Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
The Effect of Neutrophil/Lymphocyte Ratio in Peripheral Blood and Tumor on Prognosis and Survival in Oral Squamous Cell Carcinomas
*Corresponding author: Neslihan Kaya Terzi, Faculty of Medicine, Department of Pathology, Canakkale Onsekiz Mart University, Canakkale, Turkey.
Received: February 04, 2025; Published: February 06, 2025
DOI: 10.34297/AJBSR.2025.25.003361
Abstract
Objective: The aim of this study was to evaluate the prognostic significance of the neutrophil-to-lymphocyte ratio in peripheral blood (NLR) and in the cancer nest (iNLR) in patients with oral squamous cell carcinoma (OSCC).
Methods: A retrospective analysis was performed on 59 OSCC patients who underwent radical surgery at a University Hospital between January 2017 and December 2023. Baseline assessments included routine peripheral blood tests and histopathologic examination of the carcinoma nest. Survival outcomes were analyzed using Kaplan-Meier curves, and univariate and multivariate analyses were performed to identify potential prognostic factors.
Results: The median postoperative survival time for patients with low iNLR was 43 months compared to 19.5 months for patients with high iNLR. Although the overall survival difference did not reach statistical significance (P=0.09), patients with high NLR exhibited lower survival times. Furthermore, the median postoperative survival time for patients with low NLR was 38 months, compared to 20 months for patients with high NLR, a statistically significant difference (P=0.013). Univariate analysis identified NLR and tumor-node-metastasis staging as independent prognostic factors for postoperative survival in OSCC patients (P<0.05). Multivariate analysis revealed smoking status, surgical procedure, tumor-nodemetastasis staging, pathological tumor differentiation and NLR/iNLR scores as significant factors affecting postoperative survival (P<0.05).
Conclusion: High NLR and iNLR levels are indicative of a worse prognosis in OSCC patients. Integration of NLR and iNLR assessments could potentially inform clinicians in the implementation of more aggressive therapeutic strategies for high-risk individuals.
Keywords: Oral Squamous Cell Carcinoma, Neutrophil Lymphocyte Ratio, Peripheral Blood, Tumor associated, Survival
Introduction
Primary Squamous Cell Carcinoma (SCC) in the oral cavity is the most frequent malignancy of this anatomical area and affects older male patients who smoke. For this patient population, the estimated 5 year overall survival rate varies from around 50% to 60% [1]. At present, the evaluation criterion of prognosis mostly depends on TNM staging system However clinically it is difficult for this standard to accurately predict the postoperative outcome of patients [2]. Research has shown that tumor-infiltrating leuko cytes play an essential role in the development and progression of cancers, controlling both positive as well as negative impacts on tumourigenesis [3]. Recent studies have highlighted the pro-tumorigenic and anti tumorigenic functions of neutrophils in oral squamous cell carcinomas in oncology [4]. Recent studies indicate that an elevated neutrophil count and a higher neutrophil-to-lymphocyte ratio in peripheral blood (NLR) adversely affect the prognostic course of cancer [4,5].
In head and neck carcinomas a greater neutrophilic infiltrate was seen in T4 tumors rather than those of lower stage (T1, T2). In addition, these patients with low neutrophil infiltration in grade (III and IV) diseases had better 5-year survival rates compared to those at a higher level [5]. The prognostic importance of neutrophils in Oral Squamous Cell Carcinoma (OSCC) has also been emphasized in the literature; high NLR is associated with poor prognosis, low tumor differentiation and more advanced clinical stages [6]. Furthermore, high neutrophil density has been associated with lymph node metastasis, recurrence and poor differentiation [7]. In contrast to previous studies, our research aims to evaluate the prognostic value of the neutrophil/lymphocyte ratio both within tumor tissues (iNLR) and in peripheral blood (NLR).
