Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Physio-Biochemical and Growth Attributes of Cadmium Stressed Soybean [Glycine Max (L.) Merr.] With 24-Epibrassinolide
*Corresponding author: Kamal Jit Singh, Department of Botany, Panjab University, Chandigarh (160014), India.
Received: February 19, 2025; Published: February 25, 2025
DOI: 10.34297/AJBSR.2025.25.003389
Abstract
The hyper accumulation of Cadmium (Cd) in soil rhizosphere due to anthropogenic activities have detrimental effects on the growth and development of plants. The study was aimed at investigating the efficacy of 24-epibrassinolide (24-EBL) in enhancing growth and physio-biochemical attributes of Cd-stressed soybean. Soybean seedlings at 20 DAS (Days After Sowing) were subjected to Cd treatments alone and in supplementation of 24-EBL1.0, 3.0μM. A notable reduction of growth attributes, photosynthetic pigments, and Relative Leaf Water Content (RLWC) accompanied by an increased leakage of ions, MDA (Malondialdehyde), H2O2 (Hydrogen Peroxide), and proline content resulted with Cd. 24-EBL application improved the morphological and physiological attributes including photosynthetic pigments, RLWC, biomass accumulation, and root-shoot length of plants. Largely the restoration of MDA, H2O2 and EL (Electrolyte Leakage) was accompanied by pooling up of the ascorbic acid and proline content in the leaves. Hence, up regulated content of osmolytes and antioxidants along with a significant improvement in the level of stress indicators provided an enhanced stress tolerance against Cd in soybean possibly through the osmoregulation and ROS (Reactive Oxygen Species) scavenging. Thus, our findings prove the efficacy of exogenously supplied 24-EBL in ameliorating Cd toxicity with improved photosynthesis, osmoregulation, membrane stabilization and regulation of heavy metal stress indicators.
Keywords: Heavy metals, Brassinosteroids, Osmolytes, Rhizosphere, ROS, Osmoregulation
Introduction
The presence of heavy metals in cropland areas is hazardous to productivity compromising the yield and quality of food crops. Various factors such as soil characteristics and agricultural practices using sewage sludge in farmland leads to excessive accumulation of heavy metals ions in the soil [1]. Cadmium (48Cd) from group II B of the periodic table is noxious heavy metal among others. Although present naturally in the soil, Cd tends to accumulate to toxic levels due to anthropogenic activities such as mining, smelting, use of phosphate fertilizers and sewage sludge application in agriculture. Its entry in the agricultural food chain has raised serious health concerns both for human and animals [2]. Cd accumulation results in the several stress related plant symptoms like reduced growth, mineral nutrition, altered carbohydrate metabolism [3], absorption, transport, and utilization of nutrients [4], inhibited photosynthesis [5], altered enzyme activity [6], and injury signs like necrosis, chlorosis, root tip browning [7]. As a non-redox metal, Cd lacks the ability to directly produce ROS like superoxide anion (O2 ._), Singlet Oxygen (1O2), Hydrogen Peroxide (H2O2), and Hydroxyl Radical (OH.), however, induced oxidative the stress by disrupting antioxidant defence system [8].
Brassinosteroids (BRs), a group of polyhydroxylated steroidal hormones widely spread throughout plant kingdom plays pivotal role in physiological responses such as stem elongation, pollen tube expansion, leaf curvature and epinasty, inhibition of root growth, ethylene biosynthesis, activation of proton pumps, initiation of vascular differentiation, nucleic acid and protein synthesis, and the process of photosynthesis [9]. BRs also bolster plant resilience against biotic and abiotic stresses [10]. BRs have capacity to ameliorate the impact of extreme temperatures [11], moisture [12], drought [13], salinity stress [14], and heavy metal [15] stress. 24-epibrassinolide (EBL) has a multifaceted role in metabolic processes like seed germination [16], cell division [17], cell elongation [18], root establishment [19], reproductive development [20], senescence, abscission, and maturation [21] including the gene regulation [22]. Apart from that EBL has anti-stress properties against drought, cold, salt, and heavy metals [23].
Glycine max (L.) Merr. (Soybean) in legumes is a valuable crop for its oil (13-22%) and protein (30-48%) content. The productivity and quality of soybeans is highly impacted with Cd contaminated soil. In light of the stress-alleviating properties associated with BRs especially with EBL, present study was undertaken to assess its role in regulating growth characteristics, physio-biochemical responses in Cd-stressed soybean.
