Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Photo-Catalytic Degradation of Organic Pollutants Using Zinc-Doped Cobalt Oxide: A Sustainable Approach for Environmental Remediation
*Corresponding author: Rehana Riaz, Department of Physics, International Islamic University, Islamabad, Pakistan.
Received: February 11, 2025; Published: February 20, 2025
DOI: 10.34297/AJBSR.2025.25.003383
Abstract
The increasing presence of organic pollutants in water systems necessitates the development of efficient and sustainable photo-catalysts for environmental remediation. This study focuses on the photo-catalytic properties of zinc-doped cobalt oxide (Zn-Co₃O₄) in comparison to pure cobalt oxide (Co₃O₄), emphasizing their role in the degradation of Methylene Blue (MB) under UV-visible light. Morphological and optical characterizations were conducted using Scanning Electron Microscopy (SEM) and Diffuse Reflectance Spectroscopy (DRS). SEM revealed sponge-like morphologies with enhanced porosity in the doped samples, while DRS analysis demonstrated a reduction in the band gap from 2.0 eV (pure Co₃O₄) to 1.8 eV (Zn- Co₃O₄). The reduced band gap facilitated improved absorption of visible light, significantly enhancing the photo-catalytic degradation of MB. Zinc doping proved instrumental in optimizing the structural and optical properties of cobalt oxide, establishing Zn-Co₃O₄ as a promising candidate for tackling environmental pollution through photo-catalysis.
Introduction
Bacterial infections are a grave threat to human health, necessitating the development of biocompatible antibiotics for safe clinical use [1]. In both healthcare and the food industry, microbial contamination presents significant challenges, driving research into antimicrobial agents and surface coatings [2]. Nanoparticles have emerged as promising candidates due to their antimicrobial properties, despite their potentially higher toxicity compared to larger particles of the same materials [3]. Globally, bacterial infectious diseases exert widespread impacts on health, economies, and societal well-being [4]. Challenges such as escalating outbreaks of pathogenic strains, antibiotic resistance, emergence of new bacterial mutations, insufficient vaccination in developing regions, and hospital-associated infections underscore the critical need for innovative antibacterial solutions targeting major foodborne pathogens like Escherichia coli O157:H, Campylobacter jejuni, Staphylococcus aureus, Pseudomonas aeruginosa, Enterococcus faecalis, Salmonella spp., and Clostridium perfringens [5].
Recently, nano medicine has gained attention for its antibacterial properties. Metal oxide nanoparticles (NPs) demonstrate strong antibacterial activity, influenced by factors such as particle size, surface area, crystallinity, capping/stabilizing agents, morphology, concentration, solution pH, and the type of microorganisms. Smaller nanoparticles with appropriate morphology can easily penetrate bacterial pores [6,7]. Among various semiconductor- based photo-catalysts, cobalt-based oxides, particularly Co₃O₄ nanoparticles, have garnered significant attention due to their excellent redox properties, narrow band gap, and superior photo-catalytic efficiency [8]. However, their photo-catalytic activity can be further enhanced through doping strategies, which introduce foreign elements to modulate the electronic structure, charge carrier dynamics, and light absorption properties [9]. Optimizing these parameters is essential for developing new nano materials to treat pathogenic diseases. ZnO is a remarkably versatile material with a wide range of uses.
In the pharmaceuticals and cosmetics industries, it finds its way into creams, powders, and dental pastes while the textile industry uses ZnO for UV protection, and in electronics, it’s a key component in photo-electronics, sensors, UV lasers, and solar cells. It’s also used in photo-catalysis, zinc silicate production, criminal analysis, and packaging [10]. ZnO is particularly appealing because it has a band gap energy of 3.3 eV, low production costs, and is recognized as safe by the U.S. FDA [11]. One of ZnO’s standout features is its ability to absorb UVA and UVB light, which generates electron-hole pairs and leads to significant antibacterial properties. To improve ZnO’s antibacterial properties and address challenges like UV-induced photo corrosion, electron-hole recombination, lack of visible light absorption, and agglomeration, researchers have formed hetero junctions and composites with other metal oxides and doped ZnO with other ions [12,13]. Introducing impurities is an effective way to achieve unique properties. However, zinc (Zn) doping in Co₃O₄ is considered an effective modification to improve its optical and catalytic performance by reducing recombination losses and extending light absorption into the visible region [14-19].
