Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Prospects for Rare Diseases Treatment: Insights from Autophagy
*Corresponding author: Dr. Cuicui Ji, Department of Biology, College of Chemistry and Life Science, Beijing University of Technology, 100124, Beijing, China.
Received: January 17, 2025; Published: January 23, 2025
DOI: 10.34297/AJBSR.2025.25.003344
Abstract
Rare diseases are characterized by their low incidence rates, yet there are numerous types of rare diseases that result in a significant number of individuals suffering from rare diseases globally. Although some progress has been made in the therapy of rare diseases so far, the geographic dispersion of the small population suffering from individual rare diseases, the high cost associated with orphan drug development, and other factors challenge the development of clinical therapeutics for these diseases. Autophagy, a highly conserved degradation process in eukaryotic cells, is crucial for maintaining cell homeostasis. Research has shown that dysregulation of autophagy contributes to the pathologies of many rare diseases, like Vici syndrome, Danon disease, and Mesothelioma. A clearer comprehension of how autophagy is involved in rare diseases could help to develop novel treatment approaches. In this review, we will introduce autophagy briefly, followed by a focus on the connection between rare diseases and autophagy. The prospects and challenges of targeting autophagy for rare disease treatment will also be discussed.
Keywords: Autophagy, Rare disease, Neurodegenerative disease, Cancer, Myopathy, Metabolic disease
Abbreviations: PtdIns3K: Phosphatidylinositol 3-Kinase; BPAN: Beta-Propeller Protein-Associated Neurodegeneration; OGT: O-GlcNAc transferase; MM: Malignant mesothelioma; DD: Danon disease; LAMP-2: lysosomal-associated membrane protein 2; ERT: Enzyme replacement therapy; GAA: α-glucosidase.
Introduction
Rare diseases are characterized by low incidence rates. Although they may not be prevalent, they pose significant threats to human health. Numerous types of rare diseases have been identified globally. In China, due to its large population, the total number of individuals affected by rare diseases is significant. In the past few decades, some progress have been made in the treatment of rare diseases, yet numerous challenges remain. Currently, various therapeutic approaches for rare diseases, including small molecule drugs, monoclonal antibodies, enzyme replacement therapy, and stem cell therapy, have been devised. However, the availability of these medications and therapies is restricted, leaving many patients without effective options. Macroautophagy is a cellular process that involves the formation of autophagosomes, which encapsulate substrates such as damaged organelles and protein aggregates, transporting them to lysosomes for degradation. Dysfunction of autophagy is associated with various rare diseases, including neurological, metabolic, muscular, and oncological conditions. A deeper insight into the link between rare diseases and autophagy may contribute to the developing of more effective treatment strategies. This review will describe autophagy, summarize the current progress of its role in the development of rare diseases, and discuss the prospects and challenges for rare disease treatment by modulating autophagy.
Overview of Autophagy
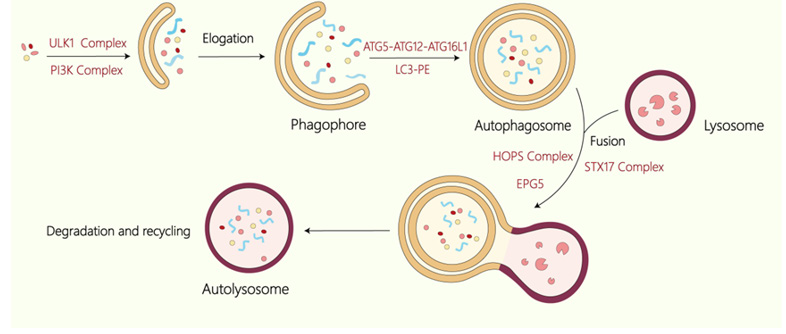
Autophagy is a conserved degradation pathway and is utilized to degrade aging and damaged organelles as well as macromolecules within the cytoplasm in eukaryotes. In mammals, autophagy has been classified into three types: microautophagy, macroautophagy, and chaperone-mediated autophagy [1]. Among them, macroautophagy is studied mostly. The feature of macroautophagy is the formation of the double-membrane autophagosomes. Under stress conditions, like nutrient deprivation, autophagy is initiated, leading to the formation of the double-membrane isolation membrane (phagophore). This structure wraps around the cargoes and expands to form an autophagosome. The autophagosome then fuses with lysosomes, forming autolysosomes, where the contents are broken down. The resulting degradation products are released into the cytoplasm for reuse, thereby maintaining normal cellular functions and homeostasis [2,3]. In the following, macroautophagy is referred to as autophagy for brevity.
