Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Safety and Efficacy of Different Anticoagulant Regimens in Patients with Cancer-Associated Thrombosis: A Clinical Research
*Corresponding author: Veronika Petrusheva, Department of cardiology, Heart and Brain Center of Excellence, Pleven, Bulgaria.
Received: January 30, 20255; Published: February 06, 2025
DOI: 10.34297/AJBSR.2025.25.003359
Abstract
Introduction: Cancer-Associated Thrombosis (CAT) is a major problem for cancer patients, worsening their prognosis and leading to increased mortality rate. Anticoagulant treatment in cancer patients carries some risks associated with both increased bleeding and recurrence rates despite optimal anticoagulant therapy.
Aim: We aim to evaluate the safety and efficacy of different anticoagulation regimens in CAT patients.
Methods: We included a retrospective and a prospective group of CAT patients in the study. Anticoagulant therapy was initiated according to individualized patients’ characteristics. We divided the patients into four groups based on the prescribed anticoagulant and we compared mortality, bleeding, and recurrent events between these groups on the sixth month of the follow-up.
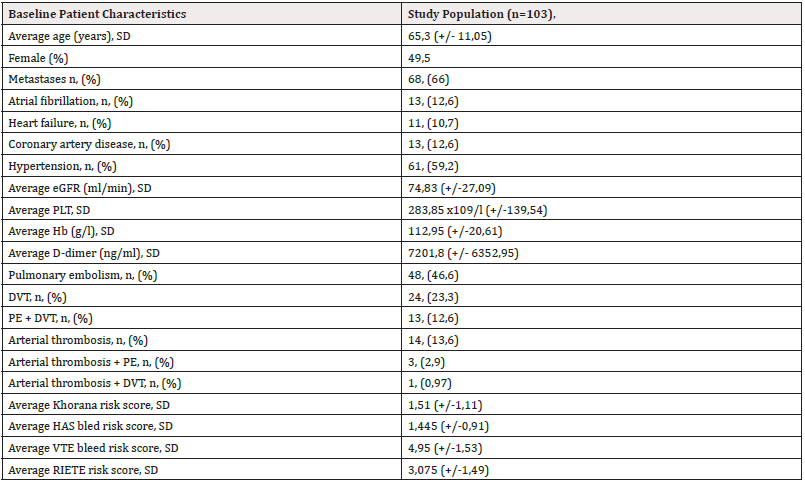
Results: The study includes 103 patients with a mean age of 65,3 (+/- 11,05); 49,5% of them are female; 66% are in stage IV (with metastatic disease at diagnosis); 12,6% have concomitant atrial fibrillation; 10,7% have heart failure; 12,6% have coronary artery disease, and 59,2% have hypertension. 82,5% were diagnosed with Venous Thromboembolism (VTE), 13,6% with arterial thrombosis, and 3,87% with both arterial and venous thrombosis. 22,33% of patients had bleeding events. 12,6% were cases of major, 5,8% Clinically Relevant Non-Major (CRNM), and 3,9% minor bleeding. Different anticoagulant regimens resulted in a similar rate of bleeding. The most frequent tumors associated with bleeding were gastrointestinal and gynecologic tumors (17,4% of each). There were 14,6% recurrent events, without a significant difference in subgroups of different anticoagulation therapies. The most common tumors associated with recurrence were lung, gastric, and bladder cancer (13,33% each). The mortality rate was high: 52,4% of patients died during follow-up, with no difference in subgroups according to therapy.
Conclusion: There is still no optimal anticoagulant suitable for all cases of CAT with a predictable effect, effectively limiting both recurrence and bleeding events. There is no statistical significance between different anticoagulation regimen groups regarding mortality, bleeding, and recurrence.
Keywords: Cardio-oncology, Cancer-associated thrombosis, Anticoagulants, Bleeding, Recurrence
Introduction
Cancer-Associated Thrombosis includes VTE and arterial thrombotic events. The most common arterial thrombotic events include myocardial infarction, cerebrovascular disease, and Peripheral Artery Disease (PAD) [1]. VTE combines Pulmonary Embolism (PE) and Deep Vein Thrombosis (DVT). CAT is a leading cause of morbidity and mortality in cancer patients. VTE and arterial thromboembolism are the second most common cause of death in cancer patients after cancer itself [2]. Cancer patients are associated with an increased risk of bleeding and recurrent thromboembolic events, despite optimal anticoagulant therapy. The overall survival of CAT patients is shorter than that of cancer patients without thrombosis.