Material and Methods
Certified seeds of soybean [Glycine max (L.) Merr. cv. SL688] were procured from Punjab Agricultural University, Ludhiana, Punjab, India. Uniform sized healthy-looking seeds were surface sterilized with 0.01% aq. HgCl2 solution followed by repeated distilled water washing and soaked overnight for inoculating with specific rhizobial strain making thick slurry of activated charcoal. Seeds were sown in earthen pots lined with perforated polyethylene bags filled using washed river sand and kept in dome-shaped outhouses under natural conditions. At the seedling stage thinning was done to maintain three healthy plants per pot and irrigated with tap water following fortnight application of nutrient medium [24].
Experimental Design and Treatments
24-EBL stock solution (10μⅯ) was prepared in ethanol and final volume prepared with distilled water containing 0.5% tween-20 as surfactant. Following 10 combination treatments were given: Control; Cd0.2ⅿⅯ, Cd0.2mⅯ + EBL1μⅯ, Cd0.2mⅯ + EBL3μⅯ; Cd0.4ⅿⅯ, Cd0.4mⅯ + EBL1μⅯ, Cd0.4mⅯ + EBL3μⅯ; Cd0.6ⅿⅯ, Cd0.6mⅯ + EBL1μⅯ, Cd0.6mⅯ + EBL3μⅯ. Two Cd treatments were given with a 15d gap along with nutrient medium. Seedling were allowed to grow and at day 20 stage, foliage was sprayed with 24-EBL for 3 consecutive days. Controls were supplied with nutrient medium only and foliage was sprayed with distilled water containing tween-20 without EBL. Fresh leaves were harvested at the reproductive stage (90 DAS) and assessed for various physiological and biochemical parameters. Growth parameters were analysed at the end of experiment.
Physiological and Biochemical Analysis
Standardized protocols were followed to determine the total chlorophyll [25] and carotenoid content [26], electrolyte leakage [27] and relative leaf water content (RLWC) [28], proline content [29], Hydrogen Peroxide (H2O2) content [30], Ascorbic Acid (AsA) content [31], MDA content [32]. Observations were made by measuring Optical Density (O.D.) with thermo-scientific Evolution-201 UV-Visible spectrophotometer.
Growth Analysis
Growth attributes such as the root-shoot length (cm), fresh and dry weight (g) were analysed at harvest. Fresh weight was recorded immediately after removing plant carefully, followed by oven drying at 60oC till weight measurement become stable.
Statistical Analysis
The data was subject to One-Way Analysis of Variance (ANOVA) using SPSS-16 software with probability level of 5%. Mean differences obtained by performing Fisher’s LSD (Least Significant Difference) post-hoc test was compared by calculating LSD value. Mean values were significant at P≤ 0.05. All values were in triplicates (n=3) and represented as mean ± SE (Standard Error).
Results
Physiological Attributes
Chlorophyll Content: Leaf chlorophyll content of Cd-treated plants dropped upto 30.77% (Cd0.2mM), 53.85% (Cd0.4mM), and 69.23% (Cd0.6mM) in comparison to control. The green pigment loss was comparatively lesser with 24-EBL1.0μM foliar application i.e., 11.54% (Cd0.2mM) and 30.77% (Cd0.4mM), and gaining +7.69% (Cd0.6mM) to that of control. The corresponding values in 24-EBL3.0μM treatments were 38.46% (Cd0.2–0.4mM) and gain of +26.92% (Cd0.6mM) reflecting the effectiveness of EBL at 1.0 and 3.0μM concentrations against higher doses of cadmium. A notable decline in the green pigment was noticed when comparing control in low Cd0.2mM + EBL3.0μM treatment (Figure 1a).
Carotenoid Content: Leaf carotenoid content also dropped upto 38.46% (Cd0.2mM), 53.85% (Cd0.4mM), and 69.23% (Cd0.6mM) in comparison to control. EBL1.0μM application checked the depletion of contents in Cd treatments upto +30.77% (Cd0.2mM), 7.69% (Cd0.4mM), and 23.08% (Cd0.6mM) when comparing control. Further, these losses in EBL3.0μM were 46.15% (Cd0.2mM), 23.08% (Cd0.4mM), and +7.69% (Cd0.6mM). The rise of carotenoid pigment with EBL application was significant in response to Cd treatments (Figure 1b).
