Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Electrocide Case Study on E. coli Food Borne Infection
*Corresponding author: The Sonnenschein Institute, USA; 6617 NW 24th Ave. Boca Raton, Florida 33496 USA.
Received: December 31, 2024; Published: January 07, 2025
DOI: 10.34297/AJBSR.2025.25.003317
Abstract
As demand for food increases with ever growing populations agriculture and indeed nature is being stressed to its limits and with this environmental pressure comes the additional rise of pathogens. Pathogens, unchecked can spread through the food supply and threaten severe consequences for human health. Oftentimes treatments for pathogens include heavy doses of antibiotics which may have long term consequences of the heath of the human gut biome. Recent studies have shown that Electrocide is very effective in reducing pathogen load of Staphylococcus aureus, Streptococcus pyogenes, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Proteus mirabilis/vulgaris strains of bacteria and indeed with regard to known antibiotic testing the Electrocide has been shown to be equally or more effective. In combination with microbiome testing the use of Electrocide with inoculation of stool of E. coli has shown to improve healthy bacteria after overnight exposure. In this case study a 30 year old who contracted a virulent form of food poisoning and was hospitalized with symptoms of HUS including high fever, skin reddening, bloody diarrhea and elevated kidney and liver metabolites and was treated with high doses of antibiotics 5 days without significant blood chemistry nor symptom reduction was given Electrocide supplement with Super Detox and returned to normal levels in 14 days including no diarrhea, normalized white blood cell and neutrophils, liver and kidney metabolites returned to normal levels proving in this case an effective supplement use to normalize gut microbiome including significant pathogen reduction.
Keywords: Haemolytic Uraemic Syndrome (HUS), Electrocide, E. coli, Food safety, Public outbreak, Severe abdominal cramps, Diarrhea, Vomiting, Fatigue, Fever, Undercooked Ground Beef, Unpasteurized Products
Abbreviations: HUS: Hemolytic Uremic Syndrome; IDSA: Infectious Disease Society of America; STEC: Shiga toxin-producing Escherichia coli; EHEC: Enterohemorrhagic Escherichia Coli
Haemolytic Uraemic Syndrome
Haemolytic Uraemic Syndrome (HUS) is a form of thrombotic microangiopathy affecting predominantly the kidney and characterized by a triad of thrombocytopenia, mechanical haemolytic anaemia, and acute kidney injury [1]. HUS encompasses disorders such as shiga toxin-induced and pneumococcus-induced haemolytic uraemic syndrome, haemolytic uraemic syndrome associated with complement dysregulation or mutation of diacylglycerol kinase ɛ, haemolytic uraemic syndrome related to cobalamin C defect, and haemolytic uraemic syndrome secondary to a heterogeneous group of causes (infections, drugs, cancer, and systemic diseases). Previously, genetic, experimental, and clinical studies have helped to decipher the pathophysiology of these various forms of haemolytic uraemic syndrome and undoubtedly improved diagnostic approaches. Moreover, a specific mechanism-based treatment has been made available for patients affected by atypical haemolytic uraemic syndrome due to complement dysregulation. Such treatment is, however, still absent for several other disease types, including shiga toxin-induced haemolytic uraemic syndrome [1]. According Riley, et al., [2], it is over thirty years since Shiga toxin-producing Escherichia coli was recognized as a human pathogen after the first outbreak investigated as haemorrhagic colitis in 47 patients in Oregon and Michigan. This E. coli O157:H7 strain was then considered to be rare, but the 1993 multistate outbreak from undercooked hamburgers at a fast-food chain gained national attention for this newly emerging pathogen [3].