In this study, Zn-doped Co₃O₄ nanoparticles were synthesized using a controlled sol-gel method, followed by thermal treatment to obtain phase-pure nanostructures. The photo-catalytic efficiency of the synthesized Zn-Co₃O₄ nanoparticles was investigated by evaluating the degradation of methylene blue under controlled conditions. The research aims to check any possible effect that a change in synthesizes technique can cause on the photo catalytic degradation of organic components. We carried forward the research work of R. Sylvia Reena et al [20]. by synthesizing cobalt oxide and zinc doped cobalt oxide via sol-gel method to examine the possible changes at the maximum dopant percentage of 5%.
Experimental Section
Cobalt chloride (CoCl₂), zinc chloride (ZnCl₂), and sodium hydroxide (NaOH) were used as precursors for the synthesis. Distilled water was employed as the solvent for all solution preparations. Initially, a 1M CoCl₂ solution was prepared and mixed with a 2M NaOH solution. This reaction resulted in the formation of a precipitate. The mixture was then heated on a hot plate at 100°C to facilitate the settling of precipitates at the bottom, while the excess water was carefully removed. The resulting gel was dried at 50°C for 30 minutes to obtain pure Co₃O₄ nano powder. The reaction is represented as:
1 M of CoCl2 + 2M of NaOH →1M of Co3O4+ 2 M of NaCl+H2O
Subsequently, the Co₃O₄ nano powder was doped with 5 wt% zinc to produce Zn-doped Co₃O₄.
The process can be summarized as:
1M of Co3O4 + 2M of NaCl +5%Zinc → Zn doped Co3O4.
Result and Discussion
X-Ray Diffraction
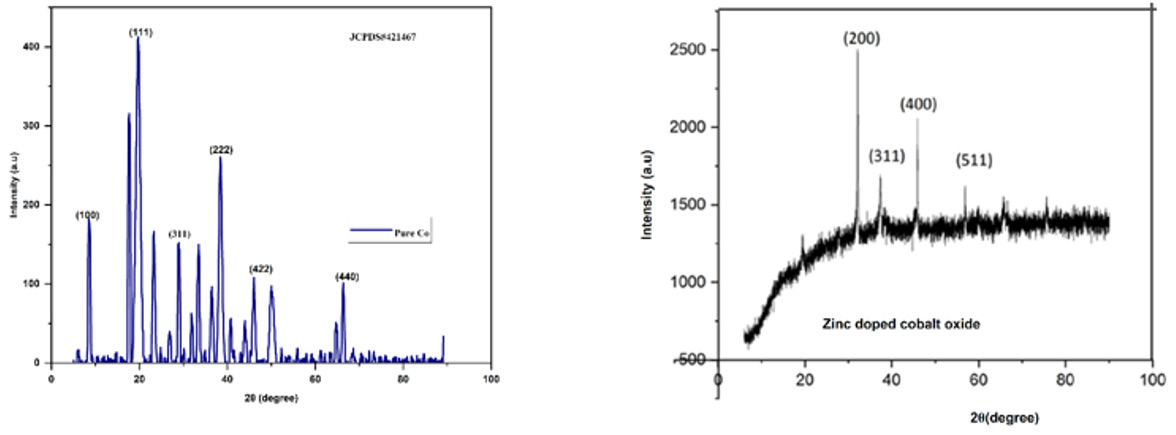
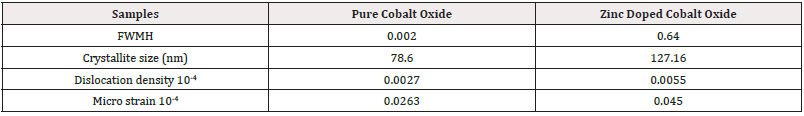
The XRD patterns for pure cobalt oxide and zinc-doped cobalt oxide showed in (Figure 1) reveal distinct structural differences. Pure cobalt oxide exhibits sharp and well-defined peaks, indicative of high crystallinity and minimal defects within the material [21]. In contrast, the zinc-doped cobalt oxide pattern shows broader peaks with slight shifts, reflecting changes in lattice parameters due to zinc incorporation [22]. (Table 1) shows several parameters for both doped and un-doped samples. The broader FWHM in the doped sample suggests reduced crystallinity and increased structural imperfections, consistent with higher dislocation density and micro strain values [23].
