Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Islet Transplantation using one’s own Differentiated stem cells: A Review and Potential Applications
*Corresponding author: Jonathan Lakey, Professor Emeritus, Surgery and Biomedical Engineering, University of California Irvine, CA.
Received: January 03, 2025; Published: January 07, 2025
DOI: 10.34297/AJBSR.2025.25.003314
Abstract
Diabetes is one of the most common chronic diseases worldwide, with around 537 million adults aged 20-79 living with the disease. It is a global epidemic and has a rapidly rising incidence. Traditional treatments for diabetes have limited efficacy in achieving long-term disease control. In recent years, the autologous infusion of bone marrow-derived mononuclear cells (BMMNC) has become a novel and effective therapeutic approach in treating autoimmune type 1 diabetes (T1DM). BMMNC contains two important types of stem cells, bone marrow-derived hematopoietic stem cells (BMHSC) and bone marrow-derived mesenchymal stem cells (BMMSC), which are currently used independently or coordinately in the treatment of T1DM. In this review, we summarize the clinical data concerning BMMNC, BMHSC, and BMMSC infusion in patients with diabetes (including Type 1, Type 2, and secondary diabetes) and diabetes-related complications. Research suggests that the autologous infusion of bone marrow stem cells is safe and effective, offering the potential to be widely used in patients with diabetes.
Keywords: Diabetes, Autologous Transplantation, Mesenchymal Stem Cell, Differentiated Stem Cells
Introduction
Diabetes is one of the most common chronic diseases worldwide, with around 537 million adults aged 20-79 living with the disease [1]. Diabetes mellitus, which includes type 1 (T1DM) and type 2 (T2DM), leads to high morbidity and mortality, and as a result, presents a huge global burden. According to the CDC (Centers for Disease Control and Prevention) [2], there are at least 37.3 million Americans (11.3% of the population) living with diabetes, and another 38% of US adult population have prediabetes. In 2017, the cost of diagnosed diabetes in the US was estimated at $327 billion [3]. Traditional therapeutic diabetes strategies such as diet control, exercise, exogenous insulin treatment, and glucoregulatory pharmacological approaches do not have effective sustained long-term outcome. Pancreatic and islet transplantation has been considered for T1DM and bariatric surgery has exceptional effects on refractory T2DM, but these applications have been limited thus far [4,5]. Furthermore, both have potential surgery-related risks [6] and long-term complications [7] associated with chronic immune suppression [8,9] e.g. histocompatability leucocyte antigen (HLA) sensitization).
In recent years, the use of bone marrow-derived mononuclear (BMMNC) cells has shown to be a promising therapeutic strategy for diabetes and some other diseases [6]. BMMNCs contain two main types of bone marrow stem cells, bone marrow-derived hematopoietic stem cells (BMHSCs) and bone marrow-derived mesenchymal stem cells (BMMSCs) [6,7,9]. Widely located in the bone marrow, BMHSCs play a key role in producing all types of blood cells. BMMSCs reside in the bone marrow stromal compartment. These cells mechanically support the hematopoietic microenvironment, and have the capacity to differentiate into a variety of cell types such as neuronal cells, cardiomyocyte, lung epithelial cells, and pancreatic beta cells [10]. A systematic review [9,10] compared different types of stem cells. The collective evidence for T1DM BMHSC infusion may help preserve or restore pancreatic β-cell function through immunomodulatory effects, reducing autoimmune-mediated β-cell destruction, while in T2DM, the combined transplantation of BMMNCs shows promise in improving insulin sensitivity and glycemic control by promoting β-cell regeneration, enhancing insulin secretion, and mitigating chronic inflammation.
Stem cell transplants can be allogeneic or autologous, depending on the source of the cells. In autologous transplantation, the patient’s own stem cells are collected, processed, and reintroduced after a conditioning regimen. This approach minimizes the risk of immune rejection or graft-versus-host disease (GVHD) because the cells are genetically identical to the recipient. Historically, for autoimmune conditions such as T1DM, where the patient’s cells may still harbor disease-causing traits and autologous transplantation was often underconsidered. However, clinical trials and preclinical studies support autologous the potential for BMMNC to play an integral role in modifying the underlying T1DM and T2DM pathophysiological processes. Compared to allogeneic transplantation, autologous infusion of bone marrow stem cells is widely accepted as it reduces graft versus host disease and engraftment syndrome [11,12]. In this review, we summarize the recent clinical data of BMMNC infusion in the treatment of T1DM and T2DM. To our knowledge, this is the first review paper of this topic.
