Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Beyond the Kidney-Klotho and the Cardiovascular System
*Corresponding author: Jorge Carlos Trainini, Hospital Presidente Perón, Buenos Aires, Argentina. Universidad Nacional de Avellaneda, Argentina.
Received: February 20, 2025; Published: February 24, 2025
DOI: 10.34297/AJBSR.2025.25.003388
Abstract
Introduction: The suction produced in the early diastolic phase of myocardial contraction cannot be explained by a passive mechanism given the low gradients reached at the inlet of the atria and should be considered as a key element to facilitate venous return. Since a variation in the ventricular suction mechanism could be an initial, even subclinical, stage of ventricular dysfunction, the objective of this analysis was to identify whether there is a relationship between the parameters that determine the deterioration of the early diastolic phase of left ventricular myocardial contraction and heart failure with preserved ejection fraction.
Material and Methods: A retrospective study was conducted on echocardiographic studies performed in the last twelve months. The population to be studied consisted of three groups. Group I: ten (10) young patients without heart disease, average age 30.3±9.2 years; Group II: ten (10) adult patients without heart disease, average age 66.2±4.1 years; Group III: ten (10) patients with heart failure with preserved ejection fraction, average age 81.1±11.3 years. Variables analyzed: cardiac cycle (ms); left ventricular systole (ms); protodiastolic phase of left ventricular myocardial contraction (ms); left ventricular diastole (ms); relative wall thickness (%); left ventricular mass (gr/m2); E/E´ ratio; left ventricular ejection fraction (%); left atrial volume (ml/m2); pulmonary artery pressure (mmHg); end-systolic volume (ml).
Results: In Group III: left ventricular mass, E/E´ ratio and left atrial volume were significant. Regarding the total duration of the cardiac cycle, systole and diastole, the cohorts were baseline equal except for the left ventricular suction time which was longer in patients with heart failure with preserved ejection fraction.
Conclusions: Heart failure with preserved ejection fraction is mainly due to a dysfunction of ventricular suction, which is excessively prolonged during protodiastolic phase of lef ventricular myocardial contraction compared to the control groups, a fact that would lead to an increase in the filling pressures of the cardiac chambers with the consequent dyspneic symptoms that characterize these patients.
Keywords: Heart failure, Ventricular suction, Helical heart
Abbreviations: LVFey: Left Ventricle Ejection Fraction; HFpEF: Heart Failure with Preserved Ejection Fraction; LVPPMC: Left Ventricular Protodiastolic Phase of Myocardial Contraction; LVM: Left Ventricular mass; PAP: Pulmonary Artery Pressure; PPMC: Protodiastolic Phase of Myocardial Contraction; RWT: Relative Wall Thickening; RV: Right Ventricle; LAV: Left Atrium Volume; LV: Left Ventricle.
Introduction
In the traditional model, cardiac filling is determined solely by venous pressure. In reality, atrial pressure is too low to explain this situation. From this “key doubt” regarding the classical explanation, the concept of an active suction pump was developed, mechanically supported by the helical organization of cardiac myocardial fibers and their electromechanical activation sequence. The results of previous research [1,2] led us to consider that cardiac function consists of three essential phases: according to our research, between systole (300ms) and diastole (400ms) a myocardial contraction phase with an average duration of 83ms in the Left Ventricle (LV) and 30ms in the Right Ventricle (RV) is inserted, which supposes a coupling between both periods and produces the Protodiastolic Phase of Myocardial Contraction (PPCM), with ventricular contraction and energy consumption in order to achieve cardiac suction. The suction produced in the PPCM cannot be explained by a passive mechanism, given the low gradients reached at the entrance of the atria, and must be considered as a key element to facilitate venous return in complementarity with the systolic impulse of the opposite ventricle [3,4]. This is supported by the sub atmospheric pressures, “depressions” recorded in these chambers during the PPCM. The driving role of the atria is minimal. Its power is 1% with respect to that of the ventricle. Obviously, this low gradient is related to a need for active ventricular suction [5,6]. Since a variation in the ventricular suction mechanism could be an initial, even subclinical, stage of ventricular dysfunction, the aim of this analysis has been to identify whether there is a relationship between the parameters that determine LVPPMC impairment and Heart Failure with Preserved Ejection Fraction (HFpEF). This should be categorized with a Left Ventricle Ejection Fraction (LVFey) above 50%, with still not wellknown underlying mechanisms for the initiation and development of this type of heart failure. Is a deficient left ventricular suction the cause of the HFpEF?
