Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
West Nile Virus Infections Unmasked from Suspected Malaria Febrile Patients in Three Northeastern States, Nigeria
*Corresponding author: Marycelin Baba, 1Department of Medical Laboratory Science, University of Maiduguri, College of Medical Sciences, University of Maiduguri, P.M.B. 1069, Maiduguri, Nigeria.
Received: December 10, 2024; Published: January 16, 2025
DOI: 10.34297/AJBSR.2025.25.003327
Abstract
The atypical symptoms of malaria and West Nile virus (WNV) infections at the initial phase could leverage their misdiagnosis. This study randomly recruited 600 malaria-suspected febrile patients who visited selected health institutions in Adamawa, Bauchi, and Borno states for malaria tests. These patients were tested for IgM by ELISA and plaque reduction neutralization (PRNT90). About 46.7% and 16.5% had WNV IgM and neutralizing antibody (nAb) respectively. Only 6.3% of the patients with IgM and 17.2% of the IgM negatives had WNV nAb indicating acute and past infections respectively. WNV nAb in Adamawa and Bauchi states was significant different from that of Borno while the residents of Bauchi were more likely to have experienced more WNV infections than the other states. No significant difference was observed between WNV nAb and age, gender , settlement and occupation of the patients. However, patients ≥ 60 years old and rural dwellers were more likely to have experienced WNV infections than others. Serum samples collected 7-10 days after onset of symptoms are more likely to yield nAb than 1-7days. Similarly, WNV nAb and patients who received antibiotics/antimalaria treatments and Yellow Fever vaccines before sample collection were significantly different. About 96.3% of WNV nAb also neutralized one or more related flaviviruses at varying degrees: dengue virus (DENV) (95%), yellow fever virus (YFV) (12.3%) and Zika virus (11.10%), indicating co-infections. Also, 59.3% of the patients tested were co-infected with chikungunya virus while only
The persistent misdiagnosis of WNV infections poses a global public health threat to both humans and animals in the phase of climate change if unchecked and underscores the need for differential diagnosis of febrile illness for Flaviviruses in Nigeria.
Keywords: West Nile virus, ELISA IgM, Neutralizing antibody, PRNT90, Flaviviruses, Febrile illness, Malaria, co-infections, Monotypic infections, Nigeria
Abbreviations: WHO: World Health Organization; FMC: Federal Medical Center; ATBUTH : Abubakar Tafawa Balewa University of Maiduguri Teaching Hospital; Bauchi; SSH: Specialist State Hospital; UMTH: University of Maiduguri Teaching Hospital; nAb: Neutralizing antibody; PRNT: Plaque reduction neutralizing antibody test; IgM: Immunoglobulin M antibody; IgG: Immunoglobulin G antibody; ELISA: Enzyme-Linked Immunosorbent assay; EMEM: Earle’s Minimum Essential Medium; IHD: In-house Dilution; ICGEB: International Center for Genetic Engineering and Biotechnology; DENV: Dengue virus; YFV: Yellow Fever Virus; WNV: West Nile virus; CHKV: Chikungunya virus; WNVF: West Nile virus fever; WNVE: West Nile virus encephalitis; IDP: Internally Displaced persons; PBS: Phosphate buffered saline; OD: Optical density; OR: Odd ratio.
Introduction
West Nile Virus (WNV), a mosquito-borne virus was first isolated from a febrile illness case in Uganda in 1937 [1]. Thereafter, the virus caused infrequent outbreaks from 1950s through the 1980s in Israel, Egypt, India, France, and South Africa [2-4]. Outbreaks of WNV infections increased in frequency, severity, and geographical spread from the mid-1990s. In 1996, outbreaks of WNV meningitis and encephalitis involving adults primarily hit Bucharest and Romania but in Volograd and Russia in 1999 and Israel in 2000 [5]. It crossed the Atlantic and invaded the Western hemisphere causing encephalitis in New York City and within three years, the virus spread to most contiguous United States of America and neighboring countries of Canada and Mexico [6].The circulation of WNV in northern and sub-Saharan Africa throughout the late 20th and early 21st century led to outbreaks in Algeria, Morocco, Tunisia, the Democratic Republic of the Congo, and South Africa, along with sporadic cases and seropositivity in humans and/or horses distributed throughout the continent [7-9]. In 2010 and 2012, WNV outbreaks occurred in Morocco and Tunisia respectively and persistent sporadic transmissions were reported in Egypt and Algeria [7,10-12].