The last decade has revolutionized our understanding of the molecular mechanisms associated with the increased risk of thrombosis in cancer patients. Despite recent advances, there is still a gap in the knowledge of the most appropriate approaches for thromboprophylaxis and treatment in cancer-related thrombosis. The VTE risk in cancer patients should be regularly reassessed. The Khorana score has been developed to assess the need for prophylaxis of VTE in cancer patients. Based on the calculated score, patients are divided into three groups: low risk (0 points), intermediate risk (1 or 2 points), and high risk (≥3 points) according to cancer type, leukocyte, hemoglobin and platelet levels, erythrocyte growth factor usage and body mass index [3].
According to Tzu-Fei Wang, the 6-month risk of developing VTE in cancer patients is up to 12-fold higher than that risk in the general population. That risk is even higher in patients receiving anticancer treatment (up to 23-fold higher) [4]. The pathophysiology of CAT is multifactorial. Cancer induces a pro-inflammatory and pro-thrombotic state and is a strong risk factor for thromboembolic events. Risk factors associated with thrombosis can be divided into three groups. The first group are patient related risk factors (such as increasing age, female sex for VTE and male sex for arterial thromboembolism, immobility, prior VTE history, some hereditary coagulation defects, comorbidities such as chronic kidney disease, obesity, acute infection, cardio-vascular disease, and pulmonary diseases). The second group are cancer-related risk factors, including cancer type and stage. Cancers of the pancreas, stomach, ovaries, brain, lung, and myeloma are associated with an increased risk for VTE. Advanced or metastatic disease is also a risk factor for VTE. Histology (e.g., adenocarcinoma) as well as some genetic characteristics of the tumor, such as JAK2 or K-ras mutations, also increase the risk. The third group are treatment-related risk factors-presence of central venous catheters, hospitalization, major surgery, cancer therapy, including chemotherapy (platinum-based chemotherapeutic agents such as cisplatin and carboplatin; anthracyclines, taxanes, cyclophosphamide, and antimetabolites); anti-angiogenic agents such as bevacizumab, sorafenib, and sunitinib; immunomodulatory drugs (e.g., thalidomide, lenalidomide); proteasome inhibitors (carfilzomab); hormonal therapy and erythropoiesis-stimulating agents. Blood transfusions also increase the thrombotic risk [5,6].
CAT is a major problem among the population with malignan cies, leading to a poor prognosis, and that is why there are several clinical trials, randomized controlled trials, and meta-analyses regarding treatment options in this field. We are focused on cardio- oncology in order to advance the care of cancer patients. We have a large database of CAT patients and which is why we would like to present our experience on this topic.
Aim
The aim of our project is to evaluate the safety and efficacy of different anticoagulation regimens in patients with cancer-associated thrombosis.
Methods
We included a retrospective and a prospective cohort in the study. We searched for the patients in the retrospective cohort in our database for a period of one year dating back from the start of the study (from November 1, 2021 to November 1, 2022). All oncology patients with newly diagnosed CAT were included in the prospective cohort for a period of one year (from November 1, 2022 to November 1, 2023).
Anticoagulant therapy was initiated. The choice of anticoagulant was based on the patient’s baseline characteristics, age, Estimated Glomerular Filtration Rate (eGFR), Hemoglobin (Hb) and Platelet (Plt) levels, concomitant diseases, bleeding risk, the type and stage of the tumor, the presence of brain lesions, the patients’ preferences, etc. We divided the patients into four groups: a group who do not take anticoagulant therapy (called group 0, which includes 30 patients), a group with Direct Oral Anticoagulants (DOACs) (group 1, which includes 55 patients), a group on parenteral anticoagulants (group 2, which includes 14 patients), and a group of patients prescribed Vitamin K Antagonists (VKAs) (group 3, which includes 4 patients).
We collected a sample of 103 patients (50 patients in the retrospective group and 53 patients in the prospective group), and we followed them up until the end of the study. The follow-up included assessing adherence to therapy, bleeding events, and the recurrence of thrombotic events.
We compared mortality, bleeding, and recurrent events between different groups of anticoagulants on the sixth month of the follow-up. The bleeding events were classified as major, CRNM and minor bleeding. The International Society on Thrombosis and Haemostasis (ISTH) defines major bleeding as fatal bleeding, or bleeding causing a fall in hemoglobin level of 20 g/l or more, or requiring transfusion of 2 or more units of blood or symptomatic bleeding in a critical area (intracranial, intraspinal, intraocular, retroperitoneal, intraarticular, pericardial, or intramuscular with compartment syndrome) [6,7]. CRNM bleeding is defined as hemorrhage that does not satisfy the criteria for the ISTH definition of major bleeding but leads to hospitalization or requires medical or surgical intervention [8]. Minor bleeding events are bleedings not classified as major or CRNM bleeding.