Relative Leaf Water Content: The percentage loss in RLWC noticed was upto 7.73% (Cd0.2ⅿⅯ), 13.16% (Cd0.4ⅿⅯ), and 17.23% (Cd0.6ⅿⅯ) with Cd treatment. This loss was comparatively lesser with EBL1.0μM i.e., upto 2.31% (Cd0.2mⅯ), 4.61% (Cd0.4mⅯ), and 12.21% (Cd0.6mⅯ); and with EBL3.0μM upto 10.04% (Cd0.2mⅯ), 8.14% (Cd0.4mⅯ), and 10.45% (Cd0.6mM) in comparison to control. Thus, the application of EBL has significantly improved the RLWC under Cd stress (Figure 1c).
Electrolyte Leakage: Cadmium treatments have raised the electrolyte leakage of membranes up to 18.97% (Cd0.2ⅿⅯ), 40.21% (Cd0.4ⅿⅯ), and 59.38% (Cd0.6ⅿⅯ) in comparison to control. Such rise in EBL supplementation was comparatively lesser i.e., upto 10.31% (Cd0.2ⅿⅯ), +8.04% (Cd0.4ⅿⅯ), and 39.79% (Cd0.6ⅿⅯ) with EBL1.0μM and upto 32.78% (Cd0.2ⅿⅯ), 1.22% (Cd0.4ⅿ), and 13.20% (Cd0.6ⅿⅯ) with EBL3.0μM (Figure 1d).
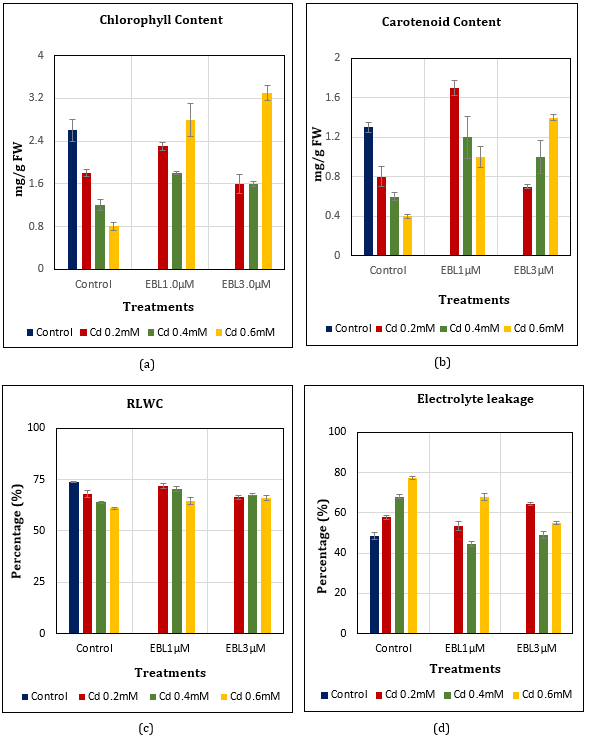
Figure 1 (a-d): Effect of Cd alone and in combination with 24-EBL on (a) total chlorophyll content (LSD0.05=0.44) (b) Total carotenoid content (LSD0.05=0.29) (c) RLWC (LSD0.05=3.13) (d) Electrolyte leakage (LSD0.05=4.06) in soybean plants. Each value represents the mean ± SE of three replicates (n=3). Mean values are significant at P≤0.05.
Morphological Attributes
Root Length: The overall length of roots reduced with cadmium and the percentage decline was 23.64% (Cd0.2ⅿⅯ), 34.15% (Cd0.4ⅿⅯ), and 41.28% (Cd0.6ⅿⅯ) in comparison to control. The loss of root length was comparatively lesser with EBL supplementation i.e., with EBL1.0μM upto 12.57% (Cd0.2mⅯ), 12.20% (Cd0.4mⅯ), and 28.71% (Cd0.6mⅯ); and with EBL3.0μM 12.57% (Cd0.2mⅯ), 12.20% (Cd0.4mⅯ), and 28.71% (Cd0.6mⅯ) to that of control (Figure 2a).
Shoot Length: The overall length of shoots also reduced with cadmium upto 17.48% (Cd0.2ⅿⅯ), 44.72% (Cd0.4ⅿⅯ), and 52.20% (Cd0.6ⅿⅯ) to that of control. EBL supplementation has lowered the plant height losses to 10.33% (Cd0.2mⅯ), 16.26% (Cd0.4mⅯ), and 26.67% (Cd0.6mⅯ) using EBL1.0μM; and 23.41% (Cd0.2mⅯ), +2.44% (Cd0.4mⅯ), and 47.40% (Cd0.6mM) using EBL3.0μM treatments in comparison to control (Figure 2b).