Autologous infusion of BMMNC in T1DM
T1DM is caused by an autoimmune process leading to islet beta-cell destruction that results in insulin deficiency and hyperglycemia. While it was once believed that islet damage resulting in beta cell destruction was irreversible stem cell therapy has been shown to restore functional β-cell mass. Compared to pancreatic transplantation, stem cell transplantation has fewer limitations and has wider applications due to its accessibility. The cells, such as BMMNCs, can be obtained with relative ease from the individual. BMMNCs can be infused through veins, arteries, or directly into tissues. Jawale [13] harvested 7.86 x 107 bone marrow stem cells and divided these into thirds: one third of the isolated cells were delivered into the omental pouch, another one third was delivered into peritoneal cavity, and the remaining third was given intravenously. This method was reported to be safe and effective for the long-term treatment of T1DM (Figure 1).
Cai, et al [14]. conducted a pilot randomized controlled trial (RCT) in patients with T1DM. One year after a co-transplantation of autologous BMMNCs plus umbilical cord mesenchymal stem cells (MSCs) through the pancreatic artery, patients showed moderate improvement of metabolic measures such as the levels of endogenous C-peptide, insulin, glucose, and hemoglobin A1C (HbA1c) compared to controls. Mesples, et al [15] treated 2 patients with recently diagnosed T1DM by infusing BMMNCs into the liver via an ultrasound guided needle. The follow up at 12 months after treatment exhibited negative values in anti-pancreatic islets cells antibodies (ICAs), glutamic acid decarboxylase (GAD) antibodies, and anti-insulin antibodies, with increased C-peptide concentration and decreased glycemic levels [15]. In addition, the anti-T1DM effects of BMMNC can be further improved by the addition of exercise in combination with the autologous BMMNC transplantation, which showed better glycemic control than stem cell alone in patients with T1DM [13].
The combined transplantation of MSCs and hematopoietic stem cells (HSCs) has been used in treatments of diseases [10] including T1DM. The combination of BMHSCs and other types of MSCs have been addressed in T1DM treatment. Thakkar, et al [16] performed a prospective trial for patients with T1DM and found that autologous BMHSCs plus adipose-derived insulin-secreting mesenchymal stromal cells offered satisfactory long-term hyperglycemic control. The co-infusion of BMMSC and other types of HSCs has not been studied and warrants further research in clinical trials.
BMHSC therapy has also been used as stand-alone treatment for autoimmune diseases including T1DM [15]. The rationale of this therapy is that BMHSC are likely to influence immunity and correct immune aberration [16]. Voltarelli, et al [17]. conducted a prospective study of 15 patients with T1DM (aged 14-31 years) diagnosed within the previous 6 weeks by clinical findings and hyperglycemia and confirmed with positive antibodies against glutamic acid decarboxylase. During a follow-up period of 7 to 36 months (mean 18.8), 14 patients achieved insulin independence, with 1 patient resuming insulin use 1 year after AHST. At 6 months, C-peptide response curves were significantly improved, and anti-glutamic acid decarboxylase antibody levels decreased, while 13 out of 14 patients maintained HbA1c levels < 7% with minimal adverse effects. Although there was no mortality, numerous adverse events were reported in a significant percentage of patients. Snarski, et al [18-20]. demonstrated similar findings in a study of eight patients with newly diagnosed T1DM after performing BMHSC transplantation. Following transplantation, all patients were less dependent on exogenous insulin and exhibited lower HbA1c levels. Li, et al [21]. did BMHSC transplantation in patients with T1DM who developed symptoms within 12 months of diagnosis. In 31-54 months, 11 out of 15 patients had decreased HbA1c and increased C-peptide concentrations, along with reduced doses of insulin for glycemic control, indicating an improvement of beta-cell function. A follow-up study by Couri, et al also exhibited an increase in C-peptide and a reduction in insulin consumption [22].