Statistical Analysis
We present continuous variables as mean values with a Standard Deviation (SD). We present categorical variables as Numbers (N) and Percentages (%). We compare mortality, bleeding events and recurrent events between different groups of anticoagulants and express the difference between groups as a p-value. We used the two sample T.TEST function in Excel (version 2016), comparing the mean values of samples between two population groups to find the p-value. A p-value less than 0,05 was considered statistically significant.
In addition, we present baseline characteristics among the four groups of anticoagulant regimens, the prevalence of different tumors in patients with CAT, tumors most commonly associated with bleeding or with recurrent events.
Results
Patient Characteristics
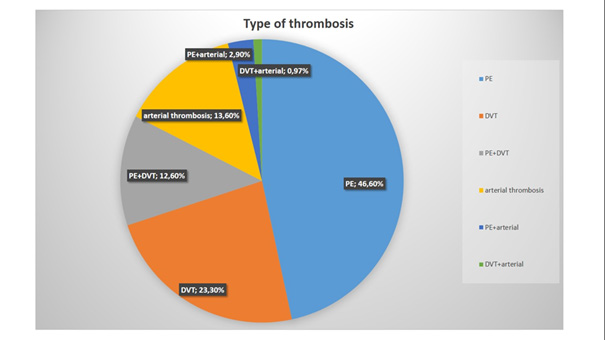
We included 103 patients-50 in the retrospective group and 53 in the prospective group and followed them up for 6 months. The average age of patients was 65,3 years. They were almost equally distributed by gender. Most of the patients had metastatic disease. Hypertension was observed in most of them. Anemia was prevalent among patients (Table 1). VTE is much more common than arterial events (Figure 1).
Based on the prescribed anticoagulant therapy we analyzed 4 different groups: the first group was on DOACs (55 people, 53,4%), 4 subjects were on VKAs (3,9 %), 14 were on parenteral anticoagulants (UFH or LMWH; 13,6%) and 30 patients (29,1 %) did not take any anticoagulant for some reason (e.g., bleeding history associated with anticoagulants, high bleeding risk, brain lesion, etc.). The oldest patients were in group 3 (patients on VKAs), and the youngest were in group 2. The platelet level is highest in group 0 and lowest in group 3. Anemia was prevalent among the four groups (Table 2).
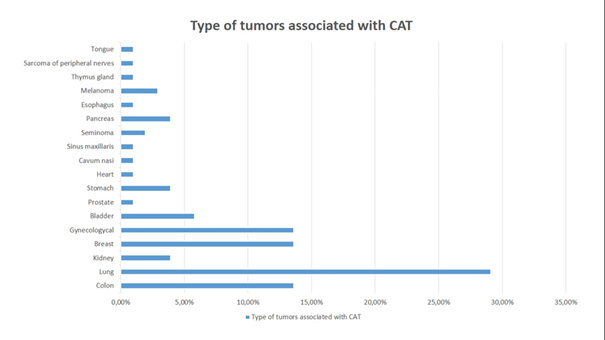
The largest proportion of CAT patients have lung cancer (30 people, 29,1%). The second place is divided between colon cancer (13,6%), breast cancer (13,6%) and carcinoma of the genital system (14 people, 13,6%). Bladder cancer takes third place (6 people, 5,8%). The next place is for kidney cancer (4 people, 3,9%), gastric cancer (3,9%) and pancreatic cancer (3,9 %). There are 3 patients with melanoma (2,9%). 2 patients have seminoma (1,94%). The rest of the tumors are one number each: prostate cancer (0,97%), heart cancer (0,97%), carcinoma of the cavum nasi (0,97%), sinus maxillaris (0,97%), esophagus (0,97%), thymus gland (0,97%), tongue (0,97%), and sarcoma of the periphery nerves (0,97%) (Figure 2).
Bleeding
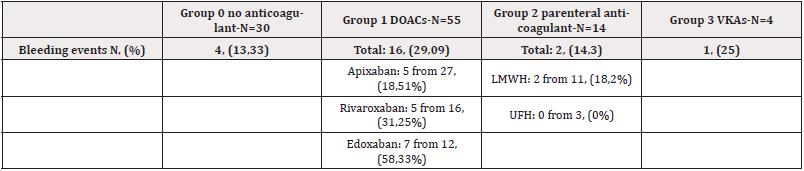
We observed 23 bleeding events (22,33%). The bleeding rate was highest in the DOAC group-29,09%, and especially among patients on edoxaban-7 out of 12 or 58,33% had bleeding events. Among the DOACs, apixaban was associated with the smallest incidents of bleeding events (Table 3). There is no statistical significance between the four groups regarding bleeding (Table 4).