Materials and Methods
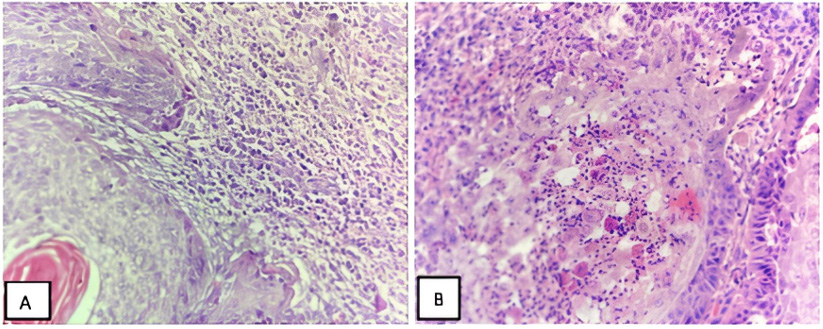
This study is a retrospective analysis of 59 patients diagnosed with OSCC who underwent radical surgery at a University Hospital between January 2017 and December 2023. It included detailed analysis of peripheral blood tests and histopathologic examinations of carcinoma tissues. Before the surgery, the NLR in peripheral blood was determined by dividing the number of neutrophils by the number of lymphocytes. Histopathologic analysis was performed by a pathologist experienced in oral pathology using light microscopy on hematoxylin and eosin stained slides. In our study, as in similar studies in the literature, tumor-associated neutrophils and neutrophil/lymphocyte ratios were detected in the intratumor, peritumor or peritumoral regions [8,9].
In our study, intratumoral, peritumoral and tumor peripheral NLR ratios were calculated in sections of tumor tissues and comparisons were made with other parameters based on their mean values. Two pathologist confirmed the iNLR count. Tumor slides were scanned in their entirety and inflammatory cells within and around 500 epithelial cells with different neutrophil/lymphocyte densities were counted and the average calculated [2]. The study attempted to correlate both iNLR and NLR as well as the proportion of neutrophils in tumor tissue and peripheral blood. Particular attention was paid to the correlation between NLR and iNLR and histopathological parameters, including histological grade, lymphatic invasion, vascular invasion, presence of necrosis, tumor-node-metastasis (TNM) stage, overall survival and disease-free survival. The study received ethics committee approval (number: 2023/1621, date: 20/12/2023).
Statistical Analysis The analytical framework employed for this investigation involved the utilization of SPSS version 21 (IBM Corp. Released 2021. IBM SPSS Statistics for Windows, Version 28.0. Armonk, NY: IBM Corp). Sequential assessment of demographic and radiological variables was conducted utilizing chi-square analysis with Yates correction, wherein a significance threshold of P≤0.05 was deemed indicative of statistical significance. Continuous variables are presented as the mean (standard deviation), and categorical variables are presented as the frequency and proportion (%). Receiver-operating characteristic (ROC) curve was used to determine the ideal cutoff values of NLR and iNLR. Comparison of the proportion of each inflammatory cell and neutrophil distribution among OSCC grades was performed using one-way ANOVA and post hoc Tukey test. Chi-square test was used to find the relationship between inflammation intensity and OSHK differentiation. The power of the study was kept at 80% and the significance level was kept at 5%. Survival outcomes were evaluated using Kaplan-Meier survival curves, and univariate and multivariate analyses were performed to identify potential prognostic factors.
Results
The ages of the patients included in the study ranged from 46 to 87 years with a mean age of 68 years. The study consisted of 47 men and 12 women. Of these, 4 patients were younger than 50 years, while the remaining 55 patients were 50 years or older. High NLR was found in the majority (84.6%) of patients with smoking habit. 23 of 26 patients (88.4%) underwent neck dissection with tumor resection and high NLR was observed in this group. According to tumor differentiation, high NLR was found in 4 (15.3%) well-differentiated squamous cell carcinomas, 19 (73%) moderately differentiated squamous cell carcinomas and 3 (11.5%) poorly differentiated squamous cell carcinomas. According to TNM stage, 16 cases (61.5%) with stage II had a high NLR relationship, while 2 cases (7.6%) with stage I and 8 cases (30.7%) with stage III had a high NLR relationship. Among 38 patients with smoking habit, 25 (65.7%) had high iNLR. Of 37 patients who underwent neck dissection with tumor resection, 26 (70.2%) had a high iNLR. The majority (68.9%) of patients with intermediate tumor differentiation had high iNLR, while only 2 (7.6%) of well-differentiated tumors had high iNLR. High iNLR association was observed in 3 of 4 poorly differentiated tumors (75%). A statistically significant association was found between high NLR and various clinical and histopathological characteristics, including smoking status, surgical technique, tumor differentiation, and TNM stage (P=0.004, P=0.0008, P=0.0006, P=0.005, respectively; see Table 1). Similarly, high iNLR was significantly associated with smoking, alcohol consumption, surgical technique, tumor differentiation, and TNM stage (P=0.0002, P=0.002, P=0.0004, P=0.0007, P=0.0006, respectively; see Table 2).