WNV belongs to the genus: Flavivirus, family: Flaviviridae, and member of the Japanese Encephalitis virus serocomplex [13,14]. It expands its range to new geographic areas mainly through migratory birds [15], globalization, land use, and travel [16]. WNV is maintained in a zoonotic transmission cycle between birds and insect vectors (mosquitoes -Culex and Aedes species). Some species of ticks (Ornithodoros moubata and Ixodes ricinus) may act as reservoirs of WNV [17] but not yet established vectors. Many other vertebrates (reptiles, amphibians, and mammals) are also susceptible but humans and horses are dead-end hosts [18] because they are unable to transmit the virus to mosquitoes after being infected [15]. However, transmission through contact with infected animals/birds, their blood, or other tissues, organ transplantation, blood transfusions, breast milk, and transplacental routes have been reported [1].
Whilst 80% of WNV infections are asymptomatic, 20-30%% are mild infections characterized by non-specific Flu-like or malaria- like symptoms classified as West Nile virus fever (WNVF) [19,20]. Less than 1% patients with WNVF could develop neuroinvasive disease characterized by meningitis (WNVM) and/or encephalitis( WNVE) [19,21]. The first WNV-associated neuroinvasive disease was reported among the elderly in Israel in 1957 [4,22] and subsequently in adults and pediatrics [2,3]. Overall, the most common neurological manifestations in WNV infections include menin gitis, encephalitis and acute flaccid paralysis [23]. According to the latest WHO data published in 2020, encephalitis deaths in Nigeria has reached 937 or 0,06% of the total deaths [24]. However, due to lack of surveillance in Nigeria, the role of WNV in neurological diseases is not yet known but few studies have reported the circulation of WNV from febrile illness cases in the country [25,26]. Although WNV and malaria are transmitted by different species of mosquitoes, the initial clinical phase of WNV infections mimics malaria resulting in the misdiagnosis of both pathogens [27]. This situation is further worsened by the lack of access to appropriate diagnostic facilities for differential diagnosis of febrile illness for arboviruses and co-infections. In Sub-Saharan Africa, Nigeria is one of the 32 countries that account for almost 93% of malaria deaths globally [28]. The availability of externally funded intervention programs against malaria in Nigeria leverages malaria diagnostics and therapeutics in the three tiers of healthcare facilities in the country as opposed to viral infections. Consequently, the virus circulates uninterrupted in the country with the possibility of facilitating the spillover events that may result in global outbreaks in both humans and animals. Thus, WNV infections are often underreported, underrecognized, and underestimated. Notably, seroprevalence data are useful for the estimation of the parameters of WNV epidemic models. These estimates may facilitate the prediction of the burden of the disease in the population and control intervention strategies. This study investigated WNV infections among malaria-suspected patients in Nigeria, highlighted possible risk factors and estimated its burden considering its association with devastating neurological disorders in humans and animals.
Materials and Methods
Study Area
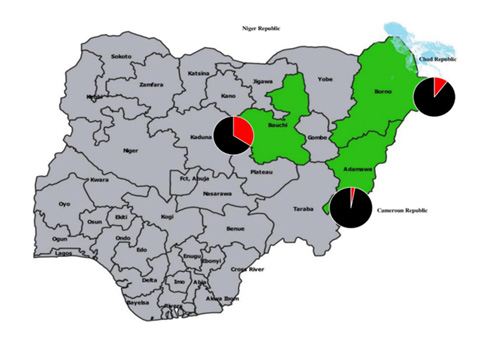
The simple random sampling technique was used to select three health institutions located in north eastern Nigeria: Federal Medical Centre, Adamawa state, Abubakar Tafawa Balewa University Teaching Hospital, Bauchi State and Specialist Hospital, Borno State (Figure 1). Adamawa state has an annual temperature of 30.8oC and spans between sub-Sudan vegetation in the north and Guinea savannah in the north [29] (Data. Info 2022). Bauchi is a Sudan savanna with annual temperatures of 28.97oC [30] (Weather Spark, 2022). Borno is a Sahel savanna and one of the warmest regions in Nigeria with annual temperature of 32.53oC 1o and shares borders with Cameron to the East, Niger to the north and Chad to the northeast [31] (World Data, 2022).