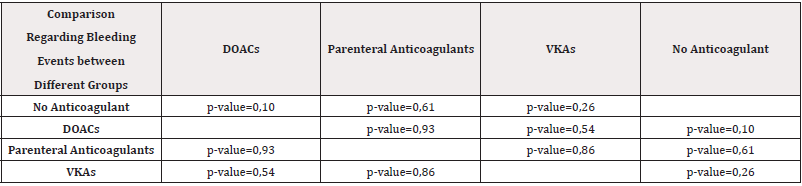
Table 4: Comparison regarding bleeding events between different groups of anticoagulants with a calculated p-value.
Bleeding events among the four anticoagulant groups are presented in Figure 4.
There are 4 cases of minor bleeding-self-limiting, that did not require hospitalization. It was mild epistaxis or hemorrhoidаl bleeding. All patients with minor bleeding were on DOACs.
There are also six cases of CRNM bleeding, that required hospitalization and specific treatment but did not require blood transfusion. In most of the cases, it was hematuria, rectorrhagia or hematemesis. Five of them were on DOACs and 1 was without anticoagulant therapy.
There are 13 cases of major bleeding: fatal bleeding or bleeding that required the transfusion of 2 or more units of red blood cells, or bleeding leading to a severe decline in hemoglobin level of more than 20 g/l. 7 of them were on DOACs, 3 were on parenteral anticoagulants, 1 was on VKAs and 2 were without anticoagulants (Table 3).
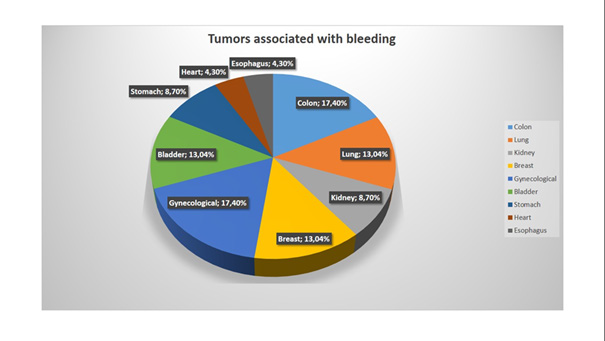
The most frequent tumors associated with bleeding events were colon cancer and gynecologic tumors (4 of 23 for each, 17,4%), the second place was divided between breast cancer, lung cancer, and bladder cancer (3 cases of each, 13,04%). Gastric and kidney cancer- 2 cases of bleeding for each, 8,7%. Tumors of the esophagus and the heart: 1 case for each, 4,3% (Figure 3). In most cases, the bleeding was from the GI tract, also there were a few cases of genitourinary bleeding and epistaxis (Table 4, Figure 4).
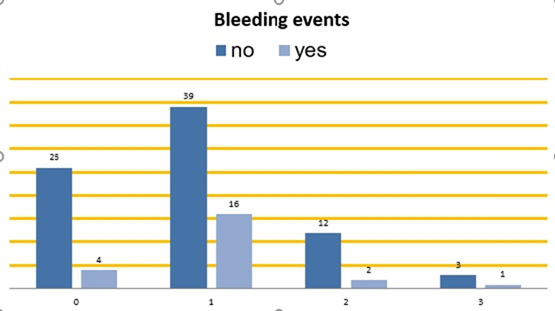
Figure 4: Bleeding events among the four anticoagulant groups. On the bottom horizontal line: Group 0: patients without anticoagulant therapy.
Group 1: patients on DOACs. Group 2: patients on parenteral anticoagulants. Group 3: patients on VKAs. Vertical columns: Dark blue – patients without bleeding during the follow-up. Light blue – patients with bleeding events during the follow-up.
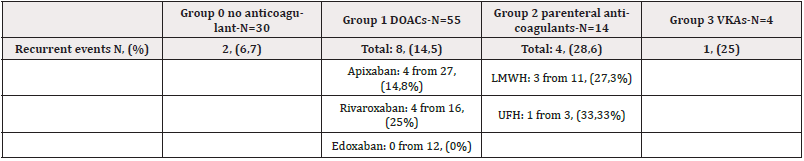
Recurrent Events
There were 15 cases of recurrent events (14,6% of the group). The recurrence rate was highest in the group 2 who were on parenteral anticoagulants- 28,6%. In the group who were on DOACs, recurrence was most common in patients on rivaroxaban (Table 5).
Recurrent Thrombotic Events Among the Four Anticoagulant Groups are Presented in Figure 6.
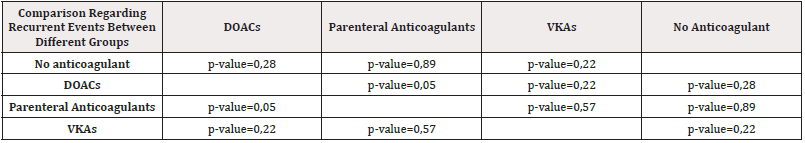
Table 6: Comparison regarding recurrent events between different groups of anticoagulants with a calculated p-value.