Fresh Weight: In Cd alone treatments (Cd0.2, 0.4, 0.6ⅿⅯ), the fresh weight of plants declined up to 22.43%, 41.12%, and 53.27% respectively. The fresh weight loss with EBL1.0μM supplementation was comparatively lesser i.e., 1.87%, 13.08%, and 25.23%; and with EBL3.0μM was 36.45%, 18.69%, and 18.69% in Cd0.2, 0.4, 0.6mM treatments, respectively (Figure 2c).
Dry Weight: Dry weight of plants also declined with Cd0.2, 0.4, 0.6ⅿⅯ alone treatments upto i.e., 31.19%, 53.61%, and 68.30%, respectively when compared to control. This loss was checked to 2.58% (Cd0.2mⅯ), 19.07% (Cd0.4mⅯ), with EBL1.0μM; and 44.33% (Cd0.2mⅯ), 14.43% (Cd0.4mⅯ), and 31.70% (Cd0.6mⅯ) with EBL3.0μM supplementation (Figure 2d) in comparison to control.
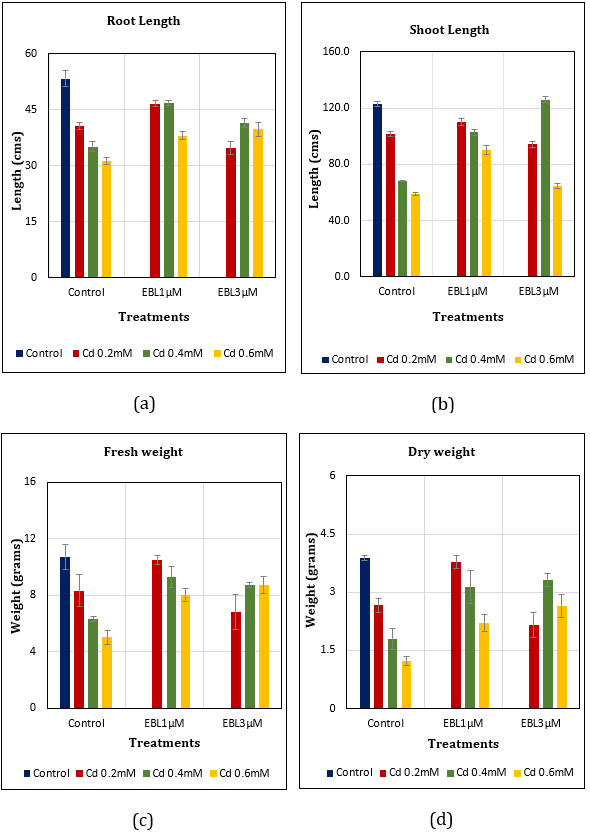
Figure 2 (a-d): Effect of Cd alone and in combination with 24-EBL on (a) Root length (LSD0.05=4.02). (b) Shoot length (LSD0.05=5.63) (c) Fresh weight (LSD0.05=2.10) (d) Dry weight (LSD0.05=0.72) in soybean plants. Each value represents the mean ±SE of three replicates (n=3). Mean values are significant at p-value ≤0.05.
Biochemical Attributes
MDA Content: MDA content of leaves as an indicator of oxidative stress increased with Cd0.2, 0.4, 0.6mM treatments up to 47.71%, 93.49%, and 152.05%, respectively when compared to control. With EBL supplementation, the capacity to reduce MDA content was more in Cd-treated plants lowering it up to 0.48%, 11.20% and 84.70% with EBL1.0μM; and up to 80.84%, 23.86%, and 34.46% EBL3.0μM treatments (Figure 3a).
H2O2 Content: Hydrogen peroxide, another indicator of oxidative stress also increased with Cd0.2, 0.4, 0.6mM treatments showing a rise of 54.84%, 104.84%, and 169.35%, respectively in comparison to control. In the combination treatments, this accumulation was lowered to 12.90%, 25.81%, and 117.74% with EBL1.0μM and 58.06%, 37.10% and 114.52% EBL3.0μM, respectively. It was noticed that EBL interaction was effective with low concentrations of Cd (0.2mM) in reducing elevated levels of H2O2 content (Figure 3b).