Study Population
A total of 600 patients with febrile illness who visited the selected hospitals to request malaria tests were recruited. The approved consent form was randomly given to 250 participants from each of the three states. Once minimum of 200 of the participants in each state endorsed the forms, we recruited them for the study. The participants also include parents of children below five years who endorsed the forms on behalf of their children. Socio-demographic characteristics and other parameters were collected using a structured questionnaire. The study was conducted between April and August 2018.
Research Ethics
The designed consent form was attached to the proposal submitted for ethical approval at each selected hospital. The research ethical clearance approved letters were obtained from the Research and Ethics Committee of ATBUTH (ref ATBUTH/ADM/42/Vol.1, dated 25 May 2017), State Specialist Hospital (SSH), Maiduguri, Borno State (ref no. SSH/GEN/641/Vol.1 dated 14 February 2018) and Federal Medical Centre, Yola (ref no FMC/YO/001/Vol.1 dated May 2018).
Sample Collection
About 3 ml of each patient’s whole blood was collected into a uniquely labeled serum separator vacutainer, test tube allowed to clot and centrifuged at 3000 rpm for 5 min. The serum was thereafter aspirated into the sterile cryovials and stored at -20oC until transported to the virology laboratory, University of Maiduguri Teaching Hospital (UMTH).
Qualitative WNV IgM ELISA
Sera samples were analyzed for human WNV IgM antibody according to the manufacturer’s protocol (MyBioSource, San Diego, CA, USA). Briefly, 50μl of undiluted serum (positive, negative controls and patients’ sera) was treated with 100μl of HRP-conjugate and incubated for 1 h at 37oC. After appropriate washing, 50μl of chromogen A and B were added to each well and incubated for 15 min at 37oC before the reaction was stopped. The plate was read at optical density (OD) of 450 nm within five minutes after the reaction was stopped. With the manufacturer’s instruction, the cut-off OD units ≥ 200 were considered positives.
Supply of Virus Stocks
The following virus stocks were obtained from the molecular Virology Unit of International Centre for Genetic Engineering and Biotechnology (ICGEB), Trieste, Italy. They include West Nile virus, yellow fever virus, dengue viruses (serotypes 1-4), chikungunya virus and zika virus.
Plaque Reduction Neutralization Test (PRNT)
The PRNT was performed as previously described for the dengue virus [32]. Each serum sample was inactivated at 56°C for 30 minutes and stored at -80oC. Vero E6 cells were grown in Eagle’s Minimum Essential Medium (EMEM) supplemented with 10% foetal bovine serum, 2mM glutamine, 2% Penicillin/Streptomycin and 1% HEPES and 2.5% sodium bicarbonate. The cells were seeded in 24-well plates at a density of 1 x 105 cells/ well and incubated at 37°C for 24-48 hours or until it reached 70-80% confluence. Each serum sample was diluted 1:8 with in-house diluent (IHD) prepared in PBS containing Penicillin/Streptomycin (PS), Gentamycin and Fungizone supplements. PS preparation involved dissolving 1gm Penincillin G (1X 106 units) and 1 gm Streptomycin sulphate (10μg/ml) in 100 ml of PBS to obtain the stock PS. Then 10ml of the stock PS was added to 35 ml of PBS. 500μl of Gentamycin (80mg/2ml) diluted 1/100 and 0.02% of Fungizone (2.5μg/ml) were added to the 35 ml of PBS to make the IHD. About 100μl WNV virus stock (obtained from Molecular Virology Laboratory, International Centre for Genetic Engineering and Biotechnology- ICGEB, Trieste, Italy) at a concentration of 100 PFU/ml was added to 100μl of the diluted serum and incubated at 37°C for 1 hour. Each serum dilution-virus mixture was prepared in duplicate and three controls including a virus dose control (100 PFU virus + cell + diluent), a cell control (diluent + cell) and positve serum control were tested simultaneously. Each serum was tested against WNV, CHIKV, Zika virus (ZIKV), dengue virus (DENV) and yellow fever virus (YFV). After incubation at 37°C for 1 hour, the 50μl virus-serum mixture was added to Vero cells and incubated for 1 hour at 37°C. The plates were rocked gently every 15 minutes for uniform distribution of the inoculum. Different concentrations of Carboxyl methylcellulose (CMC) salt (Low viscosity) (Sigma) was tested to determine the best consistency. The CMC consisted of an equal volume of 10%, 12% and 14% CMC with EMEM supplemented with 4% fetal bovine serum (FBS) (resultant concentration of the FBS became 2% while that of CMC was 5%, 6% and 7% respectively). The best consistency was 6%. 1 ml of the 6% CMC was added to each of the 24 wells and incubated at 37°C for 3-7 days. After removing the CMC, 500μl of formaldehyde solution (37.0-40.0% ) diluted in 1:10 with PBS was added to each well for 30 minutes and stained with 500 ul of 0.5% crystal violet (Sigma) for 20-30 minutes after removing the formaldehyde. The percentage of plaque reduction by the specific antibody was calculated using the formula: 100-(Number of plaques in sample/Number of plaques in control) x 100. PRNT was repeated for each positive serum diluted 1:8- 1:1024 against five different viruses (dengue viruses (DEN), West Nile virus (WNV), chikungunya virus (CHICK), zika virus (ZIKV), yellow fever virus (YFV)). Only sera with a neutralizing titer of 4-fold increase for one virus compared with those of the others were considered positive. However, two or more viruses having either equal titres or greater but not more than 4- fold difference were considered co-infections. The validity of the test was determined by the virus control (contains no serum) having a minimum of 50 plaques and cell control (no virus and no serum) with no plaque and positive serum having five or no plaques at all. The PRNT titer was defined as the reciprocal of the serum dilution that reduced the number of plaques by 90-100 % (PRNT90) [32,33].
Statistical Analysis
The data was analysed using Statistical Product and Service Solutions (SPSS), Version 25.0, from IBM SPSS. The Pearson χ2 or Fisher’s exact test for categorical variables were used when the expected count was less than 5. Binary Logistic Regression in SPSS was used to obtain odds ratio and confidence intervals for the association between WNV nAb (PRNT90 results) and other variables in the three states. The fitness of the model to correctly predict the nAb (positive and negative) by the independent variables was tested and found significant using the Omnibus test of model coefficient and insignificant with the Hosmer and Lemeshow tests. Overall, the percentage accuracy in the classification was 86.5% with 100% specificity (true negative) but 0% sensitivity (True positive). Independent variables that were not disclosed by the participants were not included in the inferential statistics. Confidence interval for our estimates was determined at 95% with 5% level of significance.
Statistical Analysis
The data was analysed using Statistical Product and Service Solutions (SPSS), Version 25.0, from IBM SPSS. The Pearson χ2 or Fisher’s exact test for categorical variables were used when the expected count was less than 5. Binary Logistic Regression in SPSS was used to obtain odds ratio and confidence intervals for the association between WNV nAb (PRNT90 results) and other variables in the three states. The fitness of the model to correctly predict the nAb (positive and negative) by the independent variables was tested and found significant using the Omnibus test of model coefficient and insignificant with the Hosmer and Lemeshow tests. Overall, the percentage accuracy in the classification was 86.5% with 100% specificity (true negative) but 0% sensitivity (True positive). Independent variables that were not disclosed by the participants were not included in the inferential statistics. Confidence interval for our estimates was determined at 95% with 5% level of significance.
