Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Marine Actinobacteria: A Potential Avenue to Novel Pharmaceutically Active Compounds
*Corresponding author: Ahmed Shuikan, Botany and Microbiology Department, College of Science, King Saud University, Riyadh, Saudi Arabia.
Received: December 26, 2024; Published: January 16, 2025
DOI: 10.34297/AJBSR.2025.25.003326
Abstract
Bioactive compounds derived from marine actinobacteria enrich the field of pharmaceutical industries. Secondary metabolites of marine actinobacteria have been tested against pathogenic bacteria, fungi, viruses, parasites, different cell lines and as immunomodulatory agents. In the marine environment, different genera of actinobacteria may live free in marine sediments or associated with several invertebrate and vertebrate organisms. Different marine actinobacterial genera have been identified such as Streptomyces, Actinomyces, Salinispora, Micrococcus, Micromonospora. Of these, Streptomyces sp. has received much attention because of its ability to produce an enormous number of secondary metabolites. The potent antimicrobial activities of these compounds make them a promising alternatives to overcome drug resistant microbes. In addition, several marine actinobacteria derived compounds have potent cytotoxic activities against several cell lines. This property has been exploited to secure save and potent anticancer alternative instead of traditional cancer therapies. In the current review, we reviewed the pharmaceutical importance of marine actinobacteria. For this purpose, we collected the most recent data regarding the bioactivities of marine actinobacteria derived compounds. The origin, distribution and diversity of marine actinobacteria are discussed. The data presented in this review emphasize the importance of marine actinobacteria and should draw the attention toward these marvellous bacteria.
Introduction
Actinobacteria are a class of Gram-positive bacteria that possess a number of features such as high genomic G/C content (79-80%), no distinctive cell wall, and the formation exospores in response to harsh conditions [1]. They are usually filamentous with slender non-septate mycelium [2]. Actinobacteria are ubiquitous where they colonize different habitats such as freshwater, marine, and soil. Actinobacteria of terrestrial origin have been extensively investigated since the early 1950s. Marine actinobacteria are among the most active producers of secondary metabolites with potential applications in pharmaceutical and biotechnological industries [3-6]. They were reported to produce a wide array of secondary metabolites with antibiotics, anticancer, and immunosuppressive activities [7]. In addition, actinobacteria-derived compounds have activities against viruses, fungi, malaria, and parasites [8].
Actinobacteria produce more than 10,000 bioactive secondary metabolites which account for up to 45% of all discovered bioactive compounds [9]. For pharmaceutical purposes, actinobacteria are the main antibiotic producers for several potent antibiotics such as chloramphenicol, macrolide, and aminoglycosides [9]. In addition, a variety of actinobacteria-derived anticancer compounds have been used clinically such as daunomycin, doxorubicin, neocarzinostatin, carzinophilin, and chalcomycin [10,11]. Such outstanding properties of Actinobacteria attract the attention of researchers around the globe. This creates a state of progression in drug discovery from these microorganisms through i) highthroughput screening and fermentation, ii) genome mining for cryptic pathways, and iii) combinatorial biosynthesis to synthesize novel secondary metabolites [12].
Marine actinobacteria reside in a complex environment in terms of pressure, salinity, and temperature. In such environment, Actinobacteria were reported to form symbiotic relationships with different fishes, seaweeds, molluscs, mangroves and sponges [2,13]. Isolation and identification of marine actinobacteria is the first step to discover new bioactive compounds. In comparison with their corresponding terrestrial partners, marine actinobacteria are difficult to be cultured. This could be attributed to the difference in growth requirements, particularly the halophilic nature of marine actinobacteria [14]. However, several studies have reported the sampling techniques and isolation of actinobacteria from shallow costal sediments to the deepest sediments [15-17]. In addition, enhancement of the fermentation techniques for the specific bioactive compounds [18,19] genetic engineering of the biosynthesis gene clusters [20] were also reported. In the current review, the following topics will be discussed; i) origin, distribution and diversity of marine actinobacteria, and iii) different biological activities of marine actinobacteria-derived compounds.
Marine Actinobacteria: Origin, Distribution and their Role in the Marine Environment
Oceans cover more 70% of the Earth’s surface with a great biological of life than in the terrestrial environment [21]. Geographically, the coastal area of the sea represents only 7-8% and the remaining is for the deep sea [22]. The nature of the marine environment particularly, the deep sea extremely differs from the terrestrial environment. The deep sea has high pressure, low temperature, darkness, and inconstant salt and oxygen concentrations [23]. With such extreme conditions, marine Actinobacteria is expected to possess unique features and consequently secrete different and novel bioactive compounds compared to their terrestrial counterparts [24]. Marine actinobacteria are believed to originate from terrigenous sediments as they are widely distributed in the soil environment. In addition, another fact that support their terrestrial origin is their ability to produce resistant spores. These spores can be transported from the land to the marine environment and can remain dormant for several years [25]. In the marine environment, actinobacteria exist in mangrove swamps, other coastal environments, and even deep ocean sediments and significantly contribute to the diversity of the actinomycetes taxa [26,27].
Actinobacteria form stable populations in different marine niches and produce novel bioactive compounds with enormous biological activities [11,28,29]. Marine actinobacteria occupies various marine habitats such as different types of sediments; marine sediments [30], Ocean sediments [31], and sediments with moderate to high salt concentrations [32]. In addition, several actinobacterial genera were recovered from seawater [4], and freshwater ecosystems [33]. In addition, marine actinobacteria have been recovered from both swimming and sessile marine vertebrates and invertebrates [34]. Several genera belong to different families such as Micrococceae, Dermatophilaceae and Gordoniaceae, were found to be associated with sponges [18]. The actinobacterium Nocardiopsis dassonvillei was isolated from ovaries of the puffer fish [35].
Although the existence of indigenous actinobacteria in the marine environs still elusive, culture-dependent studies revealed several marine indigenous actinobacterial genera. These include Dietzia, Rhodococcus [36], Streptomyces, Salinispora [37], Marinophilus, Salinibacterium, Aeromicrobium marinum [38], Verrucosispora [39], Actinomadura, Actinosynnema, Arthrobacter, Blastococcus, Brachybacterium, Frankia, Geodermatophilus, Gordonia, Kitasatospora, Micromonospora, Micrococcus, Microbacterium, Nocardioides, Nocardiopsis, Psuedonocardia, Rhodococcus, Saccharopolyspora, Serinicoccus, Solwaraspora,, Streptosporangium, Tsukamurella, Turicella [34].
Actinobacteria has a significant role in the marine environment where they contribute in the degradation and turnover of numerous materials. Marine actinomycetes were reported to have cellulolytic and chitinolytic activities [40,41]. They also participate in the degradation and recycling of organic compounds [25] and produce a wide array of enzymes of industrial importance [42]. In addition, they have a vital role in mineralization of organic matter, nitrogen fixation and immobilization of mineral nutrients [43]. Most importantly, marine actinobacteria produce enormous numbers of bioactive compounds with a wide array of applications in agricultural, industrial, biotechnology and medical fields [9,24].
Marine Actinobacteria-Derived Secondary Metabolites
The increased rate of antibiotic resistance by pathogenic bacteria and the deleterious consequences of cancer chemotherapies are pushing toward alternative approaches. Actinobacteria have long been considered the prolific producers of a wide array of secondary metabolites with potential activities against viruses, fungi, bacteria, malaria, parasites and cancers [44]. Thousands of bioactive compounds were produced by several actinobacterial genera such as Streptomyces, Actinomyces, Corynebacterium, Micrococcus, Micromonospora [9,24]. Among members of the actinobacteria, more than 500 species of the genus Streptomyces responsible for about 80% of the total actinobacterial secondary metabolites with numerous biological activities [45]. In the subsequent sections, biological activities of actinobacterial-derived secondary metabolites against different pathogens and cancer are discussed.
Actinobacterial-Derived Compounds with Anti-Microbial Activities
Actinobacterial-Derived Compounds with Antibacterial Activities: Mortalities due to Multidrug-Resistant (MDR) organisms has increased dramatically with annual costs 20 billion dollars in the United States alone [46,47]. According to the official reports of the CDC, up to 23,000 people die annually in the USA due to infection with multidrug-resistant organisms. Moreover, global estimation of premature deaths is expected to increase to 300 million by 2050, with economic losses around $100 trillion [48]. Therefore, searching for alternative drugs with no previous resistance becomes a priority. Numerous bioactive compounds of marine bacteria were found to have good antibacterial activities against several drug resistant pathogenic bacteria. Abyssomicin C, a newly discovered antibiotic, has antibacterial activity against MDR gram-positive bacteria of medical importance. It was first isolated from the marine actinobacterium Verrucosispora [39]. Abyssomicin belongs to polycyclicpolyketide group and acts by inhibiting the biosynthesis of folic acid [49].