The formation of autophagosomes demands precise regulatory mechanisms, with a series of autophagy-related core proteins participating in diverse stages of the process. Under starvation conditions, the ULK1 complex containing ULK1, FIP200, ATG13, and ATG101 is activated in mammalian cells, resulting in the induction of autophagy [4]. The next complex recruited to the isolation membrane is the PtdIns3K complex which includes Beclin 1, VPS34, VPS15, and ATG14 [5]. This complex participates in the nucleation stage by promoting the production of PI3P, which recruits WIPI proteins (WIPI1-4) to the isolation membrane. WIPI proteins, mammalian homologs of yeast Atg18, interact with Atg2. In mammals, all WIPI proteins, particularly WIPI4, interact with ATG2, which exhibits lipid transfer function essential for the expanding of isolation membrane. Two ubiquitin-like conjugation systems are pivotal for the elongation and eventual closure of the isolation membrane: the ATG12-ATG5-ATG16L1 complex [6] and the LC3-PE system [7]. The formation of the ATG12-ATG5 conjugate is facilitated by ATG7 and ATG10, after which ATG16L1 binds to ATG5, leading to the assembly of the ATG12-ATG5-ATG16L1 complex [8]. Meanwhile, proLC3 is processed by ATG4 to generate LC3-I, which is then conjugated to PE to create LC3-II. Ultimately, mature autophagosomes fuse with lysosomes, which requires multiple factors, including tethering proteins such as EPG5, the HOPS complex, and PLEKHM1, along with the SNARE complex (containing STX17-SNAP29-VAMP8 and YKT6-SNAP29-STX7), and RAB small GTPases. Figure 1 Process of macroautophagy. Macroautophagy involves the de novo formation of autophagosomes, which sequesters cellular components and transports them to lysosomes for degradation. The ULK1 complex is involved in the induction stage of autophagy and the Class III PtdIns3K complex is involved in the nucleation stage. The ATG12- ATG5-ATG16L1 and LC3-PE participate in the elogation of the isolation membrane. The autophagosome then fuses with the lysosome with the participation of HOPS complex, STX17 complex and EPG5 to form an autolysosome to degrade the substance.
The Involvement of Autophagy in Rare Diseases
Autophagy is a crucial degradative pathway in eukaryotic cells. Research has shown various rare diseases, such as Vici syndrome, Beta-Propeller Protein-Associated Neurodegeneration, Danon disease, and Mesothelioma, are closely linked to the dysfunction of autophagy. Understanding this relationship is not only essential for unraveling the pathogenesis of these conditions but also critical for developing targeted therapies. Below is the relationship between these rare diseases and autophagy.
Dysregulation of Autophagy in Neurodegenerative Disease
Vici Syndrome
Vici Syndrome is an uncommon genetic disorder that impacts various systems in the body. It is primarily marked by the absence of the corpus callosum, along with combined immunodeficiency, bilateral cataracts, hypopigmentation, and additional characteristics that significantly diminish patients’ overall health and quality of life. Although the incidence of this disease remains unclear, the count of reported cases has grown exponentially since Vici, et al. first described it in 1988 [9].