Ascorbic Acid: Ascorbic acid content of the leaves reduced with Cd treatments lowering it upto 30.65%, 50.00%, and 66.13% in Cd0.2, 0.4,0.6mM treatments, respectively, in comparison to control. The decline in EBL application was moderate i.e., 4.84%, 20.16%, and 50.81% using EBL1.0μM, and 30.65%, 34.68% and 31.45% using EBL3.0μM, respectively (Figure 3c).
Proline Content: Proline, an osmo-protectant got accumulated in Cd-stressed leaves with a rise of 22.53%, 45.77%, and 81.21% in Cd0.2, 0.4, 0.6mM treatments, respectively in comparison to control. EBL application further led to a rise upto 42.18%, 78.05%, 102.87% in case of EBL1.0μM and 25.68%, 91.10%, and 122.96% in EBL3.0μM, respectively (Figure 3d).
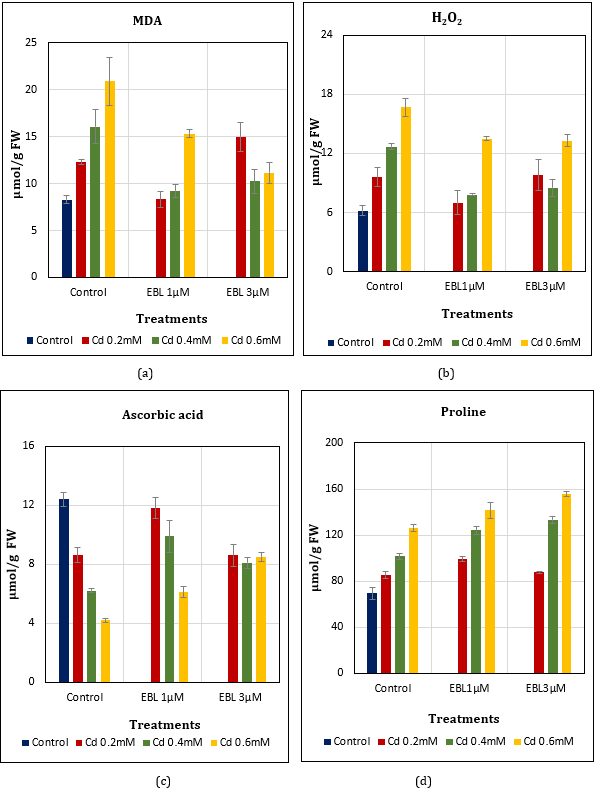
Figure 3 (a-d): Effect of Cd alone and in combination with 24-EBL on (a) MDA content (LSD0.05=3.84) (b) H2O2 content (LSD0.05=2.49) (c) Ascorbic acid content (LSD0.05=1.65) (d) Proline content (LSD0.05=10.8) in soybean plants. Each value represents the mean ±SE of three replicates (n=3). Mean differences are significant at p-value ≤ 0.05.
Discussion
Cadmium effects on the crop plants include the inhibited plant growth, seed germination and disrupted water balance [33]. Our findings clearly indicate that Cd-induced stress diminished growth parameters like root-shoot length and fresh-dry biomass accumulations, lowered photosynthetic pigments and RLWC accompanying the increased leakage of ions. Earlier reports have also indicated that elevated Cd levels impede cell and overall plant growth of crops like alfalfa, wheat, and spinach [34]. Such inhibitions by cadmium were attributed to the interference with cell division and cell elongation rate mainly through irreversible inhibition of proton pump [35]. The present study has indicated that supplementing EBL to Cd-stressed soybean minimized the loss of growth attributes. Consistent with our findings, many reports have suggested the exogenous application of EBL enhanced the growth attributes of Cd stressed crops such as tomato, radish, cucumber and chickpea [36,37]. EBL is involved in cell elongation, regulation of genes responsible for XTHs (Xyloglucan Endotransglucosylase/Hydrolase) activity that influence cell wall modification and expansion, cellulose synthesis, and sucrose synthesis [38].