As reported by Wong, et al., [4], Haemolytic Uraemic Syndrome (HUS) is a serious clinical complication of Enterohemorrhagic Escherichia Coli (EHEC) infection and the severity of a public outbreak is often discussed in terms of the HUS rate. HUS condition that can result from an infection by certain strains of E. coli, particularly E. coli O157:H7. This condition is characterized by a triad of symptoms; The destruction of red blood cells, leading to Haemolytic Anaemia, damage to the kidneys, often resulting in decreased kidney function and the need for dialysis in severe cases, and Thrombocytopenia: Low platelet count, which can increase the risk of bleeding [5]. HUS is a clinical composite of thrombocytopenia, hemolytic anemia and thrombotic microangiopathy that contributes to acute kidney injury, often requiring dialysis and can progress to acute renal failure and death. HUS often develops after a gastrointestinal infection caused by pathogenic strains of E. coli, which can be ingested through contaminated food or water. The bacteria produce toxins, such as Shiga toxin, which can damage blood vessels and lead to the symptoms associated with HUS. In addition to the classic triad, other symptoms may include: Severe abdominal cramps, Diarrhea (often bloody), Vomiting, Fatigue, and Fever. The risk factors include consumption of undercooked ground beef, unpasteurized milk or juice, and contaminated vegetables or fruits. Close contact with infected individuals or animals [6]. Epidemiology studies have shown that HUS typically develops in about 5%–15% of patients, but this varies between bacterial strains and geographic location. In terms of treatment, there is no specific treatment for HUS; care is generally supportive. Management may include fluid and electrolyte replacement, blood transfusions, and dialysis for kidney failure [7]. Preventive measures focus on proper food handling, cooking meat thoroughly, washing hands frequently, and avoiding unpasteurized dairy products [8].
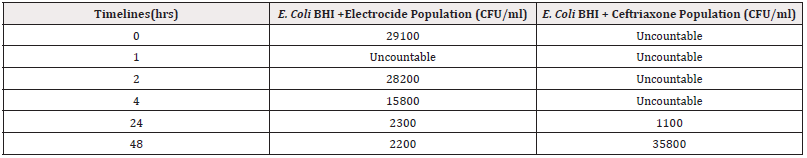
As reported by Safdar et al., [9], the use of antibiotics for treatment of Escherichia coli O157:H7 infection has become controversial since a recent small study found that it may increase the risk of HUS. However, other larger studies have reported a protective effect or no association. According to Tajiri, et al., and Michalopoulos, et al., [10,11], the use of antibiotics in Shiga Toxin-Producing Escherichia coli (STEC) infections has also been addressed in the Infectious Disease Society of America (IDSA) guidelines for the management of infectious diarrhoea. Their previous edition, published in 2001, stated that antibiotic administration should be avoided in suspected STEC infections, as their role remained unclear. The latest edition of the IDSA guidelines, published in October of 2017, strongly recommends against the use of antibiotics in infections caused by Stx2 producing STEC and considers the evidence insufficient for an analogous recommendation to be made for cases caused by non- Stx2 producing STEC strains. The assertions were further validated by a study by Sonnenschein, et al., (2024) [12] comparing the efficacy of Electrocide and a known antibiotic (Ceftriaxone)on E. Coli population over time as shown in Table 1 below in an in vitro study.
According to Varma, et al., (2003) [13], since E coli O157 can survive in the environment for more than 10 months, humans may be at risk of infection long after an environment is initially contaminated. Furthermore, Outbreaks have been linked to contamination of surfaces regularly touched by animals, such as the soil of pastures or railings in petting zoos [14-16]. There is evidence supporting airborne dispersion of E. coli O157 including attendance at the dance as an independent risk factor, anecdotes of dusty conditions during the dance, and widespread contamination of the building, including the rafters, which were out of the reach of humans and animals. As reported by Sonnenschein, et al., [17], the transmission pathways for health impacts of E. Coli contamination include foodborne illness which occurs when crops contaminated with E. coli are consumed raw or undercooked, they can cause gastrointestinal infections. E. coli can leach into drinking water sources from contaminated soil or runoff, posing health risks when ingested. Soil contaminated with E. coli can pose a risk, particularly for individuals who work in agriculture or live near contaminated areas [18]. The health risks range from gastrointestinal illness where pathogenic strains of E. coli, such as E. coli O157:H7, can cause severe diarrhea, abdominal cramps, vomiting, and fever [19,20]. In some cases, infections can lead to Haemolytic Uraemic Syndrome (HUS), a severe condition that can cause kidney failure. Certain strains of E. coli are a leading cause of Urinary Tract Infections (UTIs), which may arise from exposure to contaminated soil or water. Although rare, E. coli can potentially enter the bloodstream or other body systems, leading to more severe infections [21-23].