There is no statistically significant difference regarding recurrence between different anticoagulant groups. The only result with borderline statistical significance is the comparison between patients treated with DOACs and those treated with parenteral anticoagulants- p-value = 0,05 (Table 6).
The most frequent tumors associated with recurrent events were lung cancer (3 cases, 20%), bladder cancer, and gastric cancer (2 cases of each, 13,3%). Kidney cancer, breast cancer, prostate cancer, seminoma, melanoma, gynecologic tumors, and tumors of the cavum nasi and sinus maxillaris-1 case for each (6,7%) (Figures 5) (Table 6) (Figure 6).
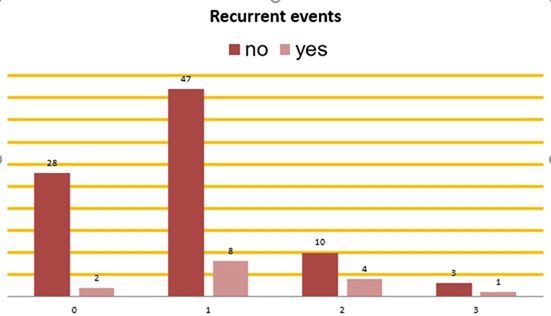
Figure 6: Recurrence among the four anticoagulant groups. On the bottom horizontal line: Group 0: patients without anticoagulant therapy. Group 1: patients on DOACs. Group 2: patients on parenteral anticoagulant. Group 3: patients on VKAs. Vertical columns: Red – patients without recurrent events during the follow-up. Pink – patients with recurrent events during the follow-up.
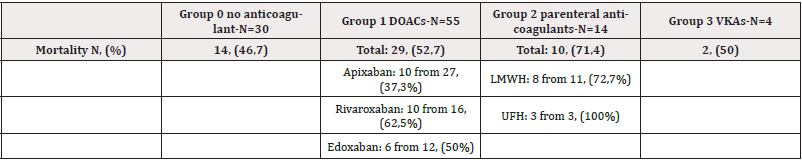
Mortality
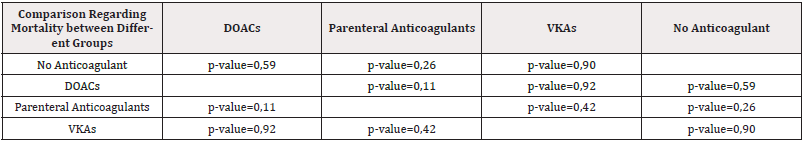
54 patients (52,4%) died during the follow-up. The mortality rate was highest in the group on UFH: 3 of 3 patients or 100% have died. Between the 4 groups, mortality was highest in the group 2 who were on parenteral anticoagulants-71,4%. In DOACs group, mortality was highest in patients on rivaroxaban and lowest in patients on apixaban. (Table 7). There is no statistical significance between the four groups regarding mortality (Table 8).
Mortality Rate Among the Four Anticoagulant Groups is Present in Figure 7.
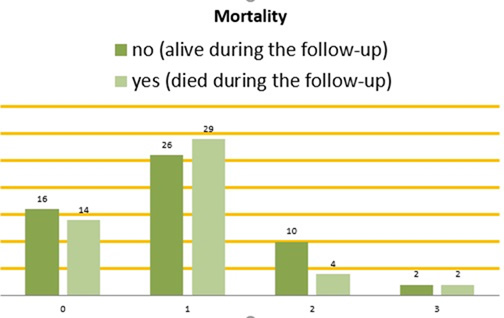
Figure 7: Mortality among the four anticoagulant groups. On the bottom horizontal line: Group 0: patients without anticoagulant therapy. Group 1: patients on DOACs. Group 2: patients on parenteral anticoagulants. Group 3: patients on VKAs. Vertical columns: Dark green – patients who were still alive during the follow-up. Light green – patients who died during the follow-up.
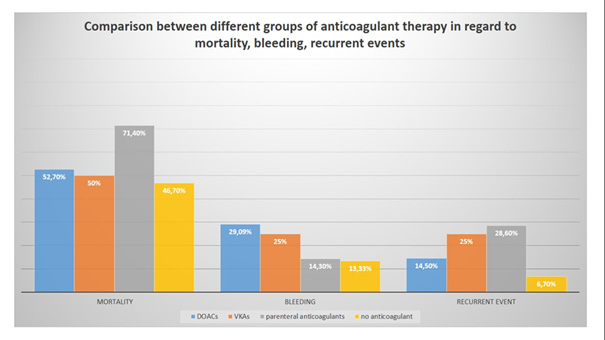
Figure 8: Comparison between different groups of anticoagulants regarding mortality, bleeding and recurrent events.