Results
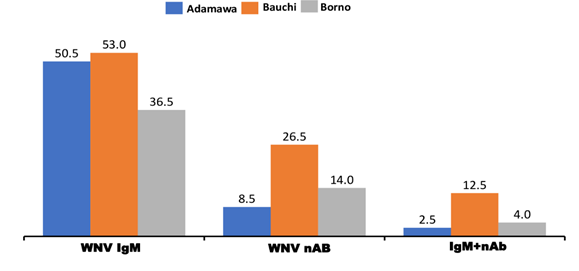
Of 600 patients suspected of malaria in the three northeastern states in Nigeria, 280 (46.7%), 99(16.5% ), 38 (6.3%%) had WNV IgM , nAb and IgM + nAb respectively. Overall, only 38 (13.5%) of 280 IgM positives and 61 (19.1%) of 320 IgM negatives had WNV nAb.
The Distribution of WNV Antibodies in Three Northeastern States of Nigeria
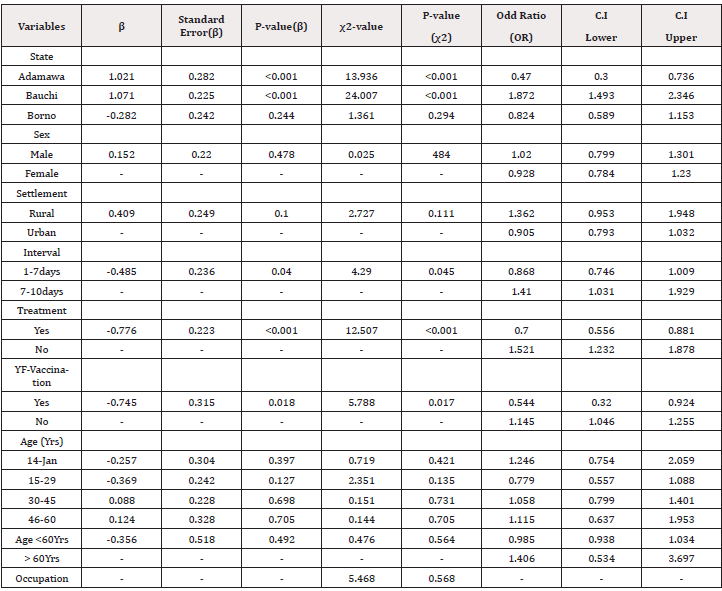
In Adamawa state, 101(50.5%) of 200 patients had IgM, 17 (8.5%) nAb and 5 (2.5%) IgM + nAb. Of 200 patients in Bauchi, 106 (53.0%) and 54 (27.0%) had IgM and nAb respectively while 25 (12.5%) had IgM + nAb. In Borno, 73 (36.5%) were IgM, 28 (14.0%) nAb and 8 (4.0%) IgM+ nAb (Figure 2). Of 99 sera with nAb, the titres obtained were 1:8 (2.0%). 1:16 (2.0%), 1:32 (13.1%), I:64 (33.3%), 1:128 (22.2%) and 1:320 (1.0%%) (Supplementary file). WNV nAb in Adamawa, Bauchi and Borno were significantly different (χ2= 24.007, df=4, p = .0.00) (Table 2). The residents of Bauchi (OR=1.872, Cl=1.493-2.346) were more likely to have experienced the WNV infections than Adamawa (OR=0.470, Cl=.300-0.736) and Borno states (OR=0.824, Cl=0.589-1.153).
Age Distribution of WNV Antibody among Febrile Patients in Northeastern, Nigeria
The prevalence of WNV IgM was obtained from children aged 1-14 years (53.1%). 15-29 years (49.1%), 30-45 years (42.1%), 46-60 year (50.0%) and 61-76 years (40.0%). Patients aged 77- 92 years had no detectable IgM. Among children aged 1-14 years, 19.8%, 13.3% of 15-29 years, 17.3% of 30-45 years, 18.1% of 46- 60 years and 20.0% at 77-92 years had WNV nAb (Table 1). Patients with the ages of < 60 years was not significantly associated (χ2=4.06, = df5, p=0.509) with WNV infections. However, patients aged >60 years (OR=1.406, Cl= 0.534-3.697) were more likely to have had WNV infections than other age groups studied (Table 2).