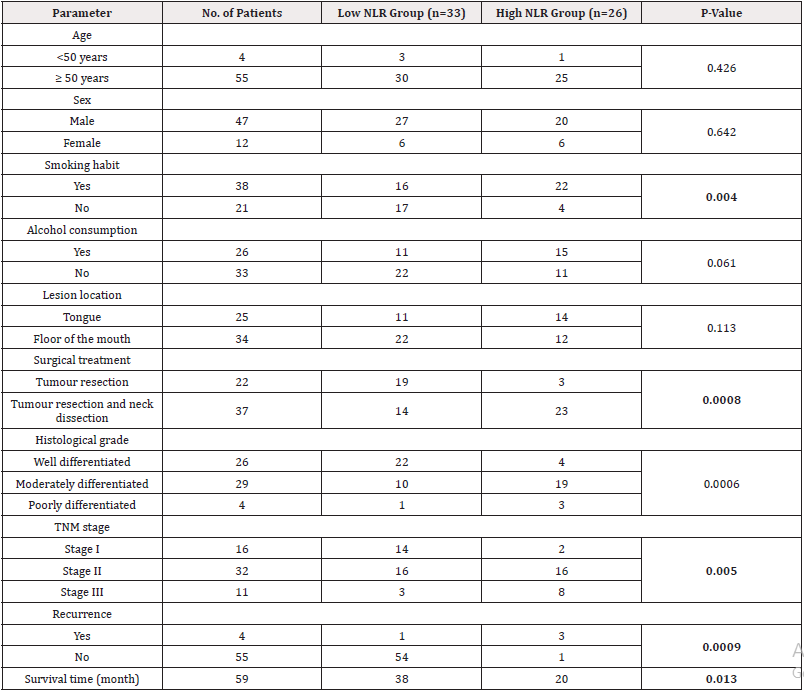
Table 1: Evaluation of clinical and microscopic features and neutrophil-lymphocyte ratio (NLR) in peripheral blood in 59 patients with oral squamous cell carcinoma.
Note*: Bold P-values <.05. NLR=neutrophils-to-lymphocyte ratio, TNM=tumor-node-metastasis.
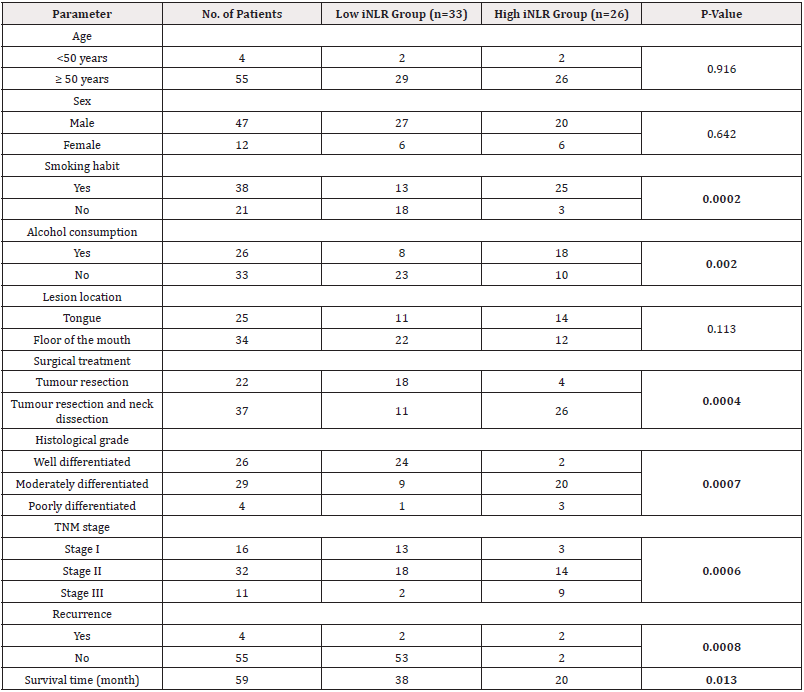
Table 2: Evaluation of clinical and microscopic features and neutrophil-lymphocyte ratio in the cancer nest (iNLR) in 59 patients with oral squamous cell carcinoma.