(Tables 7, 8) (Figure 7)
A summarized comparison between different groups of anticoagulant therapy regarding mortality, bleeding and recurrent thrombotic events is presented in Figure 8.
Discussion
Our results show that lung cancer is prevalent among CAT patients (29,1%). In our cohort, CAT is usually associated with metastatic disease (66% of patients have metastases). The most common type of thrombosis in our cohort is PE (46,6%). The most common type of tumor associated with bleeding complications is colon cancer (17,4% of all patients with bleeding). The most common tumor associated with recurrent events is lung cancer (20% of all cases). There is no statistical significance between different groups of anticoagulant treatment regarding mortality, bleeding, and recurrence events. The only result with borderline significance is the difference between patients on parenteral anticoagulants and those on DOACs regarding recurrent events (p-value = 0,05). It is also important to note that mortality in our study is very high and is highest in patients treated with parenteral anticoagulants (71,4%). The mortality rate in our group treated with UFH is 100%. Treatment of CAT is challenging because it is associated with frequent complications, including bleeding and recurrent events, despite optimal anticoagulation.
The anticoagulant treatment for VTE usually lasts from 3 to 6 months, or until the cancer is considered active. Cancer is defined as active if it is metastatic or if cancer treatment is ongoing (either chemotherapy, immunotherapy, targeted therapy, and/or radiotherapy). Cancer is non-active when it is in remission for more than 6 months [9]. In addition, it is recommended to periodically assess the bleeding risk and the recurrence risk-for this purpose, we have used several risk scores, including the HAS bled score, the VTE bleed risk score, the RIETTE risk score and the Khorana risk score (results are summarized in Tables 1 and 2).
There are a variety of treatment options, which include VKAs, unfractionated heparin, LMWH (enoxaparin and dalteparin), fondaparinux, and several DOACs that are approved for cancer patients (rivaroxaban, apixaban, and edoxaban), and some advantages and disadvantages of each of them. In these patients, anticoagulant therapy should be individualized based on the patient’s baseline characteristics, the type and stage of cancer, cancer treatment and potential drug-drug interactions, bleeding risk, and also the patient’s preference. The main problems using VKAs are multiple drug-drug interactions that can reduce or potentiate their anticoagulant effect, unpredictable GI absorption because of eventual vomiting and/or diarrhea, and also they require frequent laboratory monitoring of the coagulation status (especially the INRinternational normalized ratio). The main disadvantage of LMWH therapy is that it is expensive and requires subcutaneous injections, which discomforts patients, and that is why adherence is declining over time. However, it is suitable in case of vomiting and in cases of severe kidney dysfunction. Recently, DOACs have become the anticoagulants of choice for treatment of CAT because they are administered orally in fixed doses, do not require regular monitoring of the anticoagulant effect, and have fewer drug-drug interactions [4].
In our cohort of patients, DOACs were preferred, except in cases of contraindications (53,4% of CAT patients received DOACs). VKAs were only prescribed in four patients who had either prosthetic heart valves or severe kidney dysfunction. In patients with contraindications for oral anticoagulants, a parenteral one was prescribed.
There are some challenging subgroups of patients with CAT in which the bleeding risk is high and should be periodically assessed. These are patients with GI and Genitourinary (GU) cancer, those with GI comorbidities (e.g., gastric or duodenal ulcers, gastritis, esophagitis or colitis, GI metastases), those with intracranial lesions, patients with active or recent major bleeding, those with low body weight (<60 kg) or thrombocytopenia, and those with severe kidney dysfunction. Other bleeding risk factors include treatment with VEGFi (e.g., bevacizumab), Endothelial Growth Factor Receptor Inhibitors (EGFRi, e.g., cetuximab), and ibrutinib [6], or concomitant antiplatelet therapy. Patients with brain malignancies are at both an increased risk of thrombotic complications and a high risk of brain hemorrhage. Brain tumors have a higher tendency for spontaneous bleeding, and that is why anticoagulation should be avoided in these patients [10]. We avoid prescribing DOACs to patients with brain lesions. For this group of patients, we prefer parenteral anticoagulants. Sometimes any anticoagulant is contraindicated. Thrombocytopenia resulting from malignancy or chemotherapy is common in patients with cancer. In cases of thrombocytopenia, the risk of bleeding is increased, therefore, the use of dose-adjusted LMWH in patients with a platelet count <50×109/L should be preferred [11]. Half dose LMWH is preferred in cases of a platelet count between 25 and 50×109/L. The management of patients with VTE and a platelet count lower than 25x109/L should be individualized but anticoagulation is not recommended as long as thrombocytopenia is not compensated [6]. The average PLT level in our cohort is 283,85x109/l.