Case Study
Background, Results and Discussion
This study is based on a single patient admitted to the hospital diagnosed of a possible HUS. Based on interview, the patient exposure to improperly prepared food may have been the cause. In this study a 30-year-old who contracted a virulent form of food poisoning and was hospitalized with symptoms of HUS including high fever, skin reddening, bloody diarrhea and elevated kidney and liver metabolites and was treated with high doses of antibiotics for days without significant blood chemistry nor symptom reduction. The patient was hospitalized around July 4, 2024 until July 9, 2024 when the patient was released without being able to resolve the symptoms or make any difference to the blood chemistry results. The patient was introduced to Electrocide and Super Detox from July 10 and data collected on 24 and 30 July, 2024 where WBC (/ CMM) and Neutrophils % were compared as shown in Figure 1 and 2 respectively. The patient was blood chemistry returned to normal levels in 14 days including no diarrhea, normalized white blood cell and neutrophils, liver and kidney metabolites returned to normal levels proving in this case an effective supplement use to normalize gut microbiome including significant pathogen reduction [23,24] (Figure 1).
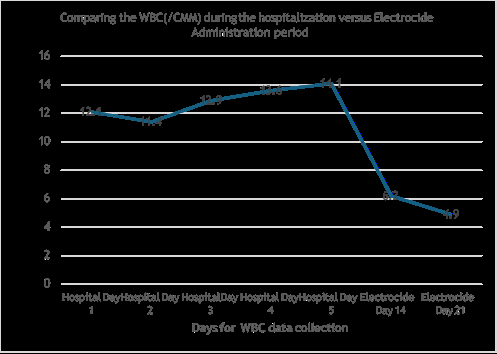
Figure 1: Comparing the WBC(/CMM) during the hospitalization versus Electrocide Administration period.
The white blood cells levels were noted to be increasing during the hospitalization period as shown in Figure 1. After being released from hospital and introduced to Electrocide and Super Detox, the levels of White blood cells reduced significantly to normal levels as shown in days 14 and 21 after the introduction of the Electrocide and Super detox as shown in Figure 1.
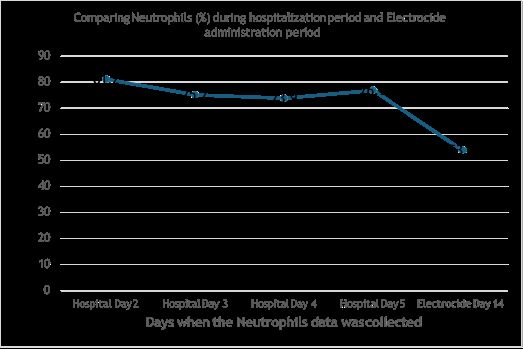
The neutrophils were high and fluctuating between 81.2% and 76.9 % during the hospitalization period. This levels significantly dropped to 53.9% as shown in Figure 2., 14 days after the patient was introduced to the Electrocide and Super detox.
Conclusion
In regard to healthy microbiome testing the use of Electrocide has shown to improve healthy bacteria after overnight exposure. In this case study a 30 year old who contracted a virulent form of food poisoning and was hospitalized with symptoms of HUS including high fever, skin reddening, bloody diarrhea and elevated kidney and liver metabolites and was treated with high doses of antibiotics 5 days without significant blood chemistry nor symptom reduction was given Electrocide supplement with Super Detox and returned to normal levels in 14 days including no diarrhea, normalized white blood cell and neutrophils, liver and kidney metabolites returned to normal levels proving in this case an effective supplement use to normalize gut microbiome including significant pathogen reduction.
Acknowledgement
None.
Conflict of Interest
None.
References
- Fakhouri F, Zuber J, Frémeaux Bacchi V, Loirat C (2017) Haemolytic uraemic syndrome. Lancet 390(10095): 681-696.
- Riley LW, Remis RS, Helgerson SD, McGee HB, Wells JG, et al. (1983) Hemorrhagic colitis associated with a rare Escherichia coli N Engl J Med 308(12): 681-685.
- Bell BP, Goldoft M, Griffin PM, Davis MA, Gordon DC, et al. (1994) A multistate outbreak of Escherichia coli O157: H7- associated bloody diarrhea and hemolytic uremic syndrome from hamburgers. The Washington experience. J Am Med Assoc 272(17): 1349-1353.
- Wong CS, Mooney JC, Brandt JR, Staples AO, Jelacic S, et al. (2012) Risk factors for the hemolytic uremic syndrome in children infected with Escherichia coli O157:H7: A multivariable analysis. Clin Infect Dis 55(1): 33-41.