Note*: Bold P-values. iNLR=neutrophil-to-lymphocyte ratio in the cancer nest, TNM=tumor-node-metastasis.
Postoperative survival analysis revealed that the median survival time was 43 months for patients with low iNLR and 19.5 months for those with high iNLR, although the difference in overall survival did not reach statistical significance (P=0.09). In contrast, patients with low peripheral blood NLR had a median postoperative survival of 38 months compared to 20 months in patients with high NLR, and this difference was statistically significant (P=0.013) (Figures 1,2 and 3).
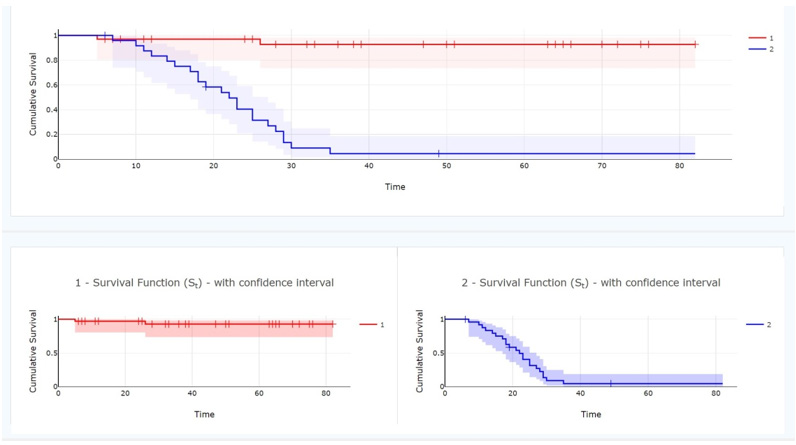
Figure 1: The relationship between survival times of patients with Oral Squamous Cell Carcinoma and neutrophil-to-lymphocyte ratio in peripheral blood (NLR). number 1 (red line): high NLR, number 2 (blue line): low NLR.
Discussion
The findings of this study underscore the complexity of oral squamous cell carcinoma (OSCC) and highlight the critical need for personalized treatment strategies that account for both tumor biology and patient specific factors. Our results demonstrate that the tumor microenvironment (TME) plays a pivotal role in OSCC progression, particularly through the dynamic interactions of tumor- associated neutrophils (TANs) and other immune cell populations. These interactions, mediated by a plethora of cytokines, contribute to the dual roles of neutrophils in either promoting or suppressing tumor growth, depending on the context. This duality aligns with previous studies that have identified neutrophils as key mediators of immune suppression and angiogenesis, while also recognizing their potential to exert anti-tumor effects under certain conditions [10-13]. One of the most significant challenges in OSCC management is the lack of reliable prognostic markers that can guide treatment decisions. Current standard therapies, including surgery, radiotherapy, and chemotherapy, often fail to account for the heterogeneity of OSCC and the unique clinical characteristics of individual patients. This can lead to overtreatment, which not only increases the physical and psychological burden on patients but also escalates healthcare costs [10,11]. Our study identifies several potential biomarkers within the TME that could serve as independent prognostic factors. These biomarkers may enable clinicians to stratify patients into distinct risk groups, thereby optimizing treatment plans and follow-up strategies. For instance, the presence of specific cytokine profiles or neutrophil subpopulations could be used to predict treatment response and disease progression. The role of neutrophils in the TME is particularly intriguing. While some studies have shown that neutrophils can promote tumor growth by enhancing angiogenesis and suppressing T cell-mediated immunity, others have demonstrated their ability to directly induce cancer cell death [14,15].