The marine species of Streptomyces produce a number of antibiotics such as bonactin [50], BD21-2, and chlorinated dihydroquinones. Structurally, chlorinated dihydroquinones can be categorized into the napyradiomycin class [51]. The three types were reported to have antibacterial activity against both Gram-positive and Gram-negative bacteria. Other antibacterial compounds produced by the marine Streptomyces species include, himalomycins [52], frigocyclinone [53], glyciapyrroles [54], essramycin [55], caboxamycin [56], marinopyrroles [57], and tirandamycin [58]. Some of these compounds are active against Gram-positive bacteria such as frigocyclinone while others have broad activity against both Gram-positive and Gram-negative bacteria such as essramycin.
In addition, antibacterial compounds such as tirandamycin displayed activity against vancomycin-resistant Enterococcus faecalis [58] and bisanthraquinone showed S. aureus resistant to methicillin and tetracycline [59]. Diazepinomicin and lynamicins are two antibiotics that produced by the marine actinobacteria Micromonospora and Marinispora, respectively. Lynamicins are chlorinated bisindole pyrroles which have broad spectrum activities against different bacteria. Moreover, lynamicins are active against methicillin-resistant S. aureus and vancomycin-resistant E. faecium [60]. Purified extracts of 20 isolates of actinomycetes including Streptomyces, Rubrobacter, Actinokineospora, Microbacterium, Micromonospora, and Rhodococcus were active against clinical isolates of E. faecalis, S. aureus, E. coli, P. aeruginosa [61].
Actinobacterial-Derived Compounds with Antifungal Activities: Although numerous types of fungi are being used in several industries, some are pathogenic to human, animals, and plants. They can cause serious human diseases such as candidiasis and others drastically affect several crops. Bioactive compounds extracted from marine actinobacteria displayed activity against both human and plant fungal pathogens. Chitinase, a bioactive compound from the marine actinobacterium Streptomyces, displayed activities against Aspergillus niger and Candida albicans. This strain of Streptomyces was first isolated from South China and was associated with a type of sponge [62]. Since chitin is a predominant component of fungal cell wall, chitinase produced by Streptomyces is expected to have a broad spectrum against several fungal species. In addition, chitinase is widely used in several fields such as biomedical, agricultural, food technology and cosmetics [63,64].
In biomedical field, chitinase has been used in wound healings, cartilage tissue engineering, and drug delivery [65,66]. In the agricultural field, antifungal compounds derived from marine actinobacteria have been used as agrochemicals [67,68]. For instance, the marine actinobacterium Streptomyces rutgersensis produces a systemic fungicide, Kasugamycin against Magnaporthe grisea [Yoshii et al., 2012]. Chandrananimycin A, is a novel compound that’s is isolated from Actinomadura sp., has a potent antifungal activity against Mucor miehei [69]. Another potent antifungal compound produced by Nocardia dassonvillei has an activity against C. albicans, with a MIC of 64 g/mL [70].
Macrolides produced by sponge-associated Streptomyces sp. and Micromonospora have activity against Cryptococcus and C. albicans at a concentration of 10 mg/ml [71,72]. Polyketides and alkaloids (e.g. caerulomycins A and C) are two anticandidal compounds produced by S. psammoticus and A. cyanogriseus, respectively [73,74]. In addition to these purified and identified compounds, extracts of some Salinispora species displayed antifungal activity against C. albicans [75]. Similarly, extracts from sponge-endosymbiotic Actinomycete strains were active against several fungal species such as C. tropicalis, A. fumigatus, and A. flavus [76, Vimal et al., 2009]. A novel antifungal protein named SAP was reported to have a broad spectrum activity against several plant fungal pathogens such as Alternaria alternata, A. fumigatus, A. versicolor, F. graminearum, M. circinelloides, and Pythium oligandrum [77]. In a recent study, Buatong et al, [78] identified a new compound with a potent antifungal activity against Pyricularia oryzae, a rice blast fungus. Extract of the marine actinobacterium Streptomyces sp. had a potent inhibitory effect on the growth of P. oryzae strains with MICs values of 8 to 16μg/ml and minimum fungicidal concentrations values of 16 to 128μg/ml. The active compound was determined by HPLC/MS as oligomycin A. Fortunately, the conditions of the submerged rice fields support the growth the marine Streptomyces sp., and thus can be used as biocontrol agent against rice blast fungus P. oryzae [78].
Actinobacterial-Derived Compounds with Antiviral Activities: Viruses have long been known to cause disastrous epidemics to the human beings. Millions of deaths were reported due to influenza viruses, human immunodeficiency virus, hepatitis viruses and many others. In fact, viruses are difficult to be treated and many viruses develop resistance to the available antiviral drugs. In this regard, several marine actinobacterial-derived compounds have antiviral activities. Benzastatin C, an alkaloid produced by Streptomyces nitrosporeus, exhibited antiviral activity herpesviruses; herpes simplex virus type 1, herpes simplex virus type 2 and Vesicular Stomatitis Virus (VSV), respectively [79]. Extracts from the marine Streptomyces increased the survival rate of shrimps’ larvae challenged with White Spot Syndrome Virus [80]. This virus causes great economic losses to the shrimp farmers due to its high mortality rate. Guanine-7-N-oxide purified from a culture of Streptococcus sp. was active against fish herpes virus, rhabdovirus, and infectious pancreatic necrovirus [81].
Replication of influenza viruses was found to be affected by bioactive compounds from Actinomadura sp [82] and Streptomyces lavendulae [83]. Raveh, et al. [84] successfully isolated a potent antiviral compound, antimycin A1a, from the marine Streptomyces kaviengensis. Antimycin A1a showed a broad spectrum activity against several viruses including western, eastern, and Venezuelan equine encephalitis viruses (Togaviridae), La Crosse virus (Bunyaviridae), cephalomyocarditis virus (Picornaviridae), vesicular stomatitis virus (Rhabdoviridae), Sendai virus (Paramyxoviridae) and hepatitis C virus (Flaviviridae) [84]. The compound (Z)-1-((1-hydroxypenta-2,4-dien-1-yl) oxy) anthracene- 9,10-dione” is produced by Nocardia alba and was report to inhibit the replication of Newcastle disease virus and infectious Brucellosis disease virus [85].
It is obvious that marine actinobacterial derived compounds have been extensively tested against bacteria and fungi. But antiviral activities of these compounds still need further investigation. This could be attributed to the high safety levels, laboratory facilities, animal models and costs required to perform viral assays. Actinobacteria of marine origin are rich in a variety of secondary metabolites that may have potent antiviral activities. We believe that screening of bioactive compounds from marine actinobacteria could reveal novel and potent antiviral compounds. This could help to treat diseases caused by these viruses and to control the serious epidemics caused by viruses of medical importance.
Actinobacterial-Derived Compounds with Anticancer Activities
Cancer is a life threatening disease with annual worldwide incidence of 10 million cases [86]. Traditional cancer therapies such as chemotherapy, surgery, and radiotherapy usually associated with serious side effects [87,88]. The deleterious consequences of such traditional therapies push toward searching for alternative approaches. In this regard, marine actinobacteria offer a plenty of bioactive compounds with anti-proliferative activities. Species of marine actinobacteria; Salinispora, Streptomyces, and Marinispora were investigated for their anti-proliferative compounds. Salinosporamide A, a bicyclic beta-lactone gammalactam compound, produced by Salinispora tropica displayed anti-proliferative activity against different cell lines [89,90]. Salinosporamide A acts by stimulating the apoptosis cascade in malignant cells [91]. The promising results of Salinosporamide A encouraged its use in clinical trials to treat different cancer types [92]. Two species of the marine marine actinobacterium Salinispora; S. arenicola and S. pacifica produce a considerable number of polyketide I with anticancer activity against human colorectal cancer cell line [93,94]. S. pacifica give rise to four new polyketides, salinipyrones A and B, and pacificanones A and B which were active against human colon cancer cells [94].
Species of Streptomyces produce an array of antiproliferative compounds such caprolactones and chinikomycin, aureoverticillactam, chalcomycin, daryamides, piperazimycins, and mansouramycin. Caprolactones and chinikomycin exhibited activity against different cell lines of human origin such as breast cancer, melanoma and renal cancer [95,96]. Chalcomycin and Daryamide A were active against HeLa cell line [97], and human colon carcinoma cell line [98], respectively. Aureoverticillactam was reported to inhibit the growth of human colorectal adenocarcnioma cell line, Jurkat leukemia and mouse melanoma cell lines [99].
Other genera of actinobacteria such as Marinispora sp. and Thermoactinomyces sp. produce two potent antitumor compounds marinomycins and mechercharmycin A, respectively [100,101]. Piperazimycins exhibited a good efficacy against human colon carcinoma cell line, leukaemia, melanoma, and prostate cell line [102]. In a similar fashion, mansouramycins displayed significant cytotoxicity to an array of cell lines including lung cancer, breast cancer, melanoma and prostate cancer cells [103]. In a recent study, extract of Streptomyces bingchenggensis was cytotoxic to different cell lines of human origin such as human acute myelocytic leukemia (K562), cervical cancer (HeLa), human gastric, breast cancer (MCF- 7), and human acute promyelocytic leukemia (HL-60). Purification and structural elucidation of S. bingchenggensis’s extract revealed two anticancer compounds; ULDF4 and ULDF5 which are structurally related to staurosporine and kigamicin [104].
