide spectrum of clinical presentation with multiple extra-intestinal manifestations, reinforces the need of awareness among health care professionals for timely diagnosis of CD and its associations. It leads to early diagnosis and treatment i.e. strict gluten restriction in diet which can lead to reversal of many clinical presentations as well as extra-intestinal manifestations.
Conclusion
Celiac disease has many associations with different systems of human body but this is the first case report which highlights the combined association of CD with AA and AHL Hence, it reemphasizes the need of screening for celiac disease in all cases of AA and AHL.
Acknowledgement
None.
Conflict of Interest
No conflict of interest and prior permission from patient and relatives was taken before publishing the case report.
References
- Harris LA (2012) Celiac disease: clinical, endoscopic and histopathologic review. Gastrointestinal Endoscopy 76(3): 625-640.
- Volta U and Villanacci V (2011) Celiac disease: diagnostic criteria in progress. Cellular and Molecular Immunology 8(2): 96-102.
- Serra S and Jani PA (2006) An approach to duodenal biopsies. Journal of Clinical Pathology 59(11): 1133-1150.
- Corazza GR, Gasbarrini G (1995) Coeliac disease in adults. Baillieres Clin Gastroenterol 9(2): 329-350.
- Capristo E, Addolorato G, Mingrone G, De Gaetano A, Greco AV, et al. (2000) Changes in body composition, substrate oxidation, and resting metabolic rate in adult celiac disease patients after a 1-y gluten-free diet treatment. Am J Clin Nutr 72(1): 76-81.
- Howard MR, Turnbull AJ, Morley P, Hollier P, Webb R, et al. (2002) A prospective study of the prevalence of undiagnosed coeliac disease in laboratory defined iron and folate deficiency. J Clin Pathol 55(10): 754-757.
- Volta U, De Franceschi L, Lari F, Molinaro N, Zoli M, et al. (1998) Coeliac disease hidden by cryptogenic hypertransaminasemia. Lancet 352(9121): 26-29.
- Corazza GR, Di Sario A, Cecchetti L, Jorizzo RA, Di Stefano M, et al. (1996) Influence of pattern of clinical presentation and of gluten-free diet on bone mass and metabolism in adult coeliac disease. Bone18(6): 525-530.
- Addolorato G, Di Giuda D, De Rossi G, Valenza V, Domenicali M, et al. (2004) Regional cerebral hypoperfusion in patients with celiac disease. Am J Med 116(5): 312-317.
- De Santis A, Addolorato G, Romito A, Caputo S, Giordano A, et al. (1997) Schizophrenic symptoms and SPECT abnormalities in a coeliac patient: regression after a gluten-free diet. J Intern Med 242(5): 421-423.
- Addolorato G, Capristo E, Ghittoni G, Valeri C, Masciana R, et al. (2001) Anxiety but not depression decreases in coeliac patients after one-year gluten-free diet: a longitudinal study. Scand J Gastroenterol 36(5): 502–506.
- Addolorato G, De Lorenzi G, Abenavoli L, Leggio L, Capristo E (2004) Psychological support counselling improves gluten-free diet compliance in coeliac patients with affective disorders. Aliment Pharmacol Ther 20(7): 777–782.
- Corazza GR, Frisoni M, Vaira D, Gasbarrini G (1983) Effect of gluten-free diet on splenic hypofunction of adult coeliac disease. Gut 24(3): 228-230.
- Cardenas A, Kelly CP (2002) Celiac sprue. Semin Gastrointestinal Dis 13:232-244.
- Zone JJ (2005) Skin manifestations of celiac disease. Gastroenterol 128: S87-S91.
- Karpati S, Burgin-Wolff A, Krieg T, Meurer M, Stolz W, et al. (1990) Binding to human jejunum of serum IgA antibody from children with coeliac disease. Lancet 336(8727):1335-1338.
- Nunzi E, Hamerlinck F, Cormane RH (1980) Immunopathological studies on alopecia areata. Arch Dermatol Res 269: 1-11.
- Volta U, Lenzi M, Lazzari R, Cassani F, Collina A, et al. (1985) Antibodies to gliadin detected by immunofluorescence and a micro-ELISA method: markers of active childhood and adult coeliac disease. Gut 26(7): 667-671.
- Brandtzaeg P, Halstensen TS, Kett K, Krajci P, Kvale D, et al. (1989) Immunobiology and immunopathology of human gut mucosa: humoral immunity and intraepithelial lymphocytes. Gastroenterology. 97(6): 1562-1584.
- Perret C, Brocker EB, Wiesner-Menzel L, Happle R (1982) In situ demonstration of T cells in alopecia areata. Arch Dermatol Res 273(1-2): 155-158.
- Corazza GR, Tabacchi P, Frisoni M, Prati C, Gasbarrini G, et al. (1985) DR and non-DR Ia allotypes are associated with susceptibility to coeliac disease. Gut 26(11): 1210-1213.
- Zhang L, Weetman AP, Friedmann PS, Oliveira DB (1991) HLA associations with alopecia areata. Tissue Antigens. 38(2): 89-91.
- Kagnoff MF, Austin RK, Hubert JJ, Bernardin JE, Kasarda DD, et al. (1984) Possible role for a human adenovirus in the pathogenesis of celiac disease. J Exp Med 160(5): 1544-1557.
- Stankler L (1979) Synchronous alopecia areata in two siblings: a possible viral aetiology. Lancet 1(8129): 1303-1304.
- Catassi C, Ratsch IM, Fabiani E, Rossini M, Bordicchia F, et al. (1994) Coeliac disease in the year 2000: exploring the iceberg. Lancet 343(8891): 200-203.
- Safavi K (1992) Prevalence of alopecia areata in the First National Health and Nutrition Examination Survey. Arch Dermatol 128(5): 702.
- Corazza GR, Andreani ML, Venturo N, Bernardi M, Tosti A, et al. (1995) Celiac disease and alopecia areata: report of a new association. Gastroenterology 109(4): 1333-1337.
- Mitchell AJ, Krull EA (1984) Alopecia areata: pathogenesis and treatment. J Am Acad Dermatol pp. 763-775.
- Naveh Y, Rosenthal E, Ben-Arieh Y, Etzioni A (1999) Celiac disease-associated alopecia in childhood. J Pediatr 134(3): 362-364.
- Hovenden AL (1987) Acquired hypertrichosis lanuginosa associated with malignancy. Arch Intern Med 147(11): 2013-2018.
- Jemec GB (1986) Hypertrichosis lanuginosa acquisita. Report of a case and review of the literature. Arch Dermatol 122(7): 805-808.
- Ikeya T, Izumi A, Suzuki M (1978) Acquired hypertrichosis lanuginosa. Dermatologica 156(5): 274-282.
- Fretzin DF (1967) Malignant down. Arch Dermatol 95(3): 294-297.
- Corazza GR, Masina M, Passarini B, Neri I, Varotti C, et al. (1988) Acquired hypertrichosis lanuginosa associated with celiac syndrome. G Ital Dermatol Venereol 123(11): 611-612.
- Lowosky MS (1983) Malabsorption. In: Wetherall DJ, Lendicham JGG, Warrel DA. Oxford text book of medicine. Ed Oxford 1983; 1:1290. Pediatr Gastroenterol Nutr 23: 29±
- Sánchez-Estella J, Yuste M, Santos JC, Alonso MT (2005) Acquired paraneoplastic hypertrichosis lanuginose. Actas Dermosifiliogr 96(7): 459-461.
- Pratt H, King L, Messenger A, Christiano A, Sundberg J, et al. (2017) Alopecia areata. Nat Rev Dis Primers 3: 17011.




 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.