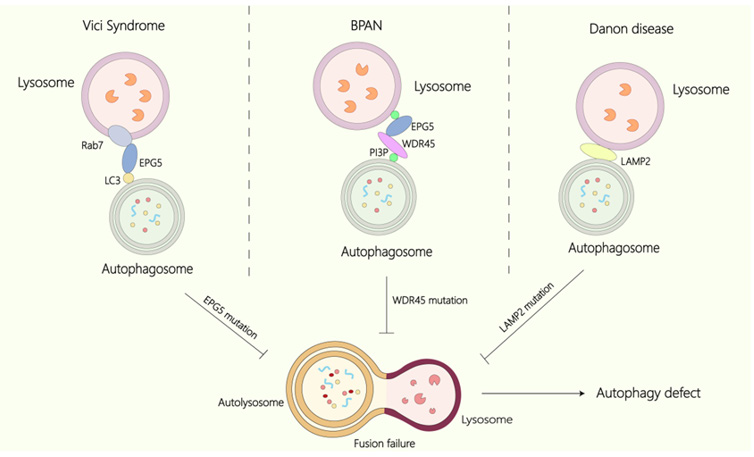
Vici syndrome results from mutations in EPG5, which is located on chromosome 18q12.3. Epg-5 was first discovered in C. elegans as a factor involved in autophagy [10]. Recessive mutations of EPG5 have been found in Vici patients [11]. Clinical studies have shown that autophagosomes accumulate in the muscles and fibroblasts of the patients [12]. In the platelets of patients, the LC3-positive puncta was accumulated, indicating a rise in the number of autophagosomes [13]. Epg5 KO mice display phenotypes similar to those of Vici syndrome patients [14], and an aggregation of non-degradative autolysosomes has been noted in Epg5 KO mice, epg-5-deficient C.elegans, and patient tissues. Nevertheless, the precise mechanism is unclear.
EPG5 has been found to be a key factor in the fusion of autophagosomes with endosomes/lysosomes [15]. Specifically, EPG5 functions as a Rab7 effector by binding to Rab7 directly, facilitating its recruitment to late endosomes/lysosomes [16]. EPG5 also engages with LC3 and the STX17-SNAP29 Qabc SNARE complex to mediate the fusion of autophagosome and lysosome. Additionally, in C.elegans, overexpression of the GDP-bound form of RAB-7 could promote lysosome biogenesis and improve autophagy defects in epg-5 mutants. The RBG-1(GAP for RAB3)–RBG-2 (GEF for RAB18) complex was found to modulate lysosomal biogenesis by regulating the dynamics of RAB-7 [17]. Vici syndrome poses a significant threat to human health, so effective treatment strategies must be identified. The current findings may provide new insights into potential therapeutic approaches for Vici syndrome.
Beta-Propeller Protein-Associated Neurodegeneration (BPAN)
BPAN is an X-linked dominant neurodegenerative disease characterized by the accumulation of iron within brain. Patients commonly exhibit clinical signs such as global developmental delay, which may progress to gait ataxia and mild spasms. WDR45 is responsible for the pathogenesis of BPAN. De novo mutations of WDR45 have been discovered in individuals with BPAN [18]. Furthermore, the autophagic activity is decreased and autophagic structures are accumulated in the lymphocytes of BPAN patients. Similar findings of decreased autophagic activity and iron accumulation have also been noted in patient-derived fibroblasts [19,20].
Since the essential role of WDR45 in BPAN was discovered, various animal and cellular models were generated to investigate its function and the role of autophagy in BPAN. For instance, knockout of the WDR45 homolog EPG6 in C. elegans leads to the early accumulation of autophagic structures, suggesting the role of EPG6 in the formation of autophagosomes [21]. WDR45 conditional knockout mice display phenotypes and autophagic abnormalities similar to those observed in BPAN patients [22]. Mechanistically, WDR45 performs several functions: first, it binds to ATG2, which has lipid transfer activity, to supply phospholipids necessary for the expanding of the isolation membrane. Second, WDR45 and WDR45B function redundantly and interact with EPG5 to mediate autophagosome- lysosome fusion. Additionally, WDR45 also regulates autophagy by interacting with the AMPK-ULK1 signaling pathway. Moreover, maintaining iron homeostasis is crucial for cellular functions. Overexpression of WDR45 mutations associated with BPAN in HeLa cells leads to iron overload [23]. These mutations in WDR45 impair the autophagic degradation of transferrin receptors, resulting in an overload of iron and subsequent iron accumulation, which in turn promotes iron-induced cell apoptosis [24]. The SH-SY5Y neuroblastoma cells with the WDR45 knocked out by CRISPR-Cas9 technology exhibit a defect in ferritinophagy [25], indicating that WDR45 also participates in ferritinophagy.