Actinobacterial-Derived Compounds with Anti-Parasitic Activities
The activities of actinobacterial bioactive compounds are extended to the parasites of medical importance such as Trypanosoma sp., Leishmania sp. and Plasmodium sp. Trypanosomatids cause three important diseases to humans; the African trypanosomiasis or sleeping sickness caused by Trypanosoma brucei, South American trypanosomiasis or Chagas disease, caused by Trypanosoma cruzi, and leishmaniasis caused by several species of Leishmania [105]. Of these diseases, Chagas disease is a systemic disease (i.e. affect multiple organs) that killed more than 10,000 individuals during the year 2008. It was estimated that T. cruzi infected more than18 million people and about 40 million people are at risk of getting the infection [106]. Similarly, leishmaniasis affects more than 88 countries with around 350 million people are at risk of acquiring infection [107]. Malaria is an endemic disease that affects several countries with high incidence and high mortality rate. The causative agent, P. falciparum, infects more than 300 million cases with an estimated 2 million deaths per annum [24].
The available drugs to treat these parasitic diseases are not efficient and some were not effective because of resistance. In addition, some anti-parasitic synthetic drugs have serious side effects due to prolonged parenteral administration [108]. Benznidazole is specific for the treatment of Chagas disease but is active for the acute phase of the disease with toxic side effects [106]. For the treatment of leishmaniasis, antimony organic compounds are commonly prescribed followed by pentamidine, miltefosine, and amphotericin B [108]. The high mortality rates of such parasitic diseases, side effects of the used drugs and drug resistance necessitate the need for novel and effective therapeutic drugs. Screening of marine actinobacteria yielded several bioactive compounds with anti-parasitic activities.
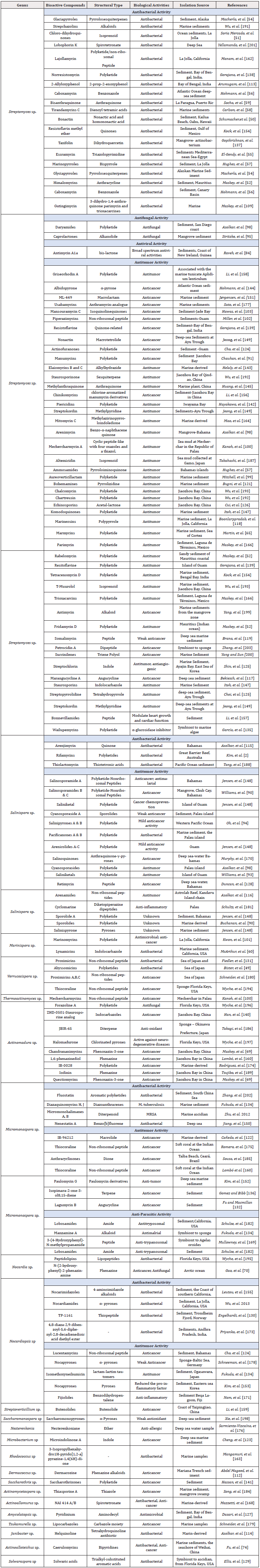
Maskey et al, reported the high efficiency of Trioxacarcins compounds against P. falciparum. The authors purified trioxacarcin A, B and C from cultures of Streptomyces ochraceus and Streptomyces bottropensis [109]. Sponge-associated Streptomyces produce three compounds with anti-parasitic activity; cyclic depsipeptide valinomycin, indolocarbazole alkaloid staurosporine and butenolide. The three compounds were active against Leishmania major and Trypanosoma brucei brucei [110]. Actinosporins, a compound produced by the sponge-associated Actinokineospora sp. was active against Trypanosoma brucei brucei [61]. In a recent study, Santos, et al. [111] successfully isolated eighteen isolates of sponges-associated marine actinomycetes. They tested the extracts of these isolates for their anti-fungal, antibacterial, anti-cancer and anti-parasitic activities. Extracts of some isolates were active against Trypanosoma cruzi [111-203] (Table 1).
Conclusion and Future Perspectives
In the medical field, two serious problems need to be resolved; i) the continual emergence of drug resistant microorganisms which increases the likelihood of life threatening infections, and ii) the deleterious side effects of using traditional cancer therapies. These two important issues challenged the researchers to search for safe and effective alternatives. In this regard, marine actinobacteria is considered a drug treasure house as they offer a tremendous number of secondary metabolites with a wide array of biological activities. Thousands of marine actinobacteria-derived compounds have been tested in in vitro for their antimicrobial and anti-proliferative activities. In addition, some compounds were evaluated in pre-clinical and clinical studies. Of the tested compounds, some compounds were of potential activity against MDR bacteria, drug resistant viral strains and several types of cancers. Among the actinobacterial genera, Streptomyces sp. is responsible for the largest number of bioactive compounds. Marine actinobacteria occupies different marine niches and can be found associated with several vertebrate and invertebrate speices. Although marine actinobacteria is a very important source for discovering novel bioactive compounds, research in this field still necessitates further improvements. Collection of deep sea samples, extraction, purification and identification techniques are needed to be developed.
Acknowledgement
None.
Conflict of Interest
None.
References
- Baguley BC, Kerr DJ (2002) Anticancer drug development. San Diego: Academic Press.
- Kim TK, Fuerst JA (2006) Diversity of polyketide synthase genes from bacteria associated with the marine sponge Pseudoceratina clavata: culture-dependent and culture-independent approaches.Environ. Microbiol 8(8): 1460-1470.
- Bull AT, Stach JE (2007) Marine actinobacteria: new opportunities for natural product search and discovery. Trends Microbiol 15(11): 491-499.
- Subramani R, Aalbersberg W (2013) Culturable rare actinomycetes: diversity, isolation and marine natural product discovery. Appl Microbiol Biotechnol 97(21): 9291-9321.
- Azman AS, Othman I, Velu SS, Chan KG, Lee LH (2015) Mangrove rare actinobacteria: taxonomy, natural compound, and discovery of bioactivity. Front Microbiol 6: 856.
- Bhim PS, Vijai KG, Ajit KP (2018) Actinobacteria: diversity and biotechnological applications. Amsterdam, Netherlands; Oxford, United Kingdom; Cambridge, Ma, United States Elsevier.
- María Julia Amoroso (2013) Actinobacteria: application in bioremediation and production of industrial enzymes. Boca Raton, Florida: Crc Press.
- Gomathi A, Gothandam KM (2016) Ocean Dwelling Actinobacteria as Source of Antitumor Compounds. Brazilian Archives of Biology and Technology 59.
- Berdy J (2005) Bioactive Microbial metabolites. J Antibiot 58(1): 1-26.
- Newman DJ, Cragg GM (2007) Natural products as sources of new drugs over the last 25 years. J Nat Prod 70: 461-477.
- Olano C, Méndez C, Salas JA (2009) Antitumor compounds from actinomycetes: from gene clusters to new derivatives by combinatorial biosynthesis. Nat Prod Rep 26: 628-660.
- Baltz RH (2008) Renaissance in antibacterial discovery from actinomycetes. Curr Opin Pharmacol 8(5): 557-563.
- Piel J (2004) Metabolites from symbiotic bacteria. Nat Prod Rep 21: 519-538.
- Tsueng G, Teisan S, Lam KS (2008) Defined salt formulations for the growth of Salinispora tropica strain NPS21184 and the production of salinosporamide A (NPI-0052) and related analogs. Appl Microbiol Biotechnol 78: 827-832.
- Erin A Gontang, William Fenical, Paul R Jensen (2007) Phylogenetic diversity of Gram-positive bacteria cultured from marine sediments. Appl Environ Microbiol 73(10): 3272-3282.
- Anzai K, Nakashima T, Kuwahara N, Suzuki R, Ohfuku Y, Takeshita S, Ando K, et al. (2008) Actinomycete bacteria isolated from the sediments at coastal and offshore area of Nagasaki Prefecture, Japan: diversity and biological activity. J Biosci Bioeng 106(2): 215-217.
- Maldonado LA, Fragoso Yáñez D, Pérez García A, Rosellón Druker J, Quintana ET (2009) Actinobacterial diversity from marine sediments collected in Mé Antonie van Leeuwenhoek 95: 111-120.
- Kin S Lam, Ginger Tsueng, Katherine A McArthur, Scott S Mitchell, Barbara CM Potts, et al. (2007) Effects of halogens on the production of salinosporamides by the obligate marine actinomycete Salinispora tropica. J Antibiot 60(1): 13-19.
- Selvin J, Shanmughapriya S, Gandhimathi R, Seghal Kiran G, Rajeetha Ravji, et al. (2009) Optimization and production of novel antimicrobial agents from sponge associated marine actinomycetes Nocardiopsis dassonvillei MAD08. Appl Microbiol Biotechnol 83: 435-445.