Numerous studies have highlighted the primary site of action of Cd as photosynthetic pigments like chlorophyll and carotenoid [39], up regulating chlorophyllase activity and degradation of chlorophyll [40]. Cadmium also inhibits the production of ẟ-amino- laevulinic acid and proto-chlorophyllide reductase, essential for the biosynthesis of chlorophyll [41]. Our study has confirmed the promotory role of EBL in synthesis of carotenoids in Cd stressed soybean leaves. The exogenous application of 24-EBL promotes accumulation of carotenoids and chlorophyll by up regulating their genes in T1 generation [42]. The reduced RLWC of cadmium stressed leaves also improved with EBL application in our case. This decreased RLWC was attributed to reduced water absorption under stress conditions [43]. It was reported earlier that EBL supplementation restores RLWC of Cd-stressed cucumber seedlings [44].
Cadmium induced ROS production cause peroxidation of the critical cellular components such as lipids, proteins, and hampers the metabolic processes [45]. Elevated levels of H2O2 followed by MDA disrupt the membrane fluidity causing the electrolyte leakage, inhibited enzyme activity and protein channelling [46]. In our study, various stress indicators were elevated like electrolyte leakage, MDA content, H2O2 content, proline content and accompanied by reduced ascorbic acid contents. MDA, the by-product of polyunsaturated fatty acid decomposition in cell membrane, is a recognised marker of the oxidative stress [47]. Brassinosteroids can modify the structure and stability of membranes under stress [48]. Therefore, EBL application promotes the membrane stability accompanying reduced lipid peroxidation [49]. Earlier reports have also indicated that application of EBL reduced the MDA, H2O2 and ion leakage under heavy metal stress [50,51]. EBL application allows proline accumulation that protects the cells from oxidative damage by maintaining nutrient uptake through water transport [52,53]. The EBL supplementation was able to restore ascorbic acid content in our case that gets oxidized to dehydroascorbic acid with ROS [54]. Both proline and ascorbic acid levels were able to increase the stress tolerance against Cd. Hence, a significant improvement of these stress indicators with 24-EBL supplementation improving the Cd stress tolerance is reported in soybean.
Conclusion
Cd above a particular threshold in the soil disrupts the normal physiological and metabolic functions of plants. The present study has indicated that 24-EBL application as a foliar spray to Cdstressed soybean plants ameliorates the toxic effects by improving growth profile, physiological and biochemical attributes. 24-EBL application has improved the level of stress indicators against Cd induced oxidative stress proving the efficacy of the treatment.
Acknowledgement
Financial assistance provided by University Grant Commission, New Delhi, India, under NET-JRF/SRF fellowship scheme is duly acknowledged for funding the present research work.
Conflict of Interests
There is no conflict of interests.
References
- Foy CD, Chaney RL, White MG (1978) The physiology of metal toxicity in plants. Annual Review of Plant Physiology 29: 511-566.
- Wagner GJ (1993) Accumulation of cadmium in crop plants and its consequences to human health. Advances in Agronomy 51: 173-212.
- Moya JL, Ros R, Picazo I (1993) Influence of cadmium and nickel on growth, net photosynthesis and carbohy-drate distribution in rice plants. Photosynthesis Research 36: 75-80.
- Sanita di Toppi L, Gabrielli R (1999) Response to cadmium in higher plants. Environmental and Experimental Botany 41(2): 105-130.
- Hayat S, Ali BS, Hasan A, Ahmad A (2007) Brassinosteroid enhanced the level of antioxidants under cadmium stress in Brassica juncea. Environmental and Experimental Botany 60: 33-41.
- Hasan SA, Hayat S, Ali B, Ahmad A (2008) 28-homobrassinolide protects chickpea (Cicer arietinum) from cadmium toxicity by stimulating antioxidant. Environment and Pollution 151: 60-66.
- Kahle H (1993) Response to roots of trees to heavy metals. Environmental and Experimental Botany 33: 99-119.
- Benavides MP, Gallego SM, Tomaro ML (2005) Cadmium toxicity in plants. Brazilian journal of plant physiology 17: 21-34.
- Hayat S, Ahmad A (2003) Brassinosteroids: Bioactivity and Crop Productivity. Kluwer Academic Publishers, Dordrecht.
- Bajguz A, Hayat S (2009) Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiology and Biochemistry 47: 1-8.
- Wilen RW, Sacco M, Lawrene VG, Krishna P (1995) Effects of 24-epibrassinolide on freezing and thermo tolerance of brome grass (Bromus inermis) cell cultures. Physiologia Plantarum 95: 195-202.
- Sairam RK (1994) Effect of homobrassinolide application on plant metabolism and grain yield under irrigated and moisture stress condition of two wheat varieties. Plant Growth Regulation 14: 173-181.