Since autophagy is impaired in BPAN, this indicates that it is possible to slow down the progression of this disease by modulating autophagy. Research indicates that the mTOR inhibitor torin 1 can activate autophagy, helping to reduce elevated iron levels in WDR45 mutant human fibroblasts or neurons. Another mTOR inhibitor, rapamycin, has been found to decrease apoptosis in WDR45-deficient cells. Furthermore, depleting O-GlcNAc transferase (OGT) has been reported to promote autophagy. Inhibiting SNAP29 O-GlcNAcylation by depleting OGT enhances the formation of the STX17-SNAP29-VAMP8 SNARE complex. Ji et al. found that knocking down OGT can rescue autophagy defects in WDR45/45B double knockout N2a cells.This implies that improving the efficiency of autophagosome-lysosome fusion may serve as a potential treatment approach for BPAN.
Figure 2 The relationship between Vici syndrome, BPAN, DD, and autophagy. In Vici Syndrome, mutations in EPG5 impair its interaction with Rab7, resulting in failure of autophagosome-lysosome fusion and leading to autophagy dysregulation and the accumulation of undegraded material. Similarly, in BPAN, mutations in WDR45 contribute to autophagic deficiencies. In DD, mutations in LAMP2 obstruct the fusion of autophagosomes with lysosomes, further resulting in autophagy defects.
Dysregulation of Autophagy in Cancer
Malignant Mesothelioma
Malignant mesothelioma (MM) is an uncommon and deadly cancer that mainly affects the pleura, peritoneum, and pericardium [26]. Patients typically present with symptoms like chest pain, cough, and shortness of breath. This tumor is closely linked to exposure to the carcinogenic mineral fiber asbestos. Upon inhalation, asbestos fibers can deposit on the pleural membrane and, in excessive amounts, may reach the abdomen, leading to apoptosis of the corresponding mesothelial cells [25]. As a consequence, these damaged mesothelial cells release pro-inflammatory mediators, predominantly HMGB1, thereby initiating an inflammatory response [27].
Current studies have shown that autophagy is deregulated in MM. Primary mesothelial cells exposed to various carcinogenic fibers exhibiting elevated levels of autophagy. HMGB1 modulates autophagy via the RAGE-mTOR-ULK pathway, as well as phosphorylating Beclin 1. This process enhances autophagy in mesothelial cells [28], which contributes to the survival and malignant transformation of mesothelioma cells, ultimately resulting in mesothelioma. Therefore, silencing HMGB1 has been demonstrated to decrease autophagy and enhance asbestos-induced mesothelial cell death, which in turn reduces the transformation of these cells [29].
Autophagy represents a potential therapeutic focus for MM. The specific silencing of STMN1 can reduce the expression of genes related to apoptosis and autophagy, including caspase-2 and LC3 [30]. The ULK1-specific inhibitor MRT 68921 effectively decreases autophagy in MM cells while enhancing the efficacy of chemotherapy. Furthermore, JSI-124 was shown to inhibit the proliferation of murine MM cells, leading to the induction of both apoptosis and autophagic cell death [31]. As the function of autophagy in mesothelioma is further investigated, novel therapeutic approaches based on autophagy modulation have the potential to be developed.
Dysregulation of Autophagy in Myopathy
Danon Disease
Danon disease (DD) was named by Danon and his colleagues in 1981. It is an uncommon X-linked multisystem genetic condition, with males typically presenting more severe phenotypes than females. Clinically, patients exhibit symptoms such as skeletal muscle weakness, psychiatric disorders, and hypertrophic cardiomyopathy.
The pathogenesis of DD is linked to autophagy, a process essential for cellular maintenance. In the skeletal muscle of patients, large LC3-positive membrane-bound structures were observed [32]. Muscle biopsies from patients showed inhibited autophagy, elevated LC3 levels, and accumulation of p62.