This apparent contradiction may be explained by the plasticity of neutrophils and their ability to adopt different phenotypes depending on the local cytokine milieu. Our findings suggest that targeting neutrophil recruitment or modulating their functional state within the TME could represent a novel therapeutic strategy for OSCC. However, further research is needed to fully elucidate the mechanisms underlying neutrophil-tumor interactions and to identify specific molecular targets for intervention. Granulocytes measures in the blood reflect numbers of matrix metalloproteinases (MMPs), whose collagenolytic activity degrades the extracellular matrix facilitating tumor invasion and dissemination. Neutrophils also release growth factor (VEGF) that promotes angiogenesis and provides new blood vessels required to maintain tumor development and survival [16]. Most of the current studies have focused on observing the correlation between NLR and iNLR and tumor prognosis. The mechanisms underlying the association between high NLR and poor outcomes in cancer patients are poorly understood. Studies have shown that tumoral immune microenvironment and tumor prognosis are correlated. Tumoral immune microenvironment is a complex network of immune cell composition, cell functions, cytokines, and chemokines. In a steady state, normal tissue may contain few immune cells, whereas in a tumor state, immune cells may be recruited from peripheral blood to tumor tissue. During carcinogenesis, immune homeostasis is disrupted, resulting in a decrease in the number and function of immunocytes [7]. The aggressive behavior of solid tumors is, in part, attributable to their capacity to evade host immune surveillance. A key mechanism underlying this immune evasion involves the expression of programmed cell death-ligand 1 (PD-L1) on tumor cells, which interacts with its receptor, programmed cell death-1 (PD-1), present on tumor-specific T cells. This interaction serves to suppress the antitumor immune response by limiting T cell activation and effector functions.
Emerging therapeutic agents that target the PD-1/PD-L1 axis are designed to block this inhibitory interaction, thereby facilitating the restoration of T cell proliferation and enhancing their antitumor activity. The development of PD-1 and PD-L1 inhibitors has notably transformed the clinical management of patients with Oral Squamous Cell Carcinoma (OSCC), offering promising therapeutic avenues in the treatment of this malignancy [17]. Studies have shown that the NLR is important for different forms of cancer [18-20]. In OSCC, Evidences suggested that in higher NLR level is associated with poorer prognosis of patients and decrease the quality of tumor related to later disease stages. Studies by Perisanidis, et al., [21] and Mascarella, et al., [22] observed a relationship between the NLR levels, and worse overall survival rates as well as nonrecurrence-free cancer specific mortality of OSCC patients. While not a truly novel assessment (the combination of NLR + iNLR has been assessed before), to our knowledge, we are the first group that endeavoured this comprehensive parameter in OSCC patients.
Our univariate analysis indicated that both NLR and TNM staging were independent prognostic factors for postoperative survival; this result is consistent with previously reported results [22]. Multivariate analysis showed that smoking status, surgical technique, TNM stage, pathological tumor differentiation and NLR/iNLR scores were significant independent predictors of postoperative survival (p<0.05). The data support the need to incorporate NLR and iNLR assessments into clinical practice for more refined patient stratification on risk categories to inform management approaches. Nevertheless, due to its retrospective nature, single-center design, and limited patient population, our study has certain limitations. Furthermore, because to the limits of our investigation, it is not possible to make firm conclusions despite the substantial relationships we identified between NLR/iNLR and numerous clinical and histopathologic characteristics. Larger cohort prospective investigations are necessary to validate our results and clarify the molecular processes underlying the correlation between neutrophil counts and OSCC outcomes.
Conclusion
Our research highlights the significance of NLR and iNLR as prognostic factors in OSCC. It might be able to identify high risk patients who might benefit from closer postoperative follow-up and shift away from more aggressive therapied by inplementign these signals into clinical practice. Future studies should concentrate on creating tailored treatments that alter the cancer-related tumor promoting roles of neutrophils and investigating the molecular pathways linking neutrophils to tumors.
Appendices
Acknowledgement
The study was presented as an oral presentation at the 8th International Congress of Medical Sciences and Multidisciplinary Approaches. Additional Information.
Disclosures
Human Subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study.
Canakkale Onsekiz Mart Universitesi Klinik Araştırmalar Kurulu issued approval 2023/16-21.
Animal Subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
Conflicts of Interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial Relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other Relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Conflicts of interest
Authors declare no conflicts of interest.
Funding
Authors received no specific funding for this work.
References
- Bagan J, Sarrion G, Jimenez Y (2010) Oral cancer: clinical features: Oral Oncol 46(6): 414-417.
- Pandya JA, Natarajan S (2019) A correlation of immunohistochemical expression of TP53 and severity of inflammation with varying grades of oral squamous cell carcinoma. J Cancer Res Ther 15(3): 564-570.