- Mayer CL, Leibowitz CS, Kurosawa S, Stearns Kurosawa DJ (2012). Shiga Toxins and the Pathophysiology of Hemolytic Uremic Syndrome in Humans and Animals. Toxins 4(11): 1261-1287.
- Bitzan M, Lapeyraque AL (2016) Postinfectious Hemolytic Uremic Syndrome. In: Geary D, Schaefer F (eds) Pediatric Kidney Disease. Springer, Berlin, Heidelberg.
- Okumi M, Tanabe K (2016) Prevention and treatment of atypical haemolytic uremic syndrome after kidney transplantation. Nephrology (Carlton) 21 Suppl 1: 9-13.
- Kimmitt PT, Harwood CR, Barer MR (2000) Toxin gene expression by Shiga toxin- producing Escherichia coli: The role of antibiotics and the bacterial SOS response. Emerg Infect Dis 6(5): 458-465.
- Safdar N, Said A, Gangnon RE, Maki DG (2002) Risk of Hemolytic Uremic Syndrome After Antibiotic Treatment of Escherichia coli O157:H7 Enteritis: A Meta-analysis. JAMA 288(8): 996-1001.
- Hitoshi Tajiri, Junichiro Nishi, Kosuke Ushijima, Toshiaki Shimizu, Takashi Ishige, et al. (2015) A role for fosfomycin treatment in children for prevention of haemolytic–uraemic syndrome accompanying Shiga toxin-producing Escherichia coli International Journal of Antimicrobial Agents 46(5): 586- 589.
- Argyris S Michalopoulos, Ioannis G Livaditis, Vassilios Gougoutas (2011) The revival of fosfomycin. International Journal of Infectious Diseases 15(11): e732-e739.
- Sonnenschein Leonard, Etyang Tiberious, James Mwesigye, Dickson Kikonkyo, Fred Brown Ayella, et al. (2024) New Pathways for Anti-Pathogen Responsiveness Using Electrocide. Am J Biomed Sci & Res 24(6).
- Varma JK, Greene KD, Reller ME, Stephanie M DeLong, Janine Trottier, et al. (2003) An Outbreak of Escherichia coli O157 Infection Following Exposure to a Contaminated Building. JAMA 290(20): 2709-2712.
- Crump JA, Sulka AC, Langer AJ, Chad Schaben, Anita S Crielly, et al. (2002) An outbreak of Escherichia coli O157:H7 infections among visitors to a dairy farm. N Engl J Med 347(8): 555-560.
- Crampin M, Willshaw G, Hancock R, T Djuretic, C Elstob, et al. (1999) Outbreak of Escherichia coli O157 infection associated with a music festival. Eur J Clin Microbiol Infect Dis 18(4): 286-288.
- Ogden ID, Hepburn NF, MacRae M, N J C Strachan, D R Fenlon, et al. (2002) Long term survival of Escherichia coli O157 on pasture following an outbreak associated with sheep at a scout camp. Lett Appl Microbiol 34(2): 100-104.
- Sonnenschein Leonard and Etyang Tiberious (2024) T Cell Activation by Natural Mechanisms. Am J Biomed Sci & Res 21(6).
- Kumar V, Singh DP (2023) Interactions of Escherichia coli with soil microbiomes: Implications for environmental and health risk assessment. Environmental Microbiology Reports 15(2): 145-158.
- Liu Y, Zhang T (2022) The role of gut microbiome in the colonization and pathogenicity of Escherichia coli. Nature Reviews Microbiology 20(2): 98-112.
- Ryu JH, Hwang SY (2023) Gut microbiota modulation as a strategy to mitigate the pathogenicity of Escherichia coli Frontiers in Microbiology, 14, Article 945302.
- Khan, MT, Zubair M (2023) Influence of gut microbiomes on antibiotic resistance genes in Escherichia coli during intestinal dysbiosis. Microbiome 11(1): 45.
- Dai J, Wang Y (2022) Examining the interplay between gut microbiome composition and virulence factors of Escherichia coli in gastrointestinal diseases. Applied and Environmental Microbiology 88(6): e02239-21.
- Clemente JC, Pehrsson EC (2022) Gut microbiota and its interplay with Escherichia coli in human health and disease. Trends in Microbiology 30(3): 233-246.
- Sonnenschein L, Etyang T, Shah R, Frischer R, Rein, et al. (2021) The Effect of An Aqueous Electricidal Solution on General Well Being. Am J Biomed Sci & Res 13(4).



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.