A primary cause of DD is LAMP2 deficiency [33]. LAMP2 mediates the fusion of autophagosomes and lysosomes [34]. Absence of LAMP-2 lead to a failure of autophagosome-lysosome fusion. Though LAMP-2 has three isoforms (LAMP-2A, LAMP-2B, and LAMP-2C) [35], only mutations in LAMP-2B are associated with DD. LAMP-2B is particularly significant in autophagy. Research studies, both in vivo and in vitro, have shown knocking out of LAMP-2B is sufficient to disrupt autophagic flux, leading to a distinct DD phenotype. This suggests LAMP-2A and LAMP-2C may play less significant roles in the pathogenesis of DD compared to LAMP-2B.
Since autophagy is vital for cell metabolism and the maintaining of homeostasis, the dysfunction of autophagy in DD not only disrupts cell function but may also predispose individuals to other comorbidities. Further investigation into the relationship between DD and autophagy is essential for understanding its pathological mechanisms and may provide new insight into future treatment strategies.
Pompe Disease
Pompe disease, often referred to as glycogen storage disease type II, is an uncommon autosomal recessive neuromuscular disease. This condition is marked by recurrent neurological disorders, muscle weakness, and progressive motor dysfunction. It is also accompanied by symptoms of other organ involvement, such as convulsions, loss of consciousness, ectropion of the eyelid, and blindness. The Pompe disease results from a lack of acid α-glucosidase (GAA) in lysosomes. GAA is the only enzyme capable of breaking down glycogen into glucose within lysosomes. Consequently, the lack of GAA results in the accumulation of glycogen in lysosomes, primarily affecting the heart and skeletal muscles. As glycogen builds up in the lysosomal lumen, the lysosomal membrane ruptures, releasing glycogen and toxic substances to the cytoplasm, resulting in muscle structural damage [36].
Autophagy has been linked to Pompe disease. Skeletal muscle relies heavily on autophagy due to their terminal differentiation state, which prohibits the dilution of abnormal proteins and organelles through cell division [37]. Studies demonstrated that autophagic substances are increased in skeletal muscle fibers of Pompe disease patients [38]. Both in Pompe disease patients’ myotubes and primary myoblasts of affected mice, the autophagosomes and autophagic substances are increased, accompanied by improper vacuolation and lysosomal acidification, leading to impaired autophagy. Furthermore, autophagy influences GAA maturation and glycogen clearance, leading to further muscle cell damage [39].
The current FDA-approved Pompe disease treatment is Enzyme Replacement Therapy (ERT), which exhibits improvement in heart and respiratory functions. ERT utilizes the mannose-6-phosphate receptor-mediated pathway for lysosomal enzyme uptake. However, it is expensive and unaffordable for many patients. Therefore, finding new treatments is particularly important. Studies have shown that combining exercise with ERT may reduce autophagy obstruction in animal models, suggesting that autophagy may be crucial in managing Pompe disease.
Discussion
The role of autophagy in the development of human disease has drawn considerable attention in recent years. As research progresses, the significance and potential of autophagy have been further emphasized. In this review, we summarized the connections between several rare diseases and autophagy. Those studies indicate that autophagy may be a potential therapeutic target. Nonetheless, it is important to highlight that the complexity of autophagy may pose significant challenges for disease treatment. For instance, the regulation of autophagy is a multifaceted process that can influence numerous biological processes. Given the dual functions of autophagy in various diseases, the question of whether it is more beneficial to induce or inhibit autophagy remains a crucial consideration. Additionally, the assessment of autophagy activity varies across studies, leading to some controversial and unreliable findings. While there is still much progress to be made in the autophagy- targeted treatment of rare diseases, we are optimistic about ongoing research that aims to bridge the gap between autophagy and human health. This review helps to further elucidate the intricate connection between autophagy and human disease.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 32200605).
Conflict of Interest
The author declares that he has no known competing financial interests or personal relationships that could have appeared to in fluence the work reported in this paper.
References
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self-digestion. Nature 451(7182): 1069-1075.
- Ohsumi Y, Nakatogawa H, Suzuki K, Kamada Y (2009) Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nature reviews. Molecular cell biology 10(7): 458-467.
- Yang Z, Klionsky DJ (2010) Eaten alive: a history of macroautophagy. Nat Cell Biol 12(9): 814-822.
- Zhao YG, Codogno P, Zhang H (2021) Machinery, regulation and pathophysiological implications of autophagosome maturation. Nat Rev Mol Cell Biol 22(11): 733-750.