Material and Methods
A retrospective study was performed on echocardiographic studies carried out in the last welve months. The study population consisted of three groups:
Group I: ten (10) patients youths (5 male, 5 female) without heart disease, with average age of 30,3±9,2 .8 years and body surface area of 1,81±0,16 m2.
Group II: ten (10) patients adults (5 female, 5 male) with heart failure, with average age of 66,2±4,1 años and body surface area of 1,73±0,16 m2.
Group III: Diez (10) patients (6 femeninos, 4 masculinos), and HFpEF, with average age of 81,1±11,3 años and body surface area of 1,76±0,20 m2.
Patients provided their informed consent for this study and the investigation was previously approved by the Ethics Committee. All patients were in sinus rhythm with electrocardiograms that showed no abnormality. They were studied with Doppler echocardiography (Vivid IQ Premium ultrasound system). The variables analyzed were: Cardiac cycle (ms); Left ventricular systole (ms), LVPPMC (ms); Left ventricular diastole (ms); Relative Wall Thickness (RWT) (%); Left Ventricular Mass (LVM) (gr/m2); E/E’ ratio; LVFey (%); Left Atrial Volume (LAV) (ml/m2); Pulmonary Artery Pressure (PAP)(mmHg) and End-systolic volume (ml).
Statistics
We define cohorts C1, C2 and C3 according to whether they belong to Group I, II or III, respectively. For each of the variables, in each of the cohorts: their values will be graphed, their mean, standard deviation, minimum, maximum, median and confidence interval for the mean will be calculated. The differences between the means will be studied by applying a Student T-Test for the Difference of Means in Paired Samples. With a confidence level of 95%. A p−value<0.05 would indicate a positive result of the test, the interpretation of which is that the means differ. Finally, confidence intervals will be established for the difference between the means of the indicators when comparing C1-C2 and C2-C3, in order to quantify their variation.
Results
Table 1 shows the results of the echocardiographic variables in the three groups. It was seen that all patients with HFpEF (Group III) exhibited a more prolonged LVPPMC time: 134±18,97ms, compared with the group without heart disease (Group I and II) lasting a significantly lower time: 83±16,36ms y 83,10±18,45ms, (p<0,01) respectively (Tables 1,2 and 3) (Figure 1 and 2). Concomitantly, an increase of LVM was also found in Group III with an average of 106gr/m2 compared with the group I (67gr/m2) and II (72gr/m2); of the RWT, which goes from 0.33% and 0.36% in groups I and II to 0.49% in III and of the VAI which reached 43 ml/m2 (Group II) for a value in the control groups I and II of 19 and 25ml/m2 respectively. In this analysis (Tables 1,2 and 3) it is clear that in Group III the LVM, E/E′, PAP and VAI are significant. Regarding the total duration of the cardiac cycle, systole and diastole, the cohorts are baseline equal (p>0.05 for all comparisons) except for the LVPPMC time which is longer in patients with HFpEF (p<0.01). The average values obtained, when we compare C1-C2 and C2-C3 to study whether they differ or coincide, are summarized in Table 4 (Table1).

Table 1: Echocardiographic values.
Note*: ms: milliseconds; LV: left ventricle; LVCMFP: left ventricular myocardial contraction protodiastolic phase; RPE: relative wall thickening; LVM: left ventricular mass; LFEY: left ventricular ejection fraction; LAV: left atrium volume; PAP: pulmonary artery pressure.
Observation: The p-values between GI and GII are those obtained by the Student T-Test for the Difference of Means in Paired Samples, with a confidence level of 95%, comparing Group I with Group II. The p-values between GI and GIII are those obtained by the test comparing Group I with Group III. Values (p<0.05) indicate a positive result of the test, and are interpreted as meaning that the compared means differ. Values (p≥0.05) indicate a negative result of the test, and are interpreted as meaning that the compared means coincide. The lower the p-value, the greater the probability that the compared means differ (Table 2-4).