With the ability to differentiate into islet cells and modulate the microenvironment, BMMSC therapy is also used stand-alone in T1DM treatment, although the reports of using BMMCS alone are fewer compared to using BMHSC alone. In an RCT [23], an IV injection of autologous BMMSC or placebo was performed in 21 patients with newly diagnosed T1DM demonstrating patients who underwent BMMSC treatment improved C-peptide levels and HbA1c. Interestingly, BMMSC induced an anti-inflammatory response as opposed to the pro-inflammatory cascade apparent prior to treatment [23]. Similarly, Carlsson, et al [24]. analyzed C-peptide concentrations in blood in response to a mixed-meal tolerance test (MMTT) at 1-year follow-up in 20 T1DM patients randomized to IV autologous BMMSC treatment or serving as controls. Among those in the control arm there was a loss in both C-peptide peak values and C-peptide when calculated as area under the curve during the 1st year while among MSC-treated patients’ responses were preserved or even increased [24].
Based on the above evidence about the use of BMMNCs as a promising therapeutic strategy for diabetes, our team recently reported a novel approach for BMMNC collection using a cohort of six young diabetic patients [25]. After using Filgrastim for 4 days, bone marrow was aspirated on day 5 and stem cells were extracted from the anterior superior iliac spine, which was followed by an IV injection. The qualified autologous BMMNC were collected and identified as mononuclear cells >180×106 /kg and CD34+ cells >0.22%. These patients had a diagnosis of T1DM for <120 days (60- 120 days) and were aged 12 years old on average. At six months after stem cell transplantation, 5 patients demonstrated decreased blood glucose and HbA1C levels along with improved values of ICA, GAD, and tyrosine phosphatase-related islet antigen 2 antibodies [25]. In this study, BMHSCs were stimulated and BMMSCs were not stimulated.
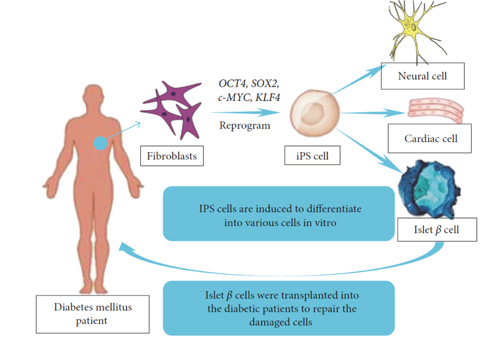
Figure 2: BMMNCs have inherent regenerative properties, they are limited in their ability to fully replace damaged β-cells.
While BMMNCs have inherent regenerative properties, they are limited in their ability to fully replace damaged β-cells. However, reprogramming BMMNCs into induced pluripotent stem cells (iPSCs) can provide a renewable source of β-like cells. BMMNC can be reprogrammed into iPSCs by introducing specific transcription factors (e.g., OCT4, SOX2, KLF4, c-MYC) [19]. iPSCs derived from BMMNCs possess the ability to differentiate into any cell type, including insulin-producing β-cells. This approach combines the immuno- compatibility of autologous BMMNCs with the pluripotency of iPSCs, potentially addressing the need for personalized diabetes therapies [20]. The immunological advantage resides in the use of BMMNCs from the patient to generate iPSCs avoids issues of immune rejection associated with allogeneic iPSCs or other stem cell sources, especially critical in autoimmune conditions like T1DM [21]. In essence, BMMNCs can serve as an autologous, readily available cell source for generating iPSCs, offering a path to more personalized and effective regenerative therapies for diabetes and other conditions (Figure 2).
Our group has capitalized on the immunological advantage. In preclinical research showing the effects of engineered islets in diabetic murine models, histological evaluation shows successful integration of engineered islets into the host tissue post-transplantation. These studies demonstrate E-ISLET-treated groups exhibit significantly reduced HbA1c levels compared to untreated diabetic mice, nearing levels seen in healthy controls in six weeks, emphasizing the ability of these modified islets to reduce both blood glucose and HbA1c levels, demonstrating their functional efficacy in restoring glucose regulation. Omentum implantation of alginate double coated (APA) human PSC islet cell capsule into a pig with total pancreatectomy model, ~60% c-peptide was detected at oneweek post-transplantation [22]. In the primate STZ T1DM model, we show injection of human iPCS, c-peptide is detected as early as two weeks post-transplantation [23].