- Sun Q, Fan W, Chen K, Ding X, Chen S, et al. (2008) Identification of Barkor as a mammalian autophagy-specific factor for Beclin 1 and class III phosphatidylinositol 3-kinase. Proc Natl Acad Sci U S A 105(49): 19211-19216.
- Fujioka Y, Noda NN, Nakatogawa H, Ohsumi Y, Inagaki F (2010) Dimeric Coiled-coil Structure of Saccharomyces cerevisiae Atg16 and Its Functional Significance in Autophagy. The Journal of biological chemistry 285(2): 1508-1515.
- Kirisako T, Ichimura Y, Okada H, Kabeya Y, Mizushima N, et al. (2000) The reversible modification regulates the membrane-binding state of Apg8/Aut7 essential for autophagy and the cytoplasm to vacuole targeting pathway. J Cell Biol 151(2): 263-276.
- Mizushima N (2007) Autophagy: process and function. Genes Dev 21(22): 2861-2873.
- Vici CD, Sabetta G, Gambarara M, Vigevano F, Bertini E, et al. (1988) Agenesis of the corpus callosum, combined immunodeficiency, bilateral cataract, and hypopigmentation in two brothers. American journal of medical genetics 29(1): 1-8.
- Tian Y, Li Z, Hu W, Ren H, Tian E, et al. (2010) C. elegans screen identifies autophagy genes specific to multicellular organisms. Cell 141(6): 1042-1055.
- Ehmke N, Parvaneh N, Krawitz P, Ashrafi MR, Karimi P, et al. (2014) First description of a patient with Vici syndrome due to a mutation affecting the penultimate exon ofEPG5 and review of the literature. Am J Med Genet A 164(12): 3170-3175.
- Cullup T, Kho AL, Dionisi-Vici C, Brandmeier B, Smith F, et al. (2013) Recessive mutations in EPG5 cause Vici syndrome, a multisystem disorder with defective autophagy. Nat Genet 45(1): 83-87.
- Schwertz H, Rowley JW, Portier I, Middleton EA, Tolley ND, et al. (2022) Human platelets display dysregulated sepsis-associated autophagy, induced by altered LC3 protein-protein interaction of the Vici-protein EPG5. Autophagy 18(7): 1534-1550.
- Zhao H, Zhao YG, Wang X, Xu L, Miao L, et al. (2013) Mice deficient inEpg5 exhibit selective neuronal vulnerability to degeneration. J Cell Biol 200(6): 731-741.
- Hori I, Otomo T, Nakashima M, Miya F, Negishi Y, et al. (2017) Defects in autophagosome-lysosome fusion underlie Vici syndrome, a neurodevelopmental disorder with multisystem involvement. Sci Rep 7(1): 3552.
- Wang Z, Miao G, Xue X, Guo X, Yuan C, et al. (2016) The Vici Syndrome Protein EPG5 Is a Rab7 Effector that Determines the Fusion Specificity of Autophagosomes with Late Endosomes/Lysosomes. Mol Cell 63(5): 781-795.
- Wang Z, Zhao H, Yuan C, Zhao D, Sun Y, et al. (2019) The RBG-1/RBG-2 complex modulates autophagy activity by regulating lysosomal biogenesis and function. J Cell Sci 32(19): jcs234195.
- Haack TB, Hogarth P, Kruer MC, Gregory A, Wieland T, et al. (2012) Exome sequencing reveals de novo WDR45 mutations causing a phenotypically distinct, X-linked dominant form of NBIA. Am J Hum Genet 91(6): 1144-1149.
- Seibler P, Burbulla LF, Dulovic M, Zittel S, Heine J, et al. (2018) Iron overload is accompanied by mitochondrial and lysosomal dysfunction in WDR45 mutant cells. Brain 141(10): 3052-3064.
- Lee HE, Jung MK, Noh SG, Choi HB, Chae SH, et al. (2021) Iron Accumulation and Changes in Cellular Organelles in WDR45 Mutant Fibroblasts. Int J Mol Sci 22(21): 11650.