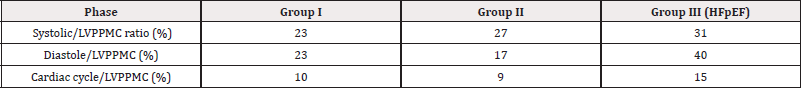
Table 2: Relationship of percent left ventricular cardiac cycle, systolic and diastolic duration with respect to the protodiastolic phase of myocardial contraction.
Discussion
A significant increase of LVPPMC time was found in Group III patients with HFpEF compared with the group without heart disease (Group I and II). Moreover, in these cases the tissue strain curve loses its sharp slope and becomes irregular needing a more prolonged time to generate the necessary pressure difference to open the mitral valve (Figure 1 and 2). This variable correlates with the E/E’ ratio, since in Group I this was 6,34±1,46 compared with Group II that reached a value of 7,50±1,53 and the Group III which reached a value of 16.13±6.47 (p<0.01) (Table 1). An abnormal effect can be interpreted in the negative pressure generated in this phase, evidencing a delayed process with longer time to open the mitral valve [7] (Figure 1 and 2). We also observed that the diastolic duration (passive filling phase without energy consumption) was maintained with scant variation in all groups (354ms in the Group I; 471ms in the Group II and 333ms in the Group III), confirming that the altered suction mechanism occurring in the LVPPMC mainly participates of the dysfunctional process. The increase in LVM, RWT and VAI in Group III, all with significance, are measurements that correspond to an increase in PAP to 32mmHg in Group III in relation to Groups I and II of 22mmHg. These concepts would explain why in HFpFE a pulmonary wedge pressure ≥15 mmHg or a left ventricular end-diastolic pressure ≥16 mmHg is usually found [8]. The possible interpretation is that as the LV mass increases it does not reach suitable detorsion in a normal time to generate a drop in pressure with adequate slope to open the mitral valve.
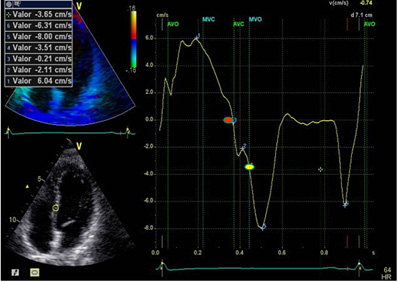
Figure 1: LVPPMC curve in a normal patient. The red dot indicates the beginning of the phase and the yellow dot its completion. Duration: 80ms; left ventricular mass: 67 gr/m2; relative wall thickness: 0.33%.
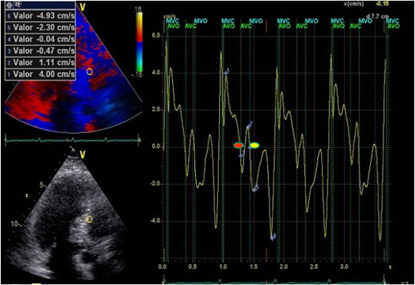
Figure 2: LVPPMC curve in a patient with HFpEF. The red dot indicates the beginning of the phase and the yellow dot its completion. Duration 160ms; left ventricular mass: 106gr/m2; relative wall thickness: 0.49%.
In terms of flow when 1cc per cardiac cycle decreases inflow to the left ventricle due to suction deficit and the right ventricle continues pumping blood into the pulmonary system, then dyspnea appears. And this is understood, since at 1 cc per beat, every hundred of these represent 100cc, which are retained in the lungs. Heart Failure consensuses point out concentric left ventricular hypertrophy as a characteristic of this disease. Other characteristics found highlight reduced distensibility of the ventricular wall, and ventricular and aortic valve stiffness [9]. In addition, increased myocardial fibrosis, due to excess type 1 collagen deposition in the extracellular matrix, and an inflammatory process with increased fibroblasts and cytokines is also mentioned [10,11]. Regarding the increase in LAV, it must be understood that the atria are volume compensatory chambers that avoid ventricular overload. This increased LAV in HFpEF patients should be considered as a deficit of left ventricular suction, and is probably a mechanism to reduce the increase in wall tension and prevent a significant increase of atrial pressure. This observation, present in all the patients with this pathology, is clinically accompanied by dyspnea at rest or during exertion [12]. In the LV, the LVFey and end-systolic volume are normal in all groups, implying that the altered values corresponding to the LVPPMC are indicating the moment of the cardiac cycle where the pathophysiological change occurs. The left ventricular cardiac cycle, systolic, PPMC and diastolic durations were measured in the three groups. The data collected (Table 2) is coherent with the investigation findings [13,14], showing that in patients with HFpEF, LVPPMC duration is prolonged in relation to the total cardiac cycle, systole and diastole. This would demonstrate the possibility that patients with HFpEF evidence their problem in the LVPPMC, as it needs a longer time to achieve an adequate intraventricular pressure to open the mitral valve (Table 3).