- Schilling G, Schiller C, Otto S (1991) Influence of brssinosteroids on organ relation and enzyme activities of sugar beet plants. In: Cutler, H.G., Yokota, T., Adam, G. (Eds.), Brassinosteroids: Chemistry, Bioactivity and Application. ACS Symposium Series 474 American Chemical Society, Washington, DC, pp. 208-219.
- Ali B, Hayat S, Ahmad A (2007) 28-homobrassinosteroids ameliorates the saline stress in Cicer arietinum Environmental and Experimental Botany 59: 217-223.
- Hasan SA, Hayat S, Ali B, Ahmad A (2008) 28-homobrassinolide protects chickpea (Cicer arietinum) from cadmium toxicity by stimulating antioxidant. Environment and Pollution 151: 60-66.
- Wang B, Zhang J, Xia X, Zhang WH (2011) Ameliorative effect of brassinosteroid and ethylene on germination of cucumber seeds in the presence of sodium chloride. Plant Growth Regulation 65(2): 407-413.
- Tong H, Xiao Y, Liu D, Gao S, Liu L, et al. (2014) Brassinosteroid regulates cell elongation by modulating gibberellin metabolism in rice. Plant Cell 26(11): 4376-4393.
- Tang W, Yuan M, Wang R, Yang Y, Wang C, et al. (2011) PP2A activates brassinosteroid-responsive gene expression and plant growth by dephosphorylating BZR1. Nature Cell Biology 13(2): 124-131.
- Cai Z, Yang W, Zhang H, Luo J, Wu F, et al. (2018) Ethylene participates in the brassinolide-regulated asymmetric growth of sativa root. South African Journal of Botany 119: 86-93.
- Maita S, Sotomayor C (2015) The effect of three plant bioregulators on pollen germination, pollen tube growth and fruit set in almond [Prunus dulcis (Mill.) D.A. Webb] cvs. Non Pareil and Carmel. Electronic Journal of Biotechnology 18(5): 381-386.
- Mazorra LM, Oliveira MG, Souza AF, Silva WB, dos Santos GM, et al. (2013) Involvement of brassinosteroids and ethylene in the control of mitochondrial electron transport chain in postharvest papaya fruit. Theoretical and Experimental Plant Physiology 25: 203-212.
- Mao J, Zhang D, Li K, Liu Z, Liu X, et al. (2017) Effect of exogenous Brassinolide (BR) application on the morphology, hormone status, and gene expression of developing lateral roots in Malus hupehensis. Plant Growth Regulation 82: 391-401.
- Shahzad B, Tanveer M, Che Z, Rehman A, Cheema SA, et al. (2018) Role of 24-epibrassinolide (EBL) in mediating heavy metal and pesticide induced oxidative stress in plants: A review. Ecotoxicology and Environmental Safety 147: 935-944.
- Minchin FR, Pate JS (1975) Effects of water, aeration and salt regime on the nitrogen fixation in a nodulated legumes: definition of an optimum root environment. Journal of experimental Botany 26: 60-80.
- Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiology 24: 1-15.
- Kirk JTO, Allen RL (1965) Dependence of chloroplast pigments synthesis on protein synthetic effects on actilione. Cell Biochemistry and Biophysics 27: 523-530.
- Lutts S, Kinet JM, Bouharmont JC (1996) NaCl-induced senescence in leaves of rice (Oryza sativus) cultivars differing in salinity resistance. Annals of Botany 78(3): 389-398.
- Chen J, Shiyab S, Han FX, Monts DL, Waggoner C, et al. (2009) Bioaccumulation and physiological effects of mercury in Pteris vittate and Nephrolepis exaltata. Ecotoxicology 18(1): 110-121.
- Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant soil 39: 205-207.
- Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidative systems in acid rain treated bean plants: protective role of exogenous polyamines. Plant Science 151: 59-66.
- Mukherji SP, Chaudhari MA (1983) Implications of water stress induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna Plant Physiology 58: 166-170.
- Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplast-1: kinetics stoichiometery of fatty peroxidation. Archives of biochemistry and Biophysics 125: 189-198.
- Mishra S, Srivastava S, Tripathi RD, Govindarajan R, Kuriakose SV, et al. (2006) Phytochelatin synthesis and response of antioxidants during cadmium stress in Bacopa monnieri Plant Physiology and Biochemistry 44: 25-37.