DOACs should be used very cautiously in patients with GI cancer because of the high bleeding risk. According to recent results from clinical trials, apixaban should be the preferred DOAC in patients with malignancies of the gastrointestinal system. LMWH are still preferred for patients with upper or lower GI tumors that are not surgically removed. Vitamin K antagonists should only be used for anticoagulation when DOACs and LMWH are unsuitable, for example, in patients with mechanical heart valves, moderate or severe mitral stenosis, and in some cases with an eGFR less than 15 ml/min/1,73 m2.
This data is confirmed in our study because the prevalent tumors associated with bleeding events were tumors of the gastrointestinal tract-30,4% (17,4% colon cancer, 8,7% gastric cancer, and 4,3% esophageal cancer) and tumors of the genitourinary tract- 39,1% (17,4% gynecologic cancer, 13,04% bladder cancer and 8,7% kidney cancer). Most of the patients with such tumors and bleeding events were treated with DOACs. In our group of patients treated with DOACs, bleeding was predominant in patients on edoxaban (58,33% of this group had bleeding events). For apixaban, bleeding is observed in 18,51%, and for rivaroxaban it is observed in 31,25%. Most of our cases of bleeding were major bleeding (43,5%). Two of our patients with bleeding complications were treated with VEGFi, one with EGFRi, and one with pemetrexed; all the rest were treated with anticancer drugs that are not associated with higher bleeding risk (e.g., 5-fluoracil, platinum-based regimens, taxanes, immune checkpoint inhibitors, cyclin-dependent kinase 4/6 inhibitors, anthracyclines, hormonal therapy, denosumab, gemcitabine, and ifosfamide).
According to information published in 2022 by Andres Escobar, the incidence of major bleeding varies from 6.5 to 18% [12].
There are several trials analyzing treatment strategies for CAT patients. Compared to dalteparin, DOACs reduce the risk of VTE recurrence, however, they increase the risk of major bleeding. That is the result of a meta-analysis of four clinical trials comparing factor Xa inhibitors to dalteparin in CAT patients [13]. In the Hokusai VTE Cancer Trial comparing edoxaban with dalteparin, a higher incidence of bleeding was observed in patients treated with edoxaban, but it was predominantly GI bleeding, mainly in patients with GI malignancies [14]. In the SELECT-D Trial, rivaroxaban was compared with dalteparin, and the results showed that rivaroxaban reduced the recurrence rate but also increased the frequency of bleeding, predominantly GI bleeding [15]. In the ADAM VTE Trial, apixaban compared with dalteparin was associated with lower rates of bleeding and recurrence of VTE [16]. In the Caravaggio Trial comparing apixaban to dalteparin, it was noticed that the cases of bleeding and recurrence were similar between the two groups [17]. These results give some predominance to apixaban as a preferred DOAC in cancer patients because of its relatively safer profile.
Cancer patients are simultaneously at increased risk of both bleeding and recurrence [18]. DOACs are currently considered to be an effective and relatively safe option for the treatment of VTE in patients without contraindications. LMWHs are still the preferred treatment option for cancer patients with high bleeding risk, such as those with GI malignancies, those with chemotherapy-induced thrombocytopenia, requiring frequent dose adjustments, and those with severe kidney dysfunction, as well as those with brain metastases [19].
Limitations
The limitations of this study are the small number of patients, the short follow-up period, and the unequal distribution of patients in the different anticoagulant groups.
Conclusion
Lung cancer is prevailing among patients with cancer-associated thrombosis. The most common type of thrombosis in our study is PE. The presence of metastases is a strong risk factor for CAT and most patients have metastatic disease. Bleeding complications occur most frequently in patients with colon cancer. Thrombotic recurrences are most common in lung cancer. The management of patients with CAT is challenging. Cancer patients, even those who do not take anticoagulant therapy, have a high bleeding risk. There is still no optimal anticoagulant regime suitable for all cases of CAT with a predictable effect, limiting both recurrence and bleeding events. There is no statistical significance between different anticoagulation regimen groups regarding mortality, bleeding, and recurrence. DOACs, the most frequently prescribed anticoagulants in our group, were not associated with increased bleeding, recurrence events, or mortality compared with parenteral anticoagulants and VKAs in cancer patients.
Funding
The project was supported by Pfizer (Pfizer Grant 76166107).
Author Contributions
Prof. I. Simova designed the study. V. Petrusheva and M. Samardjieva were in charge of data collection and data analysis. V. Petrusheva writtened the manuscript and prepared the figures and the tables. Prof. I. Simova reviewed and edited the manuscript. All the authors have read and agreed to the final version of the manuscript.