Conclusions
Based on the results obtained, it can be interpreted that the HFpEF mechanism is mainly due to ventricular suction dysfunction, excessively prolonged during the LVPPMC, compared with control groups. This would increase the filling pressures of cardiac chambers with the ensuing dyspneic symptomatology characteristic of these patients.
Acknowledgements
None.
Conflict of Interest
None.
References
- Trainini Jorge, Beraudo Mario, Wernicke Mario, Trainini Alejandro, Lowenstein Jorge, et al. (2024) Fundamental Conclusions on Research into the Anatomy and Organization of the Helical Heart. Am J Biomed Sci & Res 23(5): 577-579
- Trainini JC, Elencwajg B, López Cabanillas N, Herreros J, Lago N, et al. (2016) Electrophysiological Activation and Propagation Times in the Ventricular Myocardial Band. First Study in Humans. Rev Arg Cardiol 84(5): 453-459.
- Mangione S, Sullivan P, Wagner MS (2020) Physical Diagnosis Secrets E-Book: Physical Diagnosis Secrets E-Book-Elsevier Health Sciences 576.
- Trainini J, Valle Cabezas J, Beraudo M, Elencwajg B, Wernicke M, et al. (2024) Ventricular Complementarity. I J Cardio & Card Diso 5(2): 1-14.
- Buckberg GD, Coghlan HC, Torrent-Guasp F (2001) The structure and function ofthe helical heart and its buttress wrapping. V. Anatomic and physiologic considerations inthe healthy and failing heart. Semin Thorac Cardiovasc Sur 13(4): 358-385.
- Torrent-Guasp F, Ballester M, Buckberg GD, Carreras F, Flotats A, et al. (2001) Spatial orientation of the ventricular muscle band: physiologic contribution and surgical implications. J Thorac Cardiovasc Surg 122(2): 389-392.
- Brutsaert DL, Stanislas U, Gillibert TC (1993) Diastolic failure: pathophysiology and therapeutics implications. J Am Coll Cardiol 22(1): 318-325.
- Lam CSP, Voors AA, de Boer RA, Solomon SD, van Veldhuisen DJ (2018) Heart failure with preserved ejection fraction: From mechanisms to therapies. Eur Heart J 39(30): 2780-2792.
- Herreros J, Cirugía coronaria (2005) Evolution last decade. Indications and results. Rev Esp Cardiol 58: 1107-1116
- Mann DL, Bristow MR (2005) Mechanisms and models in heart failure. The biomechanical model and beyond. Circulation 11: 2837-2849.
- Sengupta PP, Narula J (2008) Reclassifying heart failure: predominantly subendocardial, subepicardial, and transmural. Heart Fail Clin 4(3): 379-382.
- Navarro López F, de Teresa E, López- Sendón JL, Castro Beiras A (1999) “Guidelines for the diagnosis and management of heart failure and cardiogenic shock”. Informe del Grupo de Trabajo de Insuficiencia Cardíaca de la Sociedad Española de Cardiologí Rev Esp Cardiol 52(Suppl 2): 1-54.
- Trainini JC, Lowenstein JA, Beraudo M, Llabata VM, Carreras-Costa F, et al. (2022) Fulcrum and torsion of the helical myocardium. Editorial Biblos 150.
- Trainini J, Beraudo M, Wernicke M (2021) Physiology of the helical heart. Int J Anat Appl Phys 7(5): 195-204.




 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.