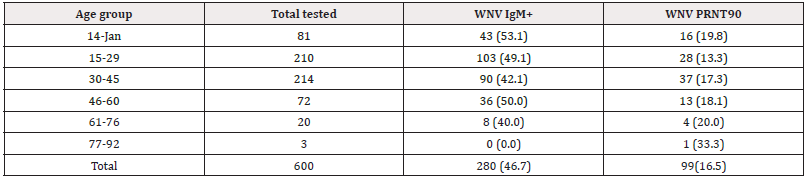
Table 1: Age distribution of West Nile virus antibodies among febrile patients in three northeastern states, Nigeria.
Gender Distribution of WNV Antibodies
Of 284 males, 137 (46.7%) and 50 (17.6%) had WNV IgM and nAb respectively. Among 316 females, 143 (45.3%) were IgM and 49 (15.6%) had nAb. Gender and WNV nAb were not significantly associated ((χ2= 0.478, df=1, p=0,501.
Distribution of WNV Antibodies According to the Occupation of the Patients
Students, housewives, civil servants, self- employed, pupils, retirees had WNV antibodies at varied rates (Supplementary file). Of 158 students, 83 (52.5%) were IgM while 21 (13.3%) had nAb. Among 102 house wives, 52(51.0%) and 16(16.6%) were IgM and nAb respectively. Of 112 Civil servants, 50(44.6%) were IgM, while 23 (20.5% had nAb. Of 37 self- employed, 17 (46.0%) were IgM and 6 (16.2%) were nAb. Of 31 under-care/pupils, 18 (58.1%) were IgM and 8(25.8%) had nAb. 2 (40.0%) of 5 public servants studied had WNV IgM but no nAb. Among 6 retirees, 2 (33.3%) were IgM and 1(16.7%) had nAb. Of 149 not disclosed cases, 56 (37.5%) and 23 (15.4%) had IgM and nAb respectively (Table 3). The occupation of the patients and WNV antibodies were not significantly associated (χ2=5.468, df=7, p = 0.568) (Table 2).
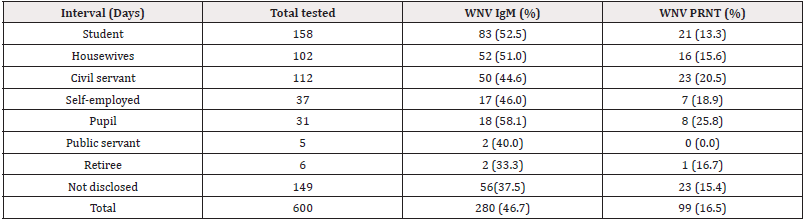
Table 3: Distribution of West Nile virus antibodies among different occupation groups in northeastern states, Nigeria.
Distribution of WNV Infection Rates According to the Interval Within which Samples were Collected after the Onset of Symptoms
Of 439 samples collected 1-7 days after onset of symptoms, 198(45.1%) and 65(13.9%) had WNV IgM and nAb respectively. Among 151 samples collected within 7-10 days, 77 (51.0%) were IgM while 34 (21.2%) had nAb. Only 10 patients with no known intervals had 5 (50.0%) IgM and 6 (60.0%) nAb (Table 4). Intervals were significantly associated (χ2=4.29 df=1, p = 0.045) (Table 2). However, serum samples collected 7-10 days (OR=1.410, Cl= 1.031- 1.929). after onset of symptoms were more likely to have yielded more nAb than 1-7days.
Distribution of WNV Infections among Rural and Urban Dwellers
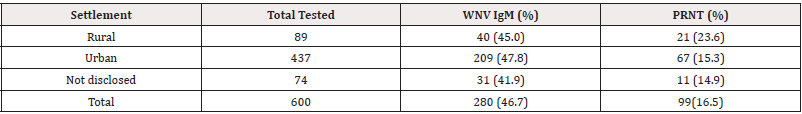
437 (72%) and 89(14.8%) of patients were urban and rural dwellers respectively. Among the 89 rural dwellers, 40 (45.0%) were IgM while 21 (23.6%) had nAb. Of 437 urban dwellers, 209(47.8%) and 67 (15.3%) had WNV IgM and nAb respectively (Table 5). Of 74 not disclosed settlements, 31 (41.9%) were IgM, while 11 (14.9%) had nAb. The type of settlement and WNVV nAb were not significantly associated (χ2=2.727, df=1, p=0.111) but the rural dwellers (OR=1.362, Cl=0.965-1.948) were more likely to have had WNV infections than the urban (Table 2).