Vertex Pharmaceuticals conducted a phase 1/2 clinical trial using iPSC-derived β-cell clusters (VX-880) in T1D patients with severe hypoglycemia unawareness. Early results showed that after transplantation, patients exhibited significant improvements in glucose control, with reduced insulin requirements and restored fasting C-peptide levels (indicating functional insulin production). One patient achieved nearly insulin independence post-transplantation, marking a major breakthrough. In addition, ViaCyte and CRISPR Therapeutics have also collaborated to leverage iPSC capacity [24]. ViaCyte developed PEC-01 cells, derived from iPSCs, which mature into β-cell precursors after implantation. Their product, VCTX210, uses gene-edited (CRISPR-modified) iPSC-derived cells to avoid immune rejection without requiring lifelong immunosuppression. Early trials indicate successful engraftment and measurable insulin production in T1D patients [25].
Recent advancements in stem cell therapy highlight a shift toward autologous approaches as an alternative to allogeneic iPSC-derived therapies like Vertex’s VX-880 and ViaCyte-CRISPR Therapeutics’ VCTX210. While VX-880, has demonstrated significant glucose control improvements in T1D patients, with some achieving near insulin independence autologous therapies, utilizing a patient’s own cells reprogrammed into β-cell precursors, offer the potential to bypass immune rejection entirely, reducing dependence on genetic modifications or immunosuppressive drugs, thus paving the way for personalized and safer solutions in T1D treatment.
Autologous infusion of BMMNCs in T2DM
T2DM, is characterized by a combination of insulin resistance and islet beta-cell dysfunction accounting for > 90% of diagnosed diabetes cases. A meta-analysis26 showed that BMMNC therapy for T2DM resulted in improved glycemic control, insulin secretion and biosynthesis in patients, and suggested that it might prevent the loss of islet cells. Bhansali, et al [26,27]. found that BMMNC transplantation led to a reduction in the required insulin dose and an improvement in C-peptide response in patients with T2DM, although insulin sensitivity remained unchanged. Hu, et al. conducted a 3-year data analysis? that indicated similar improvements [28].
The approach of infusing stem cells in T2DM varies from that used in T1DM. Sood, et al [29]. divided 21 patients with T2DM into three groups according to route of transplantation of BMMCs. Seven patients received BMMNC in the superior pancreaticoduodenal artery under fluoroscopic guidance, seven received the infusion in the splenic artery, and seven received the peripheral IV route. At 6-months post-treatment, infused through the artery had significantly reduced insulin dose requirements while no changes were observed among those who received transplantation via IV, suggesting the IV route may not be recommended for T2DM therapy [30]. Other arterial routes have also been utilized to deliver BMMNC for T2DM therapy. Wehbe, et al [7]. conducted a study in which six patients with T2DM underwent autologous infusion of BMMCs into the celiac and superior mesenteric arteries. Five patients showed normalization of fasting glucose and HbA1C with a concomitant reduction of medication required. Infusion through the great pancreatic artery has been shown to be safe and effective [31].
The effectiveness of the autologous stem cell on T2DM may be enhanced by a combination with hyperbaric oxygen treatment (HOT). Estrada, et al. studied 48 patients with T2DM [32,33]. One year after the combined treatments, the patients exhibited increased metabolic control and reduced insulin requirements compared to either the standard treatment or baseline groups. However, this conclusion contrasted with Wu, et al study [34], who found in 80 patients at 12 months after treatment significant improvement compared with the control group, but no remarkable change was observed in the HOT group. More research is needed to understand these potential synergistic effects.
BMMSCs can be used as a stand-alone therapy in patients with T2DM and demonstrated good results. There are no reports using autologous BMHSC therapy in patients with T2DM, however the use of BMMSC therapy has demonstrated satisfactory outcomes 35. Bhansali, et al [35]. randomly assigned patients with T2DM into groups receiving either BMMNCs or BMMSCs via superior pancreaticoduodenal arterial injection. At 12 months after treatment, both groups showed a reduction in HbA1C level and insulin requirements, and BMMSC infusion was shown to increase insulin sensitivity and the C-peptide response.