However, the calculated crystallite size of the doped sample is larger, which may result from grain growth induced by zinc doping during synthesis. This suggests that while doping introduces strain and defects, it simultaneously facilitates larger crystallite formation [24]. Such structural modifications can significantly influence material properties. The increase in dislocation density and micro strain in the doped sample suggests a higher density of lattice defects, which can enhance catalytic activity and electrical conductivity. Meanwhile, the increased crystallite size may improve stability and surface area interactions, potentially making the doped material more effective for applications requiring enhanced chemical or electronic performance. These structural findings correlate with prior literature [25]. The retention of the spinel structure in both samples confirms that the doping process does not disrupt the fundamental crystalline framework but modifies its structural dynamics to tailor the material’s properties.
Scanning Electron Microscopy
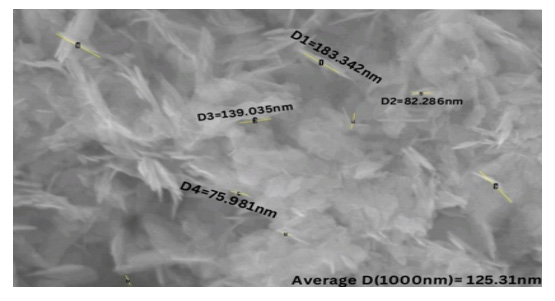
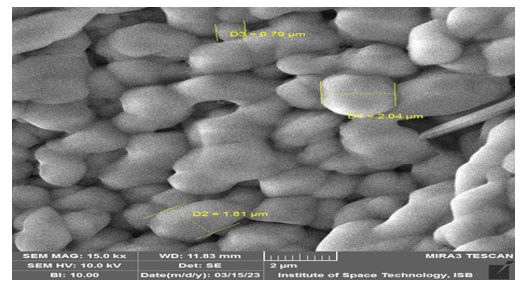
A Scanning Electron Microscope (SEM) is a powerful tool that provides high-resolution visualization of surface morphology and composition, enabling the observation of nanometer-scale details. In this study, SEM was used to analyze the morphology of pure cobalt oxide and zinc-doped cobalt oxide thin films. For pure cobalt oxide, the SEM analysis revealed a sponge-like morphology, resembling rod-like structures. Using ImageJ software, the diameters of these rod structures were calculated. The largest rod had a diameter of 183.342nm, while the smallest rod was 75.98nm, resulting in an average diameter of 125.31 nm. These findings were consistent across varying concentrations of pure cobalt oxide. The observed morphology supports high surface area and porosity, characteristics of cobalt oxide (Figure 2).
In contrast, the zinc-doped cobalt oxide thin films exhibited nano- crystalline grains with overgrown clusters. The overgrowth was attributed to nucleation processes during material synthesis. The sponge-like morphology remained evident, but the average particle size decreased to 70.80nm, with visible pores and aggregative structures. The resolution of both the particle and thin film samples was 2 micrometers. The structures are correlated by prior literature [25].
The morphological differences between pure and zinc-doped cobalt oxide influence their functional properties. The zinc doping, which resulted in smaller particle sizes and increased porosity, enhances the material’s ability to absorb visible and UV light due to its reduced band gap. The crystallite sizes measured through SEM align with XRD analysis, as SEM provides dimensions parallel to the substrate, while XRD gives dimensions perpendicular to it (Figure 3).