- Juan P Rodrigo, Mario Sánchez Canteli, Asterios Triantafyllou, Remco de Bree, Antti A Mäkitie, et al. (2023) Neutrophil to Lymphocyte Ratio in Oropharyngeal Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Cancers 15(3): 802.
- Yao Te Tsai, Chia Hsuan Lai, Geng He Chang, Cheng Ming Hsu, Ming Shao Tsai, et al. (2023) A Nomogram Incorporating Neutrophil-to-Lymphocyte Ratio and Squamous Cell Carcinoma Antigen Predicts the Prognosis of Oral Cancers. Cancers (Basel) 15(9): 2492.
- Sokratis Trellakis, Kirsten Bruderek, Claudia A Dumitru, Hossein Gholaman, Xiang Gu, et al. (2011) Polymorphonusclear granulocytes in human head and neck cancer: enhanced inflammatory activity, modulation by cancer cells and expansion in advanced disease. Int J Cancer 129(9): 2183-2193.
- Baixia Zhang, Wei Du, Kang Gan, Qigen Fang, Xu Zhang (2019) Significance of the neutrophil-to-lymphocyte ratio in young patients with oral squamous cell carcinoma. Cancer Manag Res 11: 7597-7603.
- Zixuan Hu, Jiaying Zhou, Yupeng Li, Yizhao Luan, Huan Li, et al. (2022) Peripheral immune signature resembles tumor microenvironment and predicts clinical outcomes in head and neck squamous cell carcinoma. Front Immunol 13: 915207.
- Ning Wang, Yuanyong Feng, Qingjie Wang, Shaohua Liu, Lei Xiang, et al. (2014) Neutrophils infiltration in the tongue squamous cell carcinoma and its correlation with CEACAM1 expression on tumor cells. PLoS One 9(2): 89991.
- Donskov F (2013) Immunomonitoring and prognostic relevance of neutrophils in clinical trials. Semin Cancer Biol 23(3): 200-207.
- Pabla B, Bissonnette M (2019) Neutrophils in colorectal cancer: A love-hate affair. World Journal 25: 2736 2755.
- Luai R Zarour, Sudarshan Anand, Kevin G Billingsley, William H Bisson, Andrea Cercek, et al. (2019) Colorectal cancer liver metastasis: Evolving paradigms and future directions. Cell Mol Gastroenterol Hepatol 3(2): 163-173.
- Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420: 860-867.
- Diakos CI, Charles KA, McMillan DC (2014) Cancer-related inflammation and treatment effectiveness. Lancet Oncol 15(11): 493-503.
- Wu L, Saxena S, Singh RK (2020) Neutrophils in the tumor microenvironment. Adv Exp Med Biol 1224: 1-20.
- Granot Z, Jablonska J (2015) Distinct functions of neutrophil in cancer and its regulation. Mediators Inflamm 2015: 701067.
- Samantha Solito, Ilaria Marigo, Laura Pinton, Vera Damuzzo, Susanna Mandruzzato, et al. (2014) Myeloid-derived suppressor cell heterogeneity in human cancers. Ann N Y Acad Sci 1319: 47-65.
- Jaimin J Patel, Dylan A Levy, Shaun A Nguyen, Hannah M Knochelmann, Terry A Day (2020) Impact of PD-L1 expression and human papillomavirus status in anti PD1/PDL1 immunotherapy for head and neck squamous cell carcinoma-Systematic review and meta-analysis. Head Neck 42(4): 774-786.
- Gandhi L, Zubrod C (2006) Targeting angiogenesis in cancer: the burgeoning role of bevacizumab. Nature Clinical Practice Oncology 3: 24-25.
- Zhang H, Liu H, Shen Z, et al. (2016) Tumor-infiltrating neutrophils are correlated with unfavorable prognosis in breast cancer. Tumor Biology 37: 3615-3625.
- Motz GT, Coukos G (2013) Deciphering and reversing tumor immune suppression. Immunity 39(1): 61-73.
- Perisanidis C, Kornek G, Pöschl PW, et al. (2014) High neutrophil-to-lymphocyte ratio is an independent marker of poor prognosis in patients with oral squamous cell carcinoma. British Journal of Cancer 110: 433.
- Mascarella MA, Mannard E, Silva SD, et al. (2018) Neutrophil-to-lymphocyte ratio in head and neck cancer prognosis: A systematic review and meta-analysis. Head & Neck 40(5): 1091-1100.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.