- Bakula D, Muller AJ, Zuleger T, Takacs Z, Franz-Wachtel M, et al. (2017) WIPI3 and WIPI4 beta-propellers are scaffolds for LKB1-AMPK-TSC signalling circuits in the control of autophagy. Nat Commun 8: 15637.
- Zhao YG, Sun L, Miao G, Ji C, Zhao H, et al. (2015) The autophagy gene Wdr45/Wipi4 regulates learning and memory function and axonal homeostasis. Autophagy 11(6): 881-890.
- Xiong Q, Li X, Li W, Chen G, Xiao H, et al. (2021) WDR45 Mutation Impairs the Autophagic Degradation of Transferrin Receptor and Promotes Ferroptosis. Front Mol Biosci 8: 645831.
- Aring L, Choi EK, Kopera H, Lanigan T, Iwase S, et al. (2022) A neurodegeneration gene, WDR45, links impaired ferritinophagy to iron accumulation. J Neurochem 160(3): 356-375.
- Liu G, Cheresh P, Kamp DW (2013) Molecular basis of asbestos-induced lung disease. Annu Rev Pathol 8: 161-187.
- Wagner JC, Sleggs CA, Marchand P (1960) Diffuse pleural mesothelioma and asbestos exposure in the North Western Cape Province. Br J Ind Med 17(4): 260-271.
- Yang H, Rivera Z, Jube S, Nasu M, Bertino P, et al. (2010) Programmed necrosis induced by asbestos in human mesothelial cells causes high-mobility group box 1 protein release and resultant inflammation. Proc Natl Acad Sci U S A 107(28): 12611-12616.
- Xue J, Patergnani S, Giorgi C, Suarez J, Goto K, et al. (2020) Asbestos induces mesothelial cell transformation via HMGB1-driven autophagy. Proc Natl Acad Sci U S A 117(41): 25543-25552.
- Aksoy A, Varoglu A, Onalan EE, Tektemur A, Artas G, et al. (2024) The knockdown of stathmin with si-RNA inhibits invasion of mesothelioma. Tissue Cell 87: 102303.
- Wang X, Chauhan G, Tacderas A, Muth A, Gupta V (2023) Surface-Modified Inhaled Microparticle-Encapsulated Celastrol for Enhanced Efficacy in Malignant Pleural Mesothelioma. Int J Mol Sci 24(6): 5204.
- Zhang C, Sun Q, Zhao J, Jiang N, Hao Y, et al. (2023) JSI-124 inhibits cell proliferation and tumor growth by inducing autophagy and apoptosis in murine malignant mesothelioma. Mol Carcinog 62(12): 1888-1901.
- Cuervo AM, Dice JF (2000) Unique properties of lamp2a compared to other lamp2 isoforms. J Cell Sci 113 Pt 24: 4441-4450.
- Eskelinen EL, Illert AL, Tanaka Y, Schwarzmann G, Blanz J, et al. (2002) Role of LAMP-2 in lysosome biogenesis and autophagy. Mol Biol Cell 13(9): 3355-3368.
- Tanaka Y, Guhde G, Suter A, Eskelinen EL, Hartmann D, et al. (2000) Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature 406(6798): 902-906.
- Fujiwara Y, Furuta A, Kikuchi H, Aizawa S, Hatanaka Y, et al. (2013) Discovery of a novel type of autophagy targeting RNA. Autophagy 9(3): 403-409.
- Gundersen K, Lindstedt SL, Hoppeler HH (2016) Muscle memory and a new cellular model for muscle atrophy and hypertrophy. J Exp Biol 219(Pt 2): 235-242.
- Masiero E, Sandri M (2010) Autophagy inhibition induces atrophy and myopathy in adult skeletal muscles. Autophagy 6(2): 307-309.
- Lim JA, Li L, Shirihai OS, Trudeau KM, Puertollano R, et al. (2017) Modulation of mTOR signaling as a strategy for the treatment of Pompe disease. EMBO Mol Med 9(3): 353-370.
- Palhegyi AM, Seranova E, Dimova S, Hoque S, Sarkar S (2019) Biomedical Implications of Autophagy in Macromolecule Storage Disorders. Front Cell Dev Biol 7: 179.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.