Photo-Catalysis
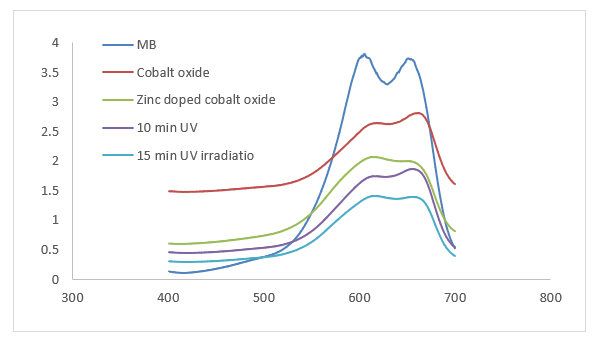
The (Figure 4) demonstrates the UV-Vis absorption spectra of Methylene Blue (MB), pure cobalt oxide, zinc-doped cobalt oxide, and their performance under UV irradiation for 10 minutes and 15 minutes. The photo catalytic activity of pure cobalt oxide and Zn-Co3O4 nanoparticles was investigated by measuring the degradation of organic Methylene blue dye. In this experiment, 10 ppm solution of methylene blue in 0.005 grams of Co & Zn-Co3O4. The following equation was used to compute the % degradation of the aqueous dye solution.
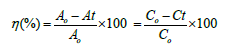
Here, ƞ is the degradation percentage (%), Co is the dye’s initial absorbance (mg/L), and Ct is the dye’s absorbance at a certain time interval (mg/L). At 0min, the percentage degradation for pure cobalt oxide was 27%. However, after doping it increases up to 46.9% and then on further increasing exposure for 10 and 15 min it increases to 52.39% and 63.2% respectively (Figure 4). The comparative analysis implies that zinc doping enhances the optical properties and photo-catalytic performance of cobalt oxide, enabling it to degrade methylene blue more effectively under UV light. This makes zinc-doped cobalt oxide a promising material for environmental remediation applications, especially in dye degradation.
Diffuse Reflectance Spectroscopy
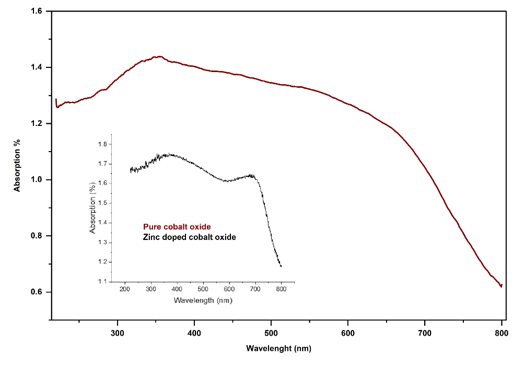
The UV-Vis Diffuse Reflectance Spectroscopy (DRS) analysis provides significant insights into the optical properties of pure cobalt oxide and zinc-doped cobalt oxide. The absorption spectra indicate that both materials exhibit strong absorption in the UV region, a characteristic feature of semiconducting metal oxides. Pure cobalt oxide shows an absorption edge corresponding to a band gap of approximately 2.0 eV, while zinc-doped cobalt oxide demonstrates a slightly red-shifted absorption edge, corresponding to a reduced band gap of 1.8eV. This reduction in the band gap upon doping can be attributed to the introduction of zinc ions, which modifies the electronic structure of cobalt oxide and enhances its light absorption capabilities in the visible range.
The enhanced absorption of zinc-doped cobalt oxide compared to pure cobalt oxide is evident in the visible and near-infrared regions, where the doped material exhibits a higher absorption percentage. This improvement in optical behavior can be explained by the incorporation of zinc ions, which may introduce localized energy states within the band gap of cobalt oxide, thereby facilitating better photon absorption [22]. The shift in the absorption edge and the increase in absorption intensity make zinc-doped cobalt oxide more suitable for applications that require efficient light absorption, such as photo-catalysis, solar energy harvesting, and UV protection [26] (Figure 5).
The reduced band gap of zinc-doped cobalt oxide, in particular, enhances its ability to absorb visible light, as materials with smaller band gaps are better at capturing lower-energy photons. This property is critical for practical applications in energy conversion devices, such as solar cells, where absorption of visible light is essential for maximizing efficiency [26]. Moreover, the increased absorption in the UV range suggests that both pure and zinc-doped cobalt oxide are effective for photo-catalytic degradation of pollutants and other environmental remediation processes.
In conclusion, the DRS analysis highlights the superior optical properties of zinc-doped cobalt oxide compared to its pure counterpart. The reduced band gap, enhanced visible light absorption, and improved UV absorption suggest that zinc doping effectively tailors the material for advanced applications in photo-catalysis. These findings reinforce the potential of zinc-doped cobalt oxide as a versatile material for light-driven technologies [27].