Autologous Infusion of BMMNC in other types of Diabetes
To date, BMMNC infusion has not been used in the treatment of gestational diabetes (GDM), although it has been applied in the prevention of pancreatogenic diabetes (also called type 3C diabetes 36). In a pilot study by Wang, et al [30], infused autologous BMMSC via the portal vein along with the islet transplantation to patients with chronic pancreatitis. Compared to untreated controls, these patients required lower doses of insulin and had lower levels of blood glucose. Thakkar, et al [16]. reported a successful treatment for one patient with pancreatogenic diabetes implanting BMHSC along with adipose tissue derived insulin-secreting MSCs into subcutaneous tissue, portal and thymic circulation. Relative to baseline measures, the patient maintained euglycemia (postprandial blood sugar from 389 mg/dl to 165 mg/dl) and HbA1C (from 8.9% to 6.8%) results with less insulin consumption (from 72 IU/day to 36 IU/day) at the 27-month follow-up [38-40].
Autologous infusion of BMMNC in Diabetic Complications
BMMNCs also play a role in attenuating the complications of T1DM and T2DM . In a study by Wu, et al., results from 8-year follow up after the co-transplantation of autologous BMMNCs and umbilical cord MSCs reduced incidence of T1DM chronic complications was observed [31]. Similarly, Gaipov, et al. revealed that the infusion of autologous BMMNCs improved nephropathy in patients with T1DM [32]. Further, findings from a phase I trial showed that intravitreal injection of autologous BMMNCs inhibited the progression of hereditary retinal dystrophy [41].
BMMNCs therapy has also be shown to relieve foot ulcers and critical limb ischemia in both T1DM and T2DM [42-45]. According to a study by Gu, et al [33], a single IV infusion of autologous BMMSCs in patients with non-proliferative diabetic retinopathy improved visual acuity and central macular and subfield thickness, with decreased fasting blood glucose and hypersensitive C-reactive protein levels. Al Demour, et al. [34] reported intracavernous autologous BMMSCs as a safe and effective treatment for diabetic patients with erectile dysfunction. Similarly, studies by Dash, et al [35] and Lu, et al [36] demonstrated that the topical application of BMMSCs in patients with chronic diabetic foot ulcers promoted blood flow and resulted in decreases pain during walking, reduced wound size and decreased ulcer recurrence rate. The role of BMMSCs in curing critical limb ischemia [37] and recurrent lower limb bullosis diabeticorum [38] in patients with T2DM was also reported. However, it was also reported that the function of BMMSCs might be compromised with a long-term exposure to chronic inflammation [46-52] or reduced due to a long history of T2DM and obesity [53-56], although these side effects need to be confirmed and explored further in future research.
Conclusion
BMMNCs and its components of BMHSCs and BMMSCs have the capacity to treat diabetes and diabetes-related complications. Several studies have been performed and different approaches have been explored. Preliminary research has shown that autologous infusion of bone marrow stem cells is feasible, safe, and effective. In the future, rigorous RCT data using larger groups and longer-term follow-up, with more comparisons between studies may be needed to standardize and optimize autologous bone marrow stem cell infusion therapies for diabetes and other diseases.
Funding
This research was funded by internal support from Betacure, Utah.
Acknowledgments
The authors acknowledge the support from the Department of Surgery at University of California Irvine for their support of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- (2023) IDF Diabetes Atlas | Tenth Edition.
- (2022) National Diabetes Statistics Report | Diabetes | CDC.
- Yang W, Dall TM and Beronjia K (2018) Economic costs of diabetes in the U.S. in 2017. Diabetes Care 41(5): 917-928.
- Gasoyan H, Tajeu G, Halpern MT and Sarwer DB (2019) Reasons for underutilization of bariatric surgery: The role of insurance benefit design. Surg Obes Relat Dis 15(1): 146-151.
- Dholakia S, Royston E, Quiroga I, Sinha S, Reddy S et al (2017) The rise and potential fall of pancreas transplantation. Br Med Bull 124(1): 171-179.
- Helman A and Melton DA (2021) A Stem Cell Approach to Cure Type 1 Diabetes. Cold Spring Harb Perspect Biol 13(1): a035741.