ORCID: https://orcid.org/0009-0003-6266-665X
Conflict of Interest
The authors declare that they have no conflict of interest to disclose.
Data Availability
All data generated or analyzed during this study are included in this published article.
Ethics Declarations
The project complied with the principles of the Helsinki Declaration.
Human Ethics and Consent to Participate Declarations
Not applicable.
References
- Tuzovic M, Herrmann J, Iliescu C, Marmagkiolis K, Ziaeian B, et al. (2018) Arterial Thrombosis in Patients with Cancer. Curr Treat Options Cardiovasc Med 20(5): 40.
- Fernandes CJ, Morinaga LTK, Alves JL Jr, Castro MA, Calderaro D, et al. (2019) Cancer-associated thrombosis: the when, how and why. Eur Respir Rev 28 (151): 180119.
- Overvad TF, Ording AG, Nielsen PB, Skjøth F, Albertsen IE, et al. (2022) Validation of the Khorana score for predicting venous thromboembolism in 40 218 patients with cancer initiating chemotherapy. Blood Adv 6(10): 2967-2976.
- Wang TF, Khorana AA, Agnelli G, Bloomfield D, Marc P Bonaca, et al. (2023) Treatment of Cancer-Associated Thrombosis: Recent Advances, Unmet Needs, and Future Direction. Oncologist 28 (7): 555-564.
- Abdol Razak NB, Jones G, Bhandari M, Berndt MC, Metharom P, et al. (2018) Cancer-Associated Thrombosis: An Overview of Mechanisms, Risk Factors, and Treatment. Cancers (Basel)10(10): 380.
- 2022 ESC Guidelines on cardio-oncology developed in collaboration with EHA, ESTRO, ICOS. Eur Heart J 2022.
- Schulman S, Kearon C (2005) Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 3(4): 692-694.
- Kaatz S, Ahmad D, Spyropoulos AC, Schulman S (2015) Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost 13(11): 2119-2126.
- Mazzolai L, Alatri A (2023) Treatment of cancer-associated venous thromboembolism. European society of cardiology.
- Lin RJ, Green DL, Shah GL (2018) Therapeutic Anticoagulation in Patients with Primary Brain Tumors or Secondary Brain Metastasis. Oncologist 23(4): 468-473.
- Wilks ML (2022) Direct Oral Anticoagulants and Cancer Thrombosis: What APs Need to Know. J Adv Pract Oncol 13(3): 253-256.
- Escobar A, Salem AM, Dickson K, Johnson TN, Burk KJ, et al. (2022) Anticoagulation and bleeding in the cancer patient. Support Care Cancer 30(10): 8547-8557.
- Moik F, Posch F, Zielinski C, Pabinger I, Ay C (2020) Direct oral anticoagulants compared to low-molecular-weight heparin for the treatment of cancer-associated thrombosis: Updated systematic review and meta-analysis of randomized controlled trials. Res Pract Thromb Haemost 4(4): 550-561.
- Raskob GE, van Es N, Verhamme P, Carrier M, Di Nisio M, et al. (2018) Hokusai VTE Cancer Investigators. Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. N Engl J Med 378(7): 615-624.
- Young AM, Marshall A, Thirlwall J, Chapman O, Lokare A, et al. (2018) Comparison of an Oral Factor Xa Inhibitor With Low Molecular Weight Heparin in Patients With Cancer With Venous Thromboembolism: Results of a Randomized Trial (SELECT-D). J Clin Oncol 36(20): 2017-2023.
- McBane RD 2nd, Wysokinski WE, Le Rademacher JG, Zemla T, Ashrani A, et al. (2020) Apixaban and dalteparin in active malignancy-associated venous thromboembolism: The ADAM VTE trial. J Thromb Haemost 18(2): 411-421.
- Agnelli G, Becattini C, Meyer G, Muñoz A, Huisman MV, et al. (2020) Caravaggio Investigators. Apixaban for the Treatment of Venous Thromboembolism Associated with Cancer. N Engl J Med 382(17): 1599-1607.
- Rygiel K (2021) Balancing Risk of Thromboembolism and Bleeding in Patients with Cancer: Selecting Anticoagulant Therapy Based on Recent Clinical Trials. EMJ Oncol.
- Frere C, Farge D, Schrag D, Prata PH, Connors JM (2022) Direct oral anticoagulant versus low molecular weight heparin for the treatment of cancer-associated venous thromboembolism: 2022 updated systematic review and meta-analysis of randomized controlled trials. J Hematol Oncol 15(1): 69.




 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.