Conclusion
This research highlights the potential of zinc-doped cobalt oxide as an efficient photo-catalyst for environmental remediation. The reduced band gap of Zn-Co₃O₄ (1.8eV) compared to pure Co₃O₄ (2.0eV) enhances its visible light absorption, while its sponge-like morphology increases surface area and light scattering. These properties collectively contribute to the material’s superior photo- catalytic degradation performance, as demonstrated by its ability to break down methylene blue under UV-visible irradiation. The findings underscore the significance of Zn-Co₃O₄ in developing cost-effective, sustainable solutions for the degradation of organic pollutants, offering a valuable tool in addressing water pollution and advancing green technologies. Future applications may include large-scale water purification systems and integration into solar- powered environmental cleanup methods.
Conflict of Interest
None.
Acknowledgement
None.
References
- Osamu Yamamoto, Kyoko Nakakoshi, Tadashi Sasamoto, Hiroyuki Nakagawa, Kouichi Miura, et al. (2001) Adsorption and growth inhibition of bacteria on carbon materials containing zinc oxide. Carbon 39(11): 1643-1651.
- Bellar TA, JJ Lichtenberg, RC Kroner (1974) The occurrence of organohalides in chlorinated drinking waters. AWWA 66(12): 703-706.
- Bruna Lallo da Silva, Bruno Leonardo Caetano, Bruna Galdorfini Chiari Andréo, Rosemeire Cristina Linhari Rodrigues Pietro, Leila Aparecida Chiavacci, et al. (2019) Increased antibacterial activity of ZnO nanoparticles: Influence of size and surface modification. Colloids Surf B Biointerfaces 177: 440-447.
- Lu E Shi, Zhen Hua Li, Wei Zheng, Yi Fan Zhao, Yong Fang Jin, et al. (2014) Synthesis, antibacterial activity, antibacterial mechanism and food applications of ZnO nanoparticles: a review. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 31(2): 173-186.
- KL Kotloff, JP Winickoff, B Ivanoff, JD Clemens, DL Swerdlow, et al. (1999) Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull World Health Organ 77(8): 651-666.
- Anu Sukhdev, Malathi Challa, Lakshmi Narayani, Adalagere Somashekar Manjunatha, PR Deepthi, et al. (2020) Synthesis, phase transformation, and morphology of hausmannite Mn3O4 nanoparticles: photocatalytic and antibacterial investigations. Heliyon 6(1): e03245.
- Toshiaki Ohira, Osamu Yamamoto, Yasuhiro Iida, Zenbe E Nakagawa (2008) Antibacterial activity of ZnO powder with crystallographic orientation. J Mater Sci Mater Med 19(3): 1407-1412.
- Ranjan Kumar, Abhishek Dhar, Ashes Banerjee, Subhabrata Mondal (2024) Scientific Frontiers: Sustainable Practices and Technologies.
- Shuqu Zhang, Xingshen Yi, Guanghua Hu, Meixue Chen, Hao Shen, et al. (2023) Configuration regulation of active sites by accurate doping inducing self-adapting defect for enhanced photocatalytic applications: A review. Coordination Chemistry Reviews 478: 214970.
- Agnieszka Kołodziejczak Radzimska, Teofil Jesionowski (2014) Zinc oxide—from synthesis to application: a review. Materials (Basel) 7(4): 2833-2881.
- Chin Boon Ong, Law Yong Ng, Abdul Wahab Mohammad (2018) A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renewable and Sustainable Energy Reviews 81: 536-551.
- Razieh Jalal, Elaheh K Goharshadi, Maryam Abareshi, Majid Moosavi, Abbas Yousefi, et al. (2010) ZnO nanofluids: green synthesis, characterization, and antibacterial activity. Materials Chemistry and Physics 121(1-2): 198-201.
- Anjani P Nagvenkar, Archana Deokar, Ilana Perelshteina, Aharon Gedanken (2016) A one-step sonochemical synthesis of stable ZnO–PVA nanocolloid as a potential biocidal agent. Journal of materials chemistry B 4(12): 2124-2132.