- Zhang F, Liu M, Li Y, Che Y, Xiao Y (2019) Effects of arbuscular mycorrhizal fungi, biochar and cadmium on the yield and element uptake of Medicago sativa. Science of the total Environment 655: 1150-1158.
- Liu D, Jiang W, Gao X (2004) Effects of cadmium on root growth, cell division and nucleoli in root tip cells of garlic. Biologia Plantarum 47: 79-83.
- Anuradha A, Rao SSR (2007) The effect of brassinosteroids on radish (Raphanus sativus ) seedlings growing under cadmium stress. Plant soil environment 53(11): 465-472.
- Shah AA, Ahmed S, Yasin NA (2019) 24-epibrassinolide triggers cadmium stress mitigation in Cucumis sativus through intonation of antioxidant system. South African Journal of Botany 127: 349-360.
- Ashraf M, Akram NA, Arteca RN, Foolad MR (2010) The physiological, biochemical and molecular roles of brassinosteroids and salicylic acid in plant processes and salt tolerance. Critical Reviews in Plant Sciences 29: 162-190.
- Prasad MNV (1995) Cadmium toxicity and tolerance in vascular plants. Environmental and Experimental Botany 35(4): 525-545.
- Reddy MP, Vora AB (1986) Changes in pigment composition, hill reaction activity and saccharide metabolism in bajra (Pennisetum typhoides S&H) leaves under NaCl salinity. Photosynthetica 20: 50-55.
- Stobart AK, Griffits W, Bukhari L, Sherwood A (1985) The effect of Cd 2+ on the biosynthesis of chlorophyll in leaves of barley. Physiologia Plantarum 63: 293-298.
- Zhang C, Liang Q, Wang Y, Liang S, Huang Z, et al. (2024) BoaBZR1.1 mediates brassinosteroid-induced carotenoid biosynthesis in Chinese kale. Horticulture Research 11(6): 1-11.
- Bayoumi TY, Eid MH, Metwali EM (2008) Application of physiological and biochemical indices as a screening technique for drought tolerance in wheat genotypes. African Journal of Biotechnology 7: 2341-2352.
- Shah AA, Ahmed S, Yasin NA (2019) 24-epibrassinolide triggers cadmium stress mitigation in Cucumis sativus through intonation of antioxidant system. South African Journal of Botany 127: 349-360.
- Ahmad P, Sarwat M, Bhat NA, Wani MR, Kazi AG, et al. (2015) Alleviation of cadmium toxicity in Brassica juncea (Czern. & Coss.) by calcium application involves various physiological and biochemical strategies. Plos One 10(1): e0114571.
- Garg N, Manchanda G (2009) ROS generation in plants: boon or bane? Plant Biosystems 143: 81-96.
- Demiral T, Turkan I (2005) Comparative lipid peroxidation, antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance. Environmental and Experimental Botany 53: 247-257.
- Hamada KM (1986) Brassinolide in crop cultivation. In: Macgrgor P (Ed.), Plant Growth Regulators in Agriculture. Food Fertility Technology, Central Asia Pacific Region 190-196.
- Rady MM (2011) Effect of 24-epibrassinolide on growth, yield, antioxidant system and cadmium content of bean (Phaseolus vulgaris L.) plants under salinity and cadmium stress. Scientia horticulturae 129(2): 232-237.
- Vardhini BV, Anjum NA (2015) Brassinosteroids make plant life easier under abiotic stresses mainly by modulating major components of antioxidant defense system. Frontiers in Environmental Sciences 2: 67.
- Soares C, de Sousa A, Pinto A, Azenha M, Teixeira J, et al. (2016) Effect of 24-epibrassinolide on ROS content, antioxidant system, lipid peroxidation and Ni uptake in Solanum nigrum under Ni stress. Environmental and Experimental Botany 122: 115-125.
- Anuradha A, Rao SSR (2007) The effect of brassinosteroids on radish (Raphanus sativus ) seedlings growing under cadmium stress. Plant soil environment 53(11): 465-472.
- Ahmad P, Abd Allah EF, Hashem A, Sarwat M, Gucel S (2016) Exogenous application of selenium mitigates cadmium toxicity in Brassica juncea (Czern & Cross) by up-regulating antioxidative system and secondary metabolites. Journal of Plant Growth Regulation 35: 936-950.
- Noctor G, Foyer CH (1998) Ascorbate and glutathione: Keeping active oxygen under control. Annual Review of Plant Physiology and Plant Molecular Biology 49: 249-279.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.