- Lim PW, Stucky CH, Wasif N, Etzioni DA, Harold KL, et al (2024) Bariatric Surgery and Longitudinal Cancer Risk: A Review. JAMA Surg 159(3): 331-338.
- Czarnecka Z, Dadheech N, Razavy H, Pawlick R and Shapiro AMJ (2023) The Current Status of Allogenic Islet Cell Transplantation. Cells 12(20): 2423.
- Suda S (2017) Bone marrow-derived mononuclear cells. Cell Ther against Cereb Stroke Compr Rev Transl Res Clin Trials 3-14.
- Wehbe T, Chahine NA, Sissi S, Abou Joaude I and Chalhoub L (2016) Bone marrow derived stem cell therapy for type 2 diabetes mellitus. Stem cell Investig 3: 87.
- Bani Hamad FR, Rahat N, Shankar K and Tsouklidis N (2021) Efficacy of Stem Cell Application in Diabetes Mellitus: Promising Future Therapy for Diabetes and Its Complications. Cureus 13(2): e13563.
- Zhao K and Liu Q (2016) The clinical application of mesenchymal stromal cells in hematopoietic stem cell transplantation. J Hematol Oncol 9(1): 46.
- Maqbool S, Nadeem M, Shahroz A, Naimat K, Khan I, et al (2022) Engraftment syndrome following Hematopoietic stem cell transplantation: a systematic approach toward diagnosis and management. Med Oncol 40(1): 36.
- Khaddour K, Hana CK and Mewawalla P (2022) Hematopoietic Stem Cell Transplantation - PubMed. StatPearls.
- Jawale S (2022) Stem cell therapy for type1 diabetes with transplantation of stem cells into the Omental pouch, peritoneum, and blood, experimental study. Ann Med Surg 81: 104468.
- Cai J, Wu Z, Xu X, Liao L and Chen J, et al (2016) Umbilical Cord Mesenchymal Stromal Cell With Autologous Bone Marrow Cell Transplantation in Established Type 1 Diabetes: A Pilot Randomized Controlled Open-Label Clinical Study to Assess Safety and Impact on Insulin Secretion. Diabetes Care 39(1): 149-157.
- Mesples A, Majeed N, Zhang Y and Xiang H (2013) Early immunotherapy using autologous adult stem cells reversed the effect of anti-pancreatic islets in recently diagnosed type 1 diabetes mellitus: preliminary results. Med Sci Monit 19: 852-857.
- Mohamed MT, Embaby EA, Labib A, Husseiny ME, Khamis H et al (2019) Effects of exercise in combination with autologous bone marrow stem cell transplantation for patients with type 1 diabetes. Physiother Theory Pract 35(12): 1233-1242.
- Thakkar UG, Trivedi HL, Vanikar AV and Dave SD (2015) Insulin-secreting adipose-derived mesenchymal stromal cells with bone marrow-derived hematopoietic stem cells from autologous and allogenic sources for type 1 diabetes mellitus. Cytotherapy 17(7): 940-947.
- Alderuccio F, Chan J, Scott DW and Toh BH (2009) Gene therapy and bone marrow stem-cell transfer to treat autoimmune disease. Trends Mol Med 15(8): 344-351.
- Zheng J, Song C and Zhang CC (2011) A new chapter: Hematopoietic stem cells are direct players in immunity. Cell Biosci 1(1): 1-5.
- Voltarelli JC, Couri CEB, Stracieri ABPL (2007) Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA 297(14): 1568-1576.
- Snarski E, Milczarczyk A, Torosian T, Paluszewska M, Urbanowska E, et al (2011) Independence of exogenous insulin following immunoablation and stem cell reconstitution in newly diagnosed diabetes type I. Bone Marrow Transplant 46(4): 562-566.
- Li L, Shen S, Ouyang J, Hu Y, Hu L, et al (2012) Autologous hematopoietic stem cell transplantation modulates immunocompetent cells and improves β-cell function in Chinese patients with new onset of type 1 diabetes. J Clin Endocrinol Metab 97(5): 1729-1736.
- Couri CEB, Oliveira MCB, Stracieri ABPL, Moraes DA, Pieroni F, et al (2009) C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA 301(15): 1573-1579.