- Yan Hua Hou, Quan Fu Wang, Ling Ding, Fu Chao Li, Song Qin (2008) attB site disruption in marine Actinomyces sp. M048 via DNA transformation of a site-specific integration vector. Biotechnol Appl Biochem 50(1): 11-16.
- Donia M, Hamann MT (2003) Marine natural products and their potential applications as anti-infective agents. Lancet Infect Dis 3(6): 338-348.
- Das S, Lyla PS, Khan SA (2006) Marine microbial diversity and ecology: importance and future perspectives. Curr Sci 90: 1325-1335.
- Bull AT, Ward AC, Goodfellow M (2000) Search and discovery strategies for biotechnology: the paradigm shift. Microbiol Mol Biol Rev 64(3): 573-606.
- Manivasagan P, Venkatesan J, Sivakumar K, Kim S K (2014) Pharmaceutically active secondary metabolites of marine actinobacteria. Microbiological Research 169: 262-278.
- Goodfellow M, Haynes JA (1984) Actinomycetes in marine sediments, in Biological, Biochemical, and Biomedical Aspects of Actinomycetes. Academic Press New York :453-472.
- Stach JEM, Maldonado LA, Ward AC, Bull AT, Goodfellow M (2004) Williamsia maris sp. nov, a novel actinomycete isolated from the Sea of Japan. Int J Syst Evol Microbiol 54: 191-194.
- Tae KK, Garson MJ, Fuerst JA (2005) Marine actinomycetes related to the ‘Salinospora’ group from the Great Barrier Reef sponge Pseudoceratina clavata. Environ Microbiol 7 (4): 509-518.
- Prudhomme J, McDaniel E, Ponts N, Bertani S, Fenical W, et al. (2008) Marineactinomycetes: a new source of compounds against the human malaria parasite. PLoS ONE 3(6): e2335.
- Rahman H, Austin B, Mitchell WJ, Morris PC, Jamieson DJ, et al. (2010) Novel Antiinfective compounds from marine Actinobacteria. Mar Drugs 8: 498-518.
- Maldonado LA, Fenical W, Jensen PR, Kauffman CA, Mincer TJ, et al. (2005) Salinispora arenicola gen. nov., sp. nov. and Salinispora tropica sp. nov., obligate marine actinomycetes belonging to the family Micromonosporaceae. Int J Syst Evol Microbiol 55: 1759-1766.
- Solano G, Jiménez KR, Jaspars M, Castillo GT (2009) Study of the diversity ofculturable actinomycetes in the North Pacific and Caribbean coasts of CostaRica. Antonie Van Leeuwenhoek 96: 71-78.
- Jinyuan Wu, Tongwei Guan, Hongchen Jiang, Xiaoyang Zhi, Shukun Tang, et al. (2009) Diversity of actinobacterial community in saline sediments from Yunnan and Xinjiang, China. Extremophiles 13(4): 623-632.
- Allgaier M, Grossart HP (2006) Diversity and seasonal dynamics of Actinobacteria populations in four lakes in northeastern Germany. Appl Environ Microbiol 72: 3489-3497.
- Ward AC, Bora N (2006) Diversity and biogeography of marine actinobacteria. Curr Opin Microbiol 9(3): 279-286.
- Wu Z, Xie L, Xia G, Zhang J, Nie Y, Hu J, et al. (2005) A new tetrodotoxin-producing actino-mycete, Nocardiopsis dassonvillei, isolated from the ovaries of puffer fish Fugurubripes. Toxicon 45(7): 851-859.
- SC Heald, PF Brandão, R Hardicre, AT Bull (2001) Physiology, biochemistry and taxonomy of deep-sea nitrile metabolising Rhodococcus strains. Antonie Van Leeuwenhoek 80(2): 169-183.
- Mincer TJ, Fenical W, Jensen PR (2005) Culture-dependent and culture-independent diversity within the obligate marine actinomycete genus Salinispora. Appl Environ Microbiol 71: 7019-7028.
- Bruns A, Philipp H, Cypionka H, Brinkhoff T (2003) Aeromicrobium marinum sp. nov., an abundant pelagic bacterium isolated from the German Wadden sea. Antonie Van Leeuwenhoek 53(6): 1917-1923.
- Riedlinger J, Reicke A, Krismer B, Zahner H, Bull AT, et al. (2004) Abyssomicins, inhibitors of para-aminobenzoic acid pathway produced by the marine Verrucosispora strain AB-18-032. J Antibiot 57: 271-279.
- Chandramohan D, Ramu S, Natarajan R (1972) Cellulolytic activity of marine streptomycetes. Curr Sci 41: 245-246.
- Pisano MA, Sommer MJ, Taras L (1992) Bioactivity of chitinolytic actinomycetes of marine origin. Appl Microbiol Biotechnol 36: 553-555.
- Ramesh S, Mathivanan N (2009) Screening of marine actinomycetes isolated from the Bay of Bengal, India for antimicrobial activity and industrial enzymes. World J Microbiol Biotechnol 25: 2103-2111.
- Goodfellow M, Williams ST (1983) Ecology of actinomycetes. Annu Rev Microbiol 37: 189-216.
- Baltz RH (2008) Renaissance in antibacterial discovery from actinomycetes. Curr Opin Pharmacol 8(5): 557-563.
- Watve MG, Tickoo R, Jog MM, Bhole BD (2001) How many antibiotics are produced by thegenus Streptomyces? Arch Microbiol 176(5): 386-390.
- Cosgrove SE (2006) The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stays, and health care costs. Clin Infect Dis 42(2): S82-89.
- Sydnor ERM, Perl TM (2011) Hospital epidemiology and Infection control in acute-care settings. Clin Microbiol Rev 24(1): 141-173.
- Centers for Disease Control (2013). Antibiotic resistance threats in the United States.
- Bister B, Bischoff D, Stroebele M, Riedlinger J, Reicke A, Wolter F, et al. (2004) Abyssomicin C-a polycyclic antibiotic from a marine Verrucosispora strain as an inhibitor ofthe p-aminobenzoic acid/tetrahydrofolate biosynthesis pathway. Angew ChemInt 43(19): 2574-2576.
- Schumacher RW, Talmage SC, Miller SA, Sarris KE, Davidson BS, et al. (2003) Isolationand structure determination of an antimicrobial ester from a marine sediment-derived bacterium. J Nat Prod 66(9): 1291-1293.
- Soria Mercado IE, Prieto Davo A, Jensen PR, Fenical W (2005) Antibiotic terpenoidchloro-dihydroquinones from a new marine actinomycete. J Nat Prod 68(6): 904-910.
- Maskey RP, Helmke E, Laatsch H (2003) Himalomycin A and B: isolation and structure elu-cidation of new fridamycin type antibiotics from a marine Streptomyces isolate. J Antibiot a;56(11): 942-949.
- Bruntner C, Binder T, Pathom-aree W, Goodfellow M, Bull AT, Potterat O, et al. (2005) Frigocyclinone, a novel Angucyclinone antibiotic produced bya Streptomyces griseus strain from Antarctica. J Antibiot 58(5): 346-349.
- Macherla VR, Liu J, Bellows C, Teisan S, Lam KS, et al. (2005) Glaciapyrroles A, B and C, pyrrolosesquiterpenes from a Streptomyces sp. isolated from an Alaskan marine sediment. J Nat Prod 68: 780-783.
- El Gendy MM, Shaaban M, Shaaban KA, El Bondkly AM, Laatsch H (2008) Essramycin: a first triazolopyrimidine antibiotic isolated from Nature. J Antibiot 61(3): 149-157.
- Hohmann C, Schneider K, Bruntner C, Irran E, Nicholson G, Bull AT, et al. (2009a) Caboxamycin, a new antibiotic of the benzoxazole family produced by the deep-sea strainStreptomyces sp NTK 937. J Antibiot 62(2): 99-104.
- Hughes CC, Prieto Davo A, Jensen PR, Fenical W (2008) The marinopyrroles, antibioticsof an unprecedented structure class from a marine Streptomyces sp. Org Lett 10(4): 629-631.
- Carlson JC, Li S, Burr DA, Sherman DH (2009) Isolation and characterizationof tirandamycins from a marine-derived Streptomyces sp. J Nat Prod 72(11): 2076-2079.
- Socha AM, LaPlante KL, Rowley DC (2006) New bisanthraquinone antibiotics andsemi-synthetic derivatives with potent activity against clinical Staphylococ-cus aureus and Enterococcus faecium isolates. Bioorg Med Chem 14(24): 8446-8454.
- McArthur KA, Mitchell SS, Tsueng G, Rheingold A, White DJ, et al. (2008) Lynamicins A-E, chlorinated bisindole pyrrole antibiotics from a novel marineactinomycete. J Nat Prod 71(10): 1732-1737.
- Abdelmohsen UR, Pimentel Elardo SM, Hanora A, Radwan M, Abou El Ela SH, et al. (2010) Isolation, phylogenetic analysis and anti-infective activity screening of marine sponge-associated actinomycetes. Mar Drugs 8(3): 399-412.