- Awais Khalid, Pervaiz Ahmad, Abdulrahman I Alharthi, Saleh Muhammad (2021) Unmodified titanium dioxide nanoparticles as a potential contrast agent in photon emission computed tomography. Crystals 11(2): 171.
- T Fukumura, Zhengwu Jin, A Ohtomo, H Koinuma, M Kawasaki, et al. (1999) An oxide-diluted magnetic semiconductor: Mn-doped ZnO. Applied physics letters 75(21): 3366-3368.
- FB Dejene, MO Onani, LF koao, AH Wako, SV Motloung, et al. (2016) Structure, morphology and optical properties of undoped and MN-doped ZnO (1− x) Sx nano-powders prepared by precipitation method. Physica B: Condensed Matter 480: 63-67.
- Pervaiz Ahmad, Mayeen Uddin Khandaker, Yusoff Mohd Amin, Nawshad Muhammad, Ghulamullah Khan, et al. (2016) Synthesis of hexagonal boron nitride fibers within two hour annealing at 5000 C and two hour growth duration at 10000 ceramics internation 42(13): 14661-14666.
- S M Ahmed, S N Kazi, G Khan, M Dahari, M N M Zubir, et al. (2017) Experimental investigation on momentum and drag reduction of Malaysian crop suspensions in closed conduit flow. IOP Conference Series: Materials Science and Engineering 210.
- Umadevi Godavarti, VD Mote, MV Ramana Reddy, P Nagaraju, Y Vijaya Kumar, et al. (2019) Precipitated cobalt doped ZnO nanoparticles with enhanced low temperature xylene sensing properties. Physica B: Condensed Matter 553: 151-160.
- R Sylvia Reena, A. Aslinjensipriya, S Grace Infantiya, J Daniel John Britto, M Jose, et al. (2022) Visible-light active zinc doped cobalt oxide (Zn-Co3O4) nanoparticles for photocatalytic and photochemical activity. Meterialstoday proceedings 68: 269-275.
- Luis Garces, Beatriz Hincapie, Richard Zerger, Steven L Suib (2015) The effect of temperature and support on the reduction of cobalt oxide: an in situ X-ray diffraction study. The Journal of Physical Chemistry C 119(10): 5484-5490.
- Shah Faisal, F Akbar Jan, Sanum Saleem, Rahat Ulla (2022) Juglans regia L. mediated synthesis of cobalt oxide and zinc-doped cobalt oxide nanoparticles: characterization and evaluation for environmental, antibacterial and cytotoxic potential. Nanotechnology for Environmental Engineering 7(3): 1-15.
- Šulčiūtė A (2016) Synthesis, structure and electrochemical properties of ZnO and Zn-Co oxide coatings. Synthesis, structure and electrochemical.
- Sunil Thakur, Pankaj Kumar, Nikesh Thakur, Kuldeep Kumar, Kamal Jeet, et al. (2024) Photocatalytic, antibacterial and antioxidant potential of spheroidal shape chromium and yttrium doped cobalt oxide nanoparticles: A green approach. Journal of the Indian Chemical Society 101(8): 101199.
- Chandra Sekhar Dash, M Sukumar, V Ravi, Anitha Gopalan, Khamael Abualnaja, et al. (2023) Effect of zinc doping on structural, optical, magnetic, and catalytic behavior of Co3O4 nanoparticles synthesized by microwave-assisted combustion method. Journal of Cluster Science 34(3): 1-9.
- Sunil Agrohiya, Ravinder Singh, Sajjan Dahiya, Ishpal Rawal, Amit Kumar, et al. (2023) Fabrication of p-ZnCo2O4/n-Si spinel heterojunction devices for self-powered ultraviolet photodetectors: Effect of Zn2+ Journal of Alloys and Compounds 968: 171855.
- Zakaria Barbouch, A El Habib, Jamal Zimou, Mustapha Beraich, Nadia Elharfaoui, et al. (2024) Unravelling the effects of Zn doping on the physical properties of Co3O4 thin films: experimental study and numerical simulation based on the AZO/ZnS/Co3O4: Zn/Mo/SLG solar cells. Optical and Quantum Electronics 56(9): 1441.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.