- Izadi M, Sadr Hashemi Nejad A, Moazenchi M, Masoumi S, et al (2022) Mesenchymal stem cell transplantation in newly diagnosed type-1 diabetes patients: a phase I/II randomized placebo-controlled clinical trial. Stem Cell Res Ther 13(1): 264.
- Carlsson PO, Schwarcz E, Korsgren O and Le Blanc K (2015) Preserved β-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes 64(2): 587-592.
- Mesples AD, Cox DCT, Lundy HD, Antonio-Collie S, Diggiss CW, et al (2023) Monitoring of Autoantibodies Following Autologous Hematopoietic Stem Cell Transplantation in 6 Children with Recently Diagnosed Type 1 Diabetes Mellitus. Med Sci Monit 29: e938979.
- Wang ZX, Cao JX, Li D, Zhang XY, Liu JL, et al (2015) Clinical efficacy of autologous stem cell transplantation for the treatment of patients with type 2 diabetes mellitus: a meta-analysis. Cytotherapy 17(7): 956-968.
- Bhansali S, Dutta P, Yadav MK, Jain A, Mudaliar S, et al (2019) Autologous bone marrow-derived mononuclear cells transplantation in type 2 diabetes mellitus: effect on β-cell function and insulin sensitivity. Diabetol Metab Syndr 9: 50.
- Bhansali A, Asokumar P, Walia R, Bhansali S, Gupta V, et al (2014) Efficacy and safety of autologous bone marrow-derived stem cell transplantation in patients with type 2 diabetes mellitus: a randomized placebo-controlled study. Cell Transplant 23(9): 1075-1085.
- Hu J, Li C, Wang L, Zhang X, Zhang M, et al (2012) Long term effects of the implantation of autologous bone marrow mononuclear cells for type 2 diabetes mellitus. Endocr J 59(11): 1031-1039.
- Sood V, Bhansali A, Mittal BR, Singh B, Marwaha N, et al (2017) Autologous bone marrow derived stem cell therapy in patients with type 2 diabetes mellitus - defining adequate administration methods. World J Diabetes 8(7): 381-389.
- Wang L, Zhao S, Mao H, Zhou L, Wang ZJ, et al (2011) Autologous bone marrow stem cell transplantation for the treatment of type 2 diabetes mellitus. Chin Med J (Engl) 124(22): 3622-3628.
- Estrada EJ, Valacchi F, Nicora E, Brieva S, Esteve C, et al (2008) Combined treatment of intrapancreatic autologous bone marrow stem cells and hyperbaric oxygen in type 2 diabetes mellitus. Cell Transplant. 17(12): 1295-1304.
- Estrada EJ, Decima JL, Bortman G, Roberti J, Romero EB, et al (2019) Combination treatment of autologous bone marrow stem cell transplantation and hyperbaric oxygen therapy for type 2 diabetes mellitus: A randomized controlled trial. Cell Transplant 28(12): 1632-1640.
- Wu Z, Cai J, Chen J, Huang L, Wu W, et al (2014) Autologous bone marrow mononuclear cell infusion and hyperbaric oxygen therapy in type 2 diabetes mellitus: an open-label, randomized controlled clinical trial. Cytotherapy 16(2): 258-265.
- Bhansali S, Dutta P, Kumar V, Yadav MK, Jain A, et al (2017) Efficacy of Autologous Bone Marrow-Derived Mesenchymal Stem Cell and Mononuclear Cell Transplantation in Type 2 Diabetes Mellitus: A Randomized, Placebo-Controlled Comparative Study. Stem Cells Dev 26(7): 471-481.
- Hart PA, Bellin MD, Andersen DK, Bradley D, Cruz Monserrate Z, et al (2016) Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. lancet Gastroenterol Hepatol 1(3): 226-237.
- Wang H, Strange C, Nietert PJ, Wang J, Turnbull TL, et al (2018) Autologous Mesenchymal Stem Cell and Islet Cotransplantation: Safety and Efficacy. Stem Cells Transl Med 7(1): 11-19.
- Thakkar U, Vanikar A and Trivedi H (2013) Co-infusion of autologous adipose tissue derived insulin-secreting mesenchymal stem cells and bone marrow derived hematopoietic stem cells: viable therapy for type III.C. a diabetes mellitus. Biomed J 36(6): 304-307.