- Han Y, Yang B, Zhang F, Miao X, Li Z (2009) Characterization of antifungal chitinase frommarine Streptomyces sp., DA11 associated with South China Sea sponge Craniellaaustraliensis. Mar Biotechnol 11(1): 132-140.
- Rabea EI, Badawy ME T, Stevens CV, Smagghe G, Steurbaut W (2003) Chitosan asantimicrobial agent: applications and mode of action. Biomacromolecules 4(6): 1457-1465.
- Lin CC, Lin HL (2005) Remediation of soil contaminated with the heavy metal (Cd2+). J Hazard Mater 122(1): 7-15.
- Shi C, Zhu Y, Ran X, Wang M, Su Y, et al. (2006) Therapeutic potential of chitosan andits derivatives in regenerative medicine. J Surg Res 133(2): 185-192.
- Yan J, Li X, Liu L, Wang F, Zhu TW, Zhang Q, er al. (2006) Potential use of collagen-chitosan-hyaluronan tri-copolymer scaffold for cartilage tissue engineering. Artif Cells Blood Substitutes Biotechnol 34(1): 27-39.
- Blunt JW, Copp BR, Munro MH, Northcote PT, Prinsep MR, et al. (2006) Marine natural products.Nat Prod Rep 23: 26-78.
- Newman DJ, Cragg GM (2012) Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod 75: 311-335.
- Maskey RP, Li F, Qin S, Fiebig HH, Laatsch H (2003) Chandrananimycins A approximately C: production of novel anticancer antibiotics from a marine Actinomadura sp. iso-late M048 by variation of medium composition and growth conditions. J Antibiot b;56(7): 622-634.
- Gao X, Lu Y, Xing Y, Ma Y, Lu J, Bao W, et al. (2012) A novel anticancer and antifun-gus phenazine derivative from a marine actinomycete BM-17. Microbiol Res 167(10): 616-622.
- N Imamura, M Nishijima, K Adachi, H Sano (1993) Novel antimycin antibiotics, urauchimycins A and B, produced by marine actinomycete. J Antibiot 46(2): 241-246.
- Lin R, Nie YL, Zhang H, Jiang H (2010) Isolation and identification on neorustmicin producing strain FIM03-1149. J Microbiol 30(1): 38-42.
- Sujatha E, Bapi Raju KVVSN, Ramana T (2005) Studies on a new marine streptomycete BT408 producing polyketide antibiotic SBR-22 effective against methicillin resistant Staphylococcus aureus. Microbiol Res 160 (2): 119-126.
- Fu P, Wang SX, Hong K, Li X, Liu PP, et al. (2011) Cytotoxic bipyridines from the marine-derived actinomycete Actinoalloteichus cyanogriseus WH1-2216-6. J Nat Prod 74(8): 1751-1756.
- Mincer TJ, Jensen PR, Kauffman CA, Fenical W (2002) Widespread and persistent populations of a major new marine actinomycete taxon in ocean sediments. Appl Environ Microbiol 68: 5005-5011.
- Gandhimathi R, Arunkumar M, Selvin J, Thangavelu T, Sivaramakrishnan S, et al. (2008) Antimicrobial potential of sponge associated marine actinomycetes. J Med Mycol 18(1): 16-22.
- Woo JH, Kitamura E, Myouga H, Kamei Y (2002) An antifungal protein from the marine bacterium Streptomyces sp. strain AP77 is specific for Pythium porphyrae, a causative agent of red rot disease in Porphyra spp. Appl Environ Microbiol 68(6): 2666-2675.
- Buatong Jirayu, Vatcharin Rukachaisirikul, Suthinee Sangkanu, Frank Surup, Souwalak Phongpaichit (2019) Antifungal Metabolites from Marine-Derived Streptomyces sp. AMA49 against Pyricularia oryzae, J Pure Appl Microbiol 13(2): 653-665.
- Lee JG, Yoo ID, Kim WG (2007) Differential antiviral activity of benzastatin C and its dechlorinated derivative from Streptomyces nitrosporeus Biol Pharm Bull 30(4): 795-797.
- Kumar SS, Philip R, Achuthankutty C (2006) Antiviral property of marine actinomycetes against white spot syndrome virus in penaeid shrimps. Curr Sci 91(6): 807–811.
- Nakagawa A, Tomoda H, Hao V M, Iwai Y, Omura S (1985) Antiviral activities of pentale nolactones. J Antibiot 8: 1114-1115.
- Hasobe M, Saneyoshi M, Isono K (1985) Antiviral activity and its mechanism of guanine 7-N oxide on DNA and RNA viruses derived from Salmonid. J Antibiot 38(11): 1581-1587.
- Sacrament ODR, Coelho RRR, Wigg MD, Linhares LFTD, Santos MGMD, et al. (2004) Antimicrobial and antiviral activities of an actinomycete (Streptomyces sp.) isolated from a Brazilian tropical forest soil. World J Microbiol Biotechnol 20(3): 225-229.
- Raveh A, Delekta PC, Dobry CJ, Peng W, Schultz PJ, et al. (2013) Discovery of Potent Broad-Spectrum Antivirals Derived from Marine Actinobacteria. PLoS ONE 8(12): e82318.
- Janardhana A, Praveen AK, Buddolla V, Gopala DS, Narasimha G (2018) Antiviral and Larvicidal Properties of Novel Bioactive Compounds Produced from Marine Actinomycetes. Russian Journal of Marine Biology 44(5): 424-428.
- Ma X, Yu H (2006) Global burden of cancer. Yale J Biol Med 79(3-4): 85-94.
- Urruticoechea A, Alemany R, Balart J, Villanueva A, Vinals F, et al. (2010) Recent advances in cancer therapy: an overview. Curr Pharm Des 16(1): 3-10.
- Nurgali K, Jagoe RT, Abalo R (2018) Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Front Pharmacol 9: 245.
- Feling RH, Buchanan GO, Mincer TJ, Kauffman CA, Jensen PR, et al. (2003) Salinospo-ramide A: a highly cytotoxic proteasome inhibitor from a novel microbialsource, a marine bacterium of the new genus Salinospora. Angew Chem Int Ed 42(3): 355-357.
- Williams PG, Buchanan GO, Feling RH, Kauffman CA, Jensen PR, Fenical W, et al. (2005) Newcytotoxic salinosporamides from the marine actinomycete Salinispora tropica. JOrg Chem 70(16): 6196-6203.
- Chauhan D, Catley L, Li G, Podar K, Hideshima T, Velankar M, et al. (2005) A novel orallyactive proteasome inhibitor induces apoptosis in multiple myeloma cells withmechanisms distinct from Bortezomib. Cancer Cell 8(5): 407-419.
- Fenical W, Jensen PR, Palladino MA, Lam KS, Lloyd GK, Potts BC, et al. (2009) Discovery and devel-opment of the anticancer agent salinosporamide A (NPI-0052). Bioorg Med Chem 17(6): 2175-2180.
- Williams PG, Miller ED, Asolkar RN, Jensen PR, Fenical W, Arenicolides AC (2007) 26-membered ring macrolides from the marine actinomycete Salinispora arenicola. J Org Chem 72(14): 5025-5034.
- Oh DC, Gontang EA, Kauffman CA, Jensen PR, Fenical W (2008) Salinipyrones and pacificanones, mixed-precursor polyketides from the marine actinomycete Salinispora pacifica. J Nat Prod 71(4): 570-575.
- Stritzke K, Schulz S, Laatsch H, Helmke E, Beil W (2004) Novel caprolactones from a marinestreptomycete. J Nat Prod 67(3): 395-401.
- Liu R, Cui CB, Duan L, Gu QQ, Zhu WM (2005) Potent in vitro anticancer activity of metacycloprodigiosin and undecylprodigiosin from a sponge-derived actinomycete Saccharopolyspora sp. Nov. Arch Pharm Res 28(12): 1341-1344.
- Asolkar RN, Maskey RP, Helmke E, Laatsch H (2002) Chalcomycin B a new macrolideantibiotic from the marine isolate Streptomyces sp. B7064 J Antibiot 55(10): 893-898.
- Asolkar RN, Jensen PR, Kauffman CA, Fenical W (2006) Daryamides AC weakly cytotoxicpolyketides from a marine-derived actinomycete of the genus Streptomycesstrain CNQ-085. J Nat Prod 69(12): 1756-1759.
- Mitchell SS, Nicholson B, Teisan S, Lam KS, Barbara C (2004) Aureoverticillactam, a novel22-atom macrocyclic lactam from the marine actinomycete Streptomyces aure-overticillatus. J Nat Prod 67(8): 1400-1402.
- Kanoh K, Matsuo Y, Adachi K, Imagawa H, Nishizawa M, et al. (2005) MechercharmycinsA and B, cytotoxic substances from marine-derived Thermoactinomyces sp. YM3-251. J Antibiot 58(4): 289-292.
- Kwon HC, Kauffman CA, Jensen PR, Fenical W, Marinomycins AD (2006) antitumor-antibiotics of a new structure class from a marine actinomycete of the recentlydiscovered genus “Marinispora”. J Am Chem Soc 128(5): 1622-1632.
- Miller ED, Kauffman CA, Jensen PR, Fenical W (2007) Piperazimycins: cytotoxic hexadep-sipeptides from a marine-derived bacterium of the genus Streptomyces. J OrgChem 72(2): 323-330.
- Hawas UW, Shaaban M, Shaaban KA, Speitling M, Maier A, Kelter G, et al. (2009) Man-souramycins A-D, cytotoxic isoquinolinequinones from a marine Streptomycete.J Nat Prod 72(12): 2120-2124.
- Davies Bolorunduro OF, Adeleye IA, Akinleye MO, Wang PG (2019) Anticancer potential of metabolic compounds from marine actinomycetes isolated from Lagos Lagoon sediment. Journal of Pharmaceutical Analysis 9(3): 201-208.
- Lopes MS, de Souza Pietra RC, Borgati TF, Romeiro CF, Júnior PA, Romanha AJ, et al. (2011) Synthesis and evaluation of the anti-parasitic activity of aromatic nitro compounds. Eur J Med Chem 46(11): 5443–5447.
- Urbina JA, Docampo R (2003) Specific chemotherapy of Chagas disease: controversies andadvances. Trends Parasitol 19(11): 495-501.
- Croft SL, Coombs GH (2003) Leishmaniasis-current chemotherapy and recent advancesin the search for novel drugs. Trends Parasitol 19(11): 502-508.
- Shukla AK, Singh BK, Patra S, Dubey VK (2010) Rational approaches for drug designingagainst leishmaniasis. Appl Biochem Biotechnol 160(8): 2208-2218.
- Maskey RP, Helmke E, Kayser O, Fiebig HH, Maier A, et al. (2004) Anti-cancerand antibacterial trioxacarcins with high anti-malaria activity from a marine Streptomycete and their absolute stereochemistry. J Antibiot b;57(12): 771-779.
- Pimentel Elardo SM, Kozytska S, Bugni TS, Ireland CM, Moll H, et al. (2010) Anti-parasitic compounds from Streptomyces sp. strains isolated from Mediterranean sponges. Mar Drugs 8(2): 373-380.
- Santos JD, Vitorino I, De la Cruz M, Díaz C, Cautain B, et al. (2019) Bioactivities and Extract Dereplication of Actinomycetales Isolated from Marine Sponges. Front Microbiol 10: 727.
- Abdel Mageed WM, Milne BF, Wagner M, Schumacher M, Sandor P, Pathom aree, W, et al. (2010) Dermacozines, a new phenazine family from deep-sea dermacocci isolated from a Mariana Trench sediment. Org. Biomol Chem 8(10): 2352-2362.
- Arumugam M, Mitra A, Jaisankar P, Dasgupta S, Sen T, Gachhui R, et al. (2009) Isolation of an unusual metabolite 2-allyloxyphenol from a marine actinobacterium, its biological activities and applications. Appl Microbiol Biotechnol 86(1): 109-117.
- Asolkar RN, Schroeder D, Heckmann R, Lang S, Wagner Doebler I, Laatsch H (2004) Helquinoline a new tetrahydroquinoline antibiotic from Janibacter limosus Hel1. J Antibiot (Tokyo) 57(1): 17-23.
- Asolkar RN, Kirkland TN, Jensen PR, Fenical W (2010) Arenimycin, an antibiotic effective against rifampin-and methicillin-resistant Staphylococcus aureus from the marine actinomycete Salinispora arenicola. J Antibiot 63(1): 37-39.
- Asolkar RN, Freel KC, Jensen PR, Fenical W, Kondratyuk TP, et al. (2009) cytotoxic NF-kappaB inhibitors from the marine actinomycete Salinispora arenicola. J Nat Prod 72(3): 396-402.
- Bekiesch P, Basitta P, Apel AK (2016) Challenges in the heterologous production of antibiotics in Streptomyces Arch. Pharm Chem Life Sci 349(8): 1-8.
- Boonlarppradab C, Kauffman CA, Jensen PR, Fenical W (2008) Marineosins A and B, cytotoxic spiroaminals from a marine-derived actinomycete. Org Lett 10(24): 5505-5508.
- Braña AF, Sarmiento Vizcaíno A, Osset M, Pérez Victoria I, Martín J, De Pedro NN, et al. (2017) Lobophorin K, a new natural product with cytotoxic activity produced by Streptomyces sp. M-207 associated with the deep-sea coral Lophelia pertusa. Mar Drugs 15(5): 144.
- Greg O Buchanan, Philip G Williams, Robert H Feling, Christopher A Kauffman, Paul R Jensen, et al. (2005) Sporolides A and B: structurally unprecedented halogenated macrolides from the marine actinomycete Salinispora tropica. Org Lett 7(13): 2731-2734.
- Tim S Bugni, Matthew Woolery, Christopher A Kauffman, Paul R Jensen, William Fenical (2006) Bohemamines from a marine-derived Streptomyces sp. J Nat Prod 69(11): 1626-1628.
- LM Cañedo, JL Fernández Puentes, JP Baz (2000) IB-96212, a novel cytotoxic macrolide produced by a marine Micromonospora. II. Physico-chemical properties and structure determination. J Antibiot 53(5): 479-483.
- Cheng C, Othman ME, Stopper H, Ebel R, Hentschel U, Abdelmohsen RU, et al. (2017) Isolation of Petrocidin A, a new cytotoxic cyclic dipeptide from the marine sponge-derived bacterium Streptomyces sp. SBT348. Mar Drugs 15(12): 383.
- Cho JY, Kwon HC, Williams PG, Kauffman CA, Jensen PR, Fenical W, et al. (2006) ActinofuranonesA and B, polyketides from a marine-derived bacterium related to the genus Streptomyces (Actinomycetales). J Nat Prod 69(3): 425-428.
- Choi IK, Shin HJ, Lee HS, Kwon HJ (2007) Streptochlorin, a marine natural product, inhibits NF-κB activation and suppresses angiogenesis in vitro. J Microbiol Biotechnol 17(8): 1338-1343.
- CB Cui, H B Liu, JY Gu, QQ Gu, B Cai, et al. (2007) Echinosporins as new cell cycle inhibitors and apoptosis inducers from marine-derived Streptomyces albogriseolus. Fitoterapia 78(3): 238-240.
- Dasari VRRK, Muthyala MKK, Nikku MY, Donthireddy SRR (2012) Novel Pyridinium com-pound from marine actinomycete, Amycolatopsis alba var. nov., DVR D4 showingantimicrobial and cytotoxic activities in vitro. Microbiol Res 167(6):346-351.
- Katherine R Duncan, Max Crüsemann, Anna Lechner, Anindita Sarkar, Jie Li, et al. (2015) Molecular networking and pattern-based genome mining improves discovery of biosynthetic gene clusters and their products from Salinispora species. Chem Biol 22(4): 460-471.
- Ellis GA, Wyche TP, Fry CG, Braun DR, Bugni TS (2014) Solwaric acids A and B, antibacterial aromatic acids from a marine Solwaraspora sp. Mar Drugs 12(2): 1013-1022.
- Engelhardt K, Degnes KF, Kemmler M, Bredholt H, Fjærvik E, Klinkenberg G, et al. (2010) Production of a New Thiopeptide Antibiotic, TP-1161, by a Marine Nocardiopsis Species. Applied and Environmental Microbiology 76(15): 4969-4976.
- Hans Peter Fiedler, Christina Bruntner, Julia Riedlinger, Alan T Bull, Gjert Knutsen, et al. (2008) Proximicin A, B and C, novel aminofuran antibiotic and anticancer compounds isolated from marine strains of the actinomycete Verrucosispora. J Antibiot 61(3): 158-163.
- Fu P, Macmillan JB (2015) Thiasporines A-C, thiazine and thiazole derivatives from a marine-derived Actinomycetospora chlora. J Nat Prod 78(3): 548-551.
- Peng Fu, Shuxia Wang, Kui Hong, Xia Li, Peipei Liu, et al. (2011) Cytotoxic bipyridines from the marine-derived actinomycete Actinoalloteichus cyanogriseus WH1–2216-6. J Nat Prod 74(8): 1751-1756.
- Takashi Fukuda, Misaki Takahashi, Kenichiro Nagai, Enjuro Harunari, Chiaki Imada (2017) Isomethoxyneihumicin, a new cytotoxic agent produced by marine Nocardiopsis alba KM6-1. J. Antibiot 70(5): 590-594.
- García CM, Bargiela R, Martínez M, Ferrer M (2018) Metagenomic protocols and strategies. Metagenomics.
- Gomez Escribano J, Bibb M (2014) Heterologous expression of natural product biosynthetic gene clusters in Streptomyces coelicolor: from genome mining to manipulation of biosynthetic pathways. J Ind Microbiol Biotechnol 41(2): 425-431.