- Wu Z, Xu X, Cai J, Chen J, Huang L, et al (2022) Prevention of chronic diabetic complications in type 1 diabetes by co-transplantation of umbilical cord mesenchymal stromal cells and autologous bone marrow: a pilot randomized controlled open-label clinical study with 8-year follow-up. Cytotherapy 24(4): 421-427.
- Gaipov A, Taubaldiyeva Z, Askarov M, Turebekov Z, Kozina L, et al (2019) Infusion of autologous bone marrow derived mononuclear stem cells potentially reduces urinary markers in diabetic nephropathy. J Nephrol 32(1): 65-73.
- Siqueira RC, Messias A, Voltarelli JC, Scott IU and Jorge R (2011) Intravitreal injection of autologous bone marrow-derived mononuclear cells for hereditary retinal dystrophy: a phase I trial. Retina 31(6): 1207-1214.
- Procházka V, Gumulec J, Jalůvka F, Salounová D, Jonszta T, et al (2010) Cell therapy, a new standard in management of chronic critical limb ischemia and foot ulcer. Cell Transplant 19(11): 1413-1424.
- Ruiz-Salmeron R, de la Cuesta Diaz A, Constantino Bermejo M, Marcos Sánchez F, Hmadcha A, et al (2011) Angiographic demonstration of neoangiogenesis after intra-arterial infusion of autologous bone marrow mononuclear cells in diabetic patients with critical limb ischemia. Cell Transplant 20(10): 1629-1639.
- Kirana S, Stratmann B, Prante C, Prohaska W, Koerperich H, et al (2012) Autologous stem cell therapy in the treatment of limb ischaemia induced chronic tissue ulcers of diabetic foot patients. Int J Clin Pract 66(4): 384-393.
- Dubsky M, Jirkovska A, Bem R, Fejfarova V, Pagacova L, et al (2013) Both autologous bone marrow mononuclear cell and peripheral blood progenitor cell therapies similarly improve ischaemia in patients with diabetic foot in comparison with control treatment. Diabetes Metab Res Rev 29(5): 369-376.
- Gu X, Yu X, Zhao C, Duan P, Tongtao Zhao T, et al (2018) Efficacy and Safety of Autologous Bone Marrow Mesenchymal Stem Cell Transplantation in Patients with Diabetic Retinopathy. Cell Physiol Biochem 49(1): 40-52.
- Al Demour S, Jafar H, Adwan S, AlSharif A, Alhawari H, et al (2018) Safety and Potential Therapeutic Effect of Two Intracavernous Autologous Bone Marrow Derived Mesenchymal Stem Cells injections in Diabetic Patients with Erectile Dysfunction: An Open Label Phase I Clinical Trial. Urol Int 101(3): 358-365.
- Dash NR, Dash SN, Routray P, Mohapatra S and Mohapatra PC (2009) Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cells. Rejuvenation Res 12(5): 359-366.
- Lu D, Jiang Y, Deng W, Zhang Y, Liang Z, et al (2019) Long-Term Outcomes of BMMSC Compared with BMMNC for Treatment of Critical Limb Ischemia and Foot Ulcer in Patients with Diabetes. Cell Transplant 28(5): 645-652.
- Lu D, Chen B, Liang Z, Deng W, Jiang Y, (2011) et al. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double-blind, randomized, controlled trial. Diabetes Res Clin Pract 92(1): 26-36.
- Chen Y, Ma Y, Li N, Wang H, Chen B, et al (2018) Efficacy and long-term longitudinal follow-up of bone marrow mesenchymal cell transplantation therapy in a diabetic patient with recurrent lower limb bullosis diabeticorum. Stem Cell Res Ther 9(1): 99.
- Van De Vyver M (2017) Intrinsic Mesenchymal Stem Cell Dysfunction in Diabetes Mellitus: Implications for Autologous Cell Therapy. Stem Cells Dev 26(14): 1042-1053.
- Nguyen LT, Hoang DM, Nguyen KT, Bui DM, Nguyen HT, et al (2021) Type 2 diabetes mellitus duration and obesity alter the efficacy of autologously transplanted bone marrow-derived mesenchymal stem/stromal cells. Stem Cells Transl Med 10(9): 1266-1278.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.