- Gopikrishnan V, Radhakrishnan M, Shanmugasundaram T, Ramakodi MP, Ramasamy Balagurunathan R (2019) Isolation, characterization and identifcation of antibiofouling metabolite from mangrove derived Streptomyces sampsonii PM33. Scientific Reports 9(1): 12975.
- Gorajana A, Kurada BV, Peela S, Jangam P, Vinjamuri S, Poluri E, Zeeck A, et al. (2005) 1-Hydroxy-1-norresistomycin, a new cytotoxic compound from a marine actinomycete, Streptomyces chibaensis AUBN1/7. J Antibiot 58(8): 526-529.
- Gorajana A, Vinjamuri S, Kurada BV, Peela S, Jangam P, Poluri E, et al. (2007) Resistoflavine,cytotoxic compound from a marine actinomycete, Streptomyces chibaensis AUBN1/7. Microbiol Res 162(4): 322-327.
- Han X, Cui C, Gu Q, Zhu W, Liu H, Gu J, et al. (2005) ZHD-0501, a novel naturally occurring staurosporine analog from Actinomadura sp. 007. Tetrahedron Lett 46: 6137-6140.
- Hassan HM, Degen D, Jang KH, Ebright RH, Fenical W (2015) Salinamide F, new depsipeptide antibiotic and inhibitor of bacterial RNA polymerase from a marine-derived Streptomyces sp. HHS Public Access 68(3): 206-209.
- Hayakawa Y, Shirasaki S, Kawasaki T, Matsuo Y, Adachi K, Shizuri Y (2007a) Structures ofnew cytotoxic antibiotics, piericidins C7 and C8. J Antibiot 60: 201-203.
- Helaly SE, Pesic A, Fiedler HP, Süssmuth RD (2011) Elaiomycins B and C: alkylhydrazide antibiotics from Streptomyces sp. BK 190. Org Lett 13 (5): 1052-1055.
- Claudia Hohmann, Kathrin Schneider, Christina Bruntner, Roselyn Brown, Amanda L Jones, et al. (2009b) Albidopyrone, a new-pyrone-containing metabolite from marine-derivedStreptomyces sp. NTK 227. J Antibiot 62(2): 75-79.
- Huang YF, Tian L, Fu HW, Hua HM, Pei YH (2006) One new anthraquinone from marine Streptomyces sp. FX-58. Nat Prod Res 20(13): 1207-1210.
- Chambers C Hughes, John B MacMillan, Susana P Gaudêncio, Paul R Jensen, William Fenical (2009) The ammosamides: structures of cell cycle modulators from a marine-derived Streptomyces species. Angew Chem Int Ed Engl 48(4): 725-727.
- Takuya Itoh, Masahiro Kinoshita, Hong Wei, Motomasa Kobayashi (2003) Stereostructure of komodoquinone A, a neuritogenic anthracycline, from marine Streptomyces sp. KS3. Chem Pharm Bull 51(12): 1402-1404.
- Paul R Jensen, Philip G Williams, Dong Chan Oh, Lisa Zeigler, William Fenical (2007) Species-specific secondary metabolite production in marine actinomycetes of the genus Salinispora. Appl Environ Microbiol 73(4): 1146-1152.
- Jeong S, Shin HJ, Kim TS, Lee HS, Park SK, et al. (2006) Streptokordin, a new cytotoxic compound of the methylpyridine class from a marine-derived Streptomyces sp. KORDI-3238. J Antibiot 59(4): 234-240.
- Jiang X, Zhang Q, Zhu Y, Nie F, Wu Z, et al. (2017) Isolation, structure elucidation and biosynthesis of benzo[b]fluorene nenestatin A from deep-sea derived Micromonospora echinospora SCSIO 0408. Tetrahedron 73(26): 3585-3592.
- Jørgensen H, Degnes KF, Dikiy A, Fjærvik E, Klinkenberg G, et al. (2010) Insights intothe evolution of macrolactam biosynthesis through cloning and comparativeanalysis of the biosynthetic gene cluster for a novel macrocyclic lactam, ML-449. Appl Environ Microbiol 76(1): 283-293.
- Jungwoo Kim, Daniel Shin, Seong Hwan Kim, Wanki Park, Yoonho Shin, et al. (2017) Borrelidins C-E: new antibacterial macrolides from a salternderived halophilic Nocardiopsis sp. Mar Drugs 15(6): 1–11.
- Min Cheol Kim, Oh Wook Kwon, Jin Soo Park, Sun Yeou Kim, Hak Cheol Kwon (2013) Nocapyrones H–J, 3, 6-disubstituted a-pyrones from the marine actinomycete Nocardiopsis sp. KMF-001. Chem Pharm Bull 61(5): 511–515.
- Kock I, Maskey RP, Biabani MA, Helmke E, Laatsch H (2005)1-Hydroxy-1-norresistomycin and resistoflavin methyl ether: new antibiotics from marine-derived streptomycetes. J Antibiot 58(8): 530-534.
- Alain S Leutou, Inho Yang, Heonjoong Kang, Eun Kyung Seo, Sang Jip Nam, et al. (2015) Nocarimidazoles A and B from a marine-derived actinomycete of the genus Nocardiopsis. J Nat Prod 78(11): 2846-2849.
- Li F, Maskey RP, Qin S, Sattler I, Fiebig HH, Maier A, et al. (2005) Chinikomycins A and B: Isolation, structure elucidation, and biological activity of novel antibiotics froma marine Streptomyces sp. isolate M045. J Nat Prod 68(3): 349-353.
- Li H, Huang H, Hou L, Ju J, Li W (2017) Discovery of antimycin- type depsipeptides from a wbl gene mutant strain of deep sea-derived Streptomyces somaliensis SCSIO ZH66 and their effects on pro-inflammatory cytokine production. Front Microbiol 8: 678.
- Li A, Piel J (2002) A gene cluster from a marine Streptomyces encoding the biosynthesis of the aromatic spiroketal polyketide griseorhodin A. Chem Biol 9(9): 1017-1026.
- Li DH, Zhu TJ, Liu HB, Fang YC, Gu QQ, et al. (2006) Four butenolides are novel cytotoxic compounds isolated from the marine-derived bacterium, Streptoverticillium luteoverticillatum 11014. Arch Pharm Res 29(8): 624-626.
- Lombó F, Velasco A, Castro A, De la Calle F, Braña AF, et al. (2006) Deciphering the biosynthesis pathway of the antitumor thiocoraline from a marine actinomycete and its expression in two Streptomyces species. Chembiochem 7(2): 366-376.
- Macherla VR, Mitchell SS, Manam RR, Reed KA, Chao TH, et al. (2005) Structure activity relationship studies of salinosporamide A (NPI-0052), a novel marine derived proteasome inhibitor. J Med Chem 48: 3684-3687.
- Manam RR, Teisan S, White DJ, Nicholson B, Grodberg J, et al. (2005) Lajollamycin, a nitro-tetraene spiro-beta-lactone-gamma-lactam antibiotic from the marine actinomycete Streptomyces nodosus. J Nat Prod 68: 240-243.
- Mangamuri UK, Vijayalakshmi M, Poda S, Manavathi B, Chitturi B, et al. (2016) Isolation and biological evaluation of N-(4-aminocyclooctyl)-3, 5-dinitrobenzamide, a new semisynthetic derivative from the mangrove-associated actinomycete Pseudonocardia endophytica VUK-10. Biotech 6(2): 1-12.
- Mao Y, Varoglu M, Sherman DH (1999) Molecular characterization and analysis of thebiosynthetic gene cluster for the antitumor antibiotic mitomycin C from Strep-tomyces lavendulae NRRL 2564. Chem Biol 6(4): 251-63.
- Martin GD, Tan LT, Jensen PR, Dimayuga RE, Fairchild CR, et al. (2007) Marmycins A and B, cytotoxic pentacyclic C-glycosides from a marine sedimentderived actinomycete related to the genus Streptomyces. J Nat Prod 70: 1406-1409.
- Maskey RP, Helmke E, Fiebig HH, Laatsch H (2002) Parimycin: isolation and structure elucidation of a novel cytotoxic 2,3-dihydroquinizarin analogue of gamma-indomycinone from a marine streptomycete isolate. J Antibiot 55: 1031-1035.
- Maskey RP, Li F, Qin S, Fiebig H H, Laatsch H (2003) Chandrananimycins A-C: production of novel anticancer antibiotics from a marine Actinomadura sp. isolate M048 by variation of medium composition and growth conditions. J Antibiot 56: 622-629.
- Mazzetti C, Ornaghi M, Gaspari E, Parapini S, Maffioli S, et al. (2012) Halogenated spirotetronates from Actinoallomurus. J Nat Prod 75: 1044-1050.
- Mullowney MW, Hainmhire E, Tanouye U, Burdette JE, Van Pham C, et al. (2015) A pimarane diterpene and cytotoxic angucyclines from a marine-derived Micromonospora sp., Vietnam’s East Sea. Mar Drugs 13(9): 5815-5827.
- Murphy B T, Narender T, Kauffman C A, Woolery M, Jensen P R, et al. (2010) Saliniquinones A-F, new members of the highly cytotoxic anthraquinone-g-pyrones from the marine actinomycete Salinispora arenicola. Aust J Chem 63(6): 10.1071.
- Nam S J, Gaudêncio S P, Kauffman C A, Jensen P R, Kondratyuk T P, et al. (2010) Fijiolides A and B, inhibitors of TNF-a-induced NFkB activation, from a marine-derived sediment bacterium of the genus Nocardiopsis. J Nat Prod 73: 1080-1086.
- Pérez M, Crespo C, Schleissner C, Rodríguez P, Zúñiga P, et al. (2009) Tartrolon D, a cytotoxic macrodiolide from the marine-derived actinomycete Streptomyces sp. MDG-04-17-069. J Nat Prod 72: 2192-2194.
- Priyanka S, Jayashree M, Shivani R, Anwesha S, Bhaskara Rao KV, et al. (2019) Characterisation and identification of antibacterial compound from marine actinobacteria: In vitro and in silico analysis. J Infect Public Health 12(1): 83-89.
- Rodríguez JC, Fernández Puentes JL, Baz JP, Cañedo LM (2003) IB-00208, a new cytotoxic polycyclic xanthone produced by a marine-derived Actinomadura. II. Isolation, physicochemical properties and structure determination. J Antibiot 56(3): 219-25.
- Romero F, Espliego F, Pérez Baz J, García de Quesada T, Grávalos D, et al. (1997) Thiocoraline, a new depsipeptide with antitumor activity produced by a marine Micromonospora. I. Taxonomy, fermentation, isolation, and biological activities. J Antibiot 50: 734-737.
- Sarmiento Vizcaíno A, Braña AF, Pérez Victoria I, Martín J, Pedro N, et al. (2017) Paulomycin G, a new natural product with cytotoxic activity against tumor cell lines produced by deep-sea sediment derived Micromonospora matsumotoense M-412 from the Avilés Canyon in the Cantabrian Sea. Mar Drugs 5(9): 271.
- Sato S, Iwata F, Yamada S, Kawahara H, Katayama M (2011) Usabamycins A-C: newanthramycin-type analogues from a marine-derived actinomycete. Bioorg MedChem Lett 21(23): 7099-101.
- Schneemann I, Ohlendorf B, Zinecker H, Nagel K, Wiese J, et al. (2010) Nocapyrones A-D, g-pyrones from a Nocardiopsis strain isolated from the marine sponge Halichondria panicea. J Nat Prod 73: 1444-1447.
- Schneider K, Nachtigall J, Ha nchen A, Nicholson G, Goodfellow M, et al. (2009) Lipocarbazoles, secondary metabolites from Tsukamurella pseudospumaeActa 1857 with antioxidative activity. J Nat Prod 72(10): 1768-1772.
- Schneider K, Keller S, Wolter FE, Röglin L, Beil W, et al. (2008) Proximicins A, B, and C-antitumor furan analogues of netropsin from the marine actinomycete Verrucosispora induce upregulation of p53 and the cyclin kinase inhibitor p21. Angew Chem Int Ed Engl 47: 3258-3261.
- Schultz A W, Oh D C, Carney J R, Williamson R T, Udwary D W, et al. (2008) Biosynthesis and structures of cyclomarins and cyclomarazines, prenylated cyclic peptides of marine actinobacterial origin. J Am Chem Soc 130: 4507-4516.
- Schulze C J, Donia M S, Siqueira Neto, J L Ray D, Raskatov J A, et al. (2015a) Genome-directed lead discovery: biosynthesis, structure elucidation, and biological evaluation of two families of polyene macrolactams against Trypanosoma brucei. ACS Chem Biol 10: 2373-2381.
- Shin HJ, Kim TS, Lee HS, Park JY, Choi IK, et al. (2008) Streptopyrrolidine, an angiogenesis inhibitor from a marine-derived Streptomyces sp. KORDI-3973. Phytochemistry 69: 2363-2366.
- Song Y, Liu G, Li J, Huang H, Zhang X, et al. (2015) Cytotoxic and antibacterial angucycline- and prodigiosinanalogues from the deep-sea derived Streptomyces sp. SCSIO 11594. Mar Drugs 13(3): 1304-1316.
- Sousa T D S, Jimenez P C, Ferreira E G, Silveira ER, Braz Filho R, et al. (2012) Anthracyclinones from Micromonospora sp. J Nat Prod 5: 489-493.
- Takagi M, Motohashi K, Khan S T, Hashimoto J, Shin ya K (2010) JBIR-65, a new diterpene, isolated from a sponge-derived Actinomadura sp. SpB081030SC-15. J Antibiot 63: 401-403.
- Takahashi A, Kurasawa S, Ikeda D, Okami Y, Takeuchi T (1989) Altemicidin, a new acaricidal and antitumor substance. I. Taxonomy, fermentation, isolation and physico-chemical and biological properties. J Antibiot 42: 1556-1561.
- Tang X, Li J, Millán Aguinaaga N, Zhang J J, ONeill, et al. (2015) Identification of thiotetronic acid antibiotic biosynthetic pathways by target-directed genome mining. ACS Chem Biol 10: 2841-2849.
- Tsujibo H, Sato T, Inui M, Yamamoto H, Inamori Y (1988) Intracellular accumulation of phenazine antibiotics produced by an alkalophilic actinomycete. I. Taxonomy, isolation and identification of the phenazine antibiotics. Agric Biol Chem 52: 301-306.
- Wan X, Zhang SY, Zhang H, Zhai J, Huang J, et al. (2017) New tenvermectin analogs obtained by microbial conversion with Saccharopolyspora erythraea. J Antibiot (Tokyo) 70(2): 190-192.
- Wu G, Nielson JR, Peterson RT, Winter JM (2017) Bonnevillamides, linear heptapeptides isolated from a Great Salt Lakederived Streptomyces sp. Mar Drugs 15(7): 1-11.
- Wu SJ, Fotso S, Li F, Qin S, Kelter G, Fiebig HH, et al. (2006) 39-N-carboxamidostaurosporine and selina-4(14),7(11)-diene-8,9-diol, new metabolites from a marine Streptomyces sp. J Antibiot 59: 331-337.
- Shao Jie Wu, Serge Fotso, Fuchao Li, Song Qin, Hartmut Laatsch (2007) Amorphane sesquiterpenes from a marine Streptomyces sp. J Nat Prod 70(2): 304-306.
- Thomas P Wyche, Yanpeng Hou, Doug Braun, Hannah C Cohen, May P Xiong, et al. (2011) First natural analogs of the cytotoxic thiodepsipeptide thiocoraline A from a marine Verrucosispora sp. J Org Chem 76(16): 6542-6547.
- Thomas P Wyche, Yanpeng Hou, Emmanuel Vazquez-Rivera, Doug Braun, Tim S Bugni (2012) Peptidolipins B-F, antibacterial lipopeptides from an ascidian-derived Nocardia sp. J Nat Prod 75(4): 735-740.
- Thomas P Wyche, Jeff S Piotrowski, Yanpeng Hou, Doug Braun, Raamesh Deshpande, et al. (2014) Forazoline A: marine-derived polyketide with antifungal in vivo Efficacy. Angew Chem Int Ed 53(43): 11583-11586.
- Thomas P Wyche, Miranda Standiford, Yanpeng Hou, Doug Braun, Delinda A Johnson, et al. (2013). Activation of the nuclear factor E2-related factor 2 pathway by novel natural products halomadurones A-D and a synthetic analogue. Mar. Drugs 11(12): 5089-5099.
- Xie CL, Liu Q, Xia JM, Gao Y, Yang Q, Shao ZZ, et al. (2017) Anti-allergic compounds from the deep-sea-derived actinomycete Nesterenkonia flava MCCC 1K00610. Mar Drugs 15(3): 1-8.
- Yang CL, Wang YS, Liu CL, Zeng YJ, Cheng P, Jiao RH, et al. (2017) Strepchazolins A and B: two new alkaloids from a marine Streptomyces chartreusis NA02069. Mar Drugs 15(8): 244.
- Yang N, Sun C (2016) The inhibition and resistance mechanisms of actinon in, isolated from marine Streptomyces sp. NHF165, against Vibrio Anguilla rum. Front Microbiol 7: 1-11.
- Yellamanda B, Vijayalakshmi M, Kavitha A, Reddy DK, Venkateswarlu Y (2016) Extraction and bioactive profile of the compounds produced by Rhodococcus sp. VLD-10. 3 Biotech 6(2): 1-9.
- Zhang W, Liu Z, Li S, Yang T, Zhang Q, et al. (2012) Spiroindimicins A-D: New Bisindole Alkaloids from a Deep-Sea-Derived Actinomycete. Org Lett 14: 3364-3367.
- Zhang YM, Li HY, Hu C, Sheng HF, Zhang Y, et al. (2016) Ergosterols from the culture broth of marine Streptomyces anandii H41-59. Mar Drugs 14(5): 1-11.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.