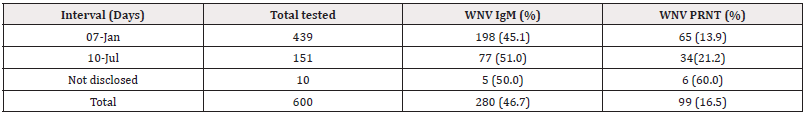
Table 5: The distribution of West Nile virus antibodies at different intervals between onset of symptoms and sample collection.
Distribution of WNV Infections among Patients who Received Antimalaria/Antibiotic Treatment before Performing Laboratory Tests for Malaria Parasites
327 (54.5%) patients received antimalaria/antibiotics treatments before seeking for a laboratory test for malaria while 262 (43.7%) did not. Of 327 treated patients, 151(46.2%), 37(11.3%) were WNV IgM and nAb respectively. Among 262 untreated patients, 123 (47.0%) were WNV IgM while 54(20.6%) had nAb. Of 11 patients with no disclosed treatment status, 6 (54.4%) had IgM while 8 (72.2%) were nAb, (Figure 3). WNV nAb was significantly associated with the antimalaria/antibiotics treatments (χ2= 12.507, df=1 p=0.001) (Table 2). Also, those who did not received these treatments were more likely to have experienced WNV infections than those treated.
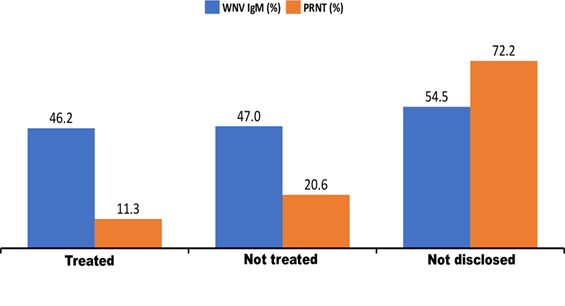
Figure 3: The distribution of West Nile virus antibodies among patients who received treatment with antibiotics/antimalaria.
Yellow Fever Vaccination Status of Patients and the Distribution of WNV Antibody
In the three states, only 134 (22.3%) patients received YF vaccination as against the majority 466 (77.7%) who did not. The YF vaccination status in Adamawa state was higher at 107 (53.5%) than in Bauchi with12 (6.0%.) and Borno having 15(7.5%) (Supplementary file). Among the vaccinated, 58 (43.3%) had WNV IgM while 13(9.7%) had nAb. Of 466 unvaccinated patients, 222 (47.6%) were IgM while 86 (18.5%) had nAb (Figure 4). WNV infections were significantly associated with the YF vaccination status of the patients studied [χ2= 5.788, df=1 p=0.017] (Table 2).
Co-infections/Cross reactions between WNV and other Flaviviruses/ Alphaviruses
Of 99 samples with WNV nAb, 95.0%, 12.3%, 11.10% also neutralized 90-100% infectivity of DENV, YFV and ZIKV respectively. Co-infections between WNV and CHIKV were observed among 59.3% of the patients.
Discussion
The results of this study have demonstrated the misdiagnosis of WNV infections for malaria in Nigeria and underscores the screening of malaria-suspected cases for arboviruses. The ELISA test which is sensitive and commonly used for the diagnosis of Flaviviruses is confounded by cross-reactivity among its members, compromising species specificity [34]. We used PRNT90 to confirm ELISA IgM results because it is considered the gold standard and its ability to neutralize the infectivity of a particular virus is considered a mark of protection against reinfection [35,36]. Also, PRNT measures the quantity of the antibody in the sample as well as the effectiveness of the antibody on the virus [33]. Although, cross‐reaction with other flaviviruses still occurs with PRNT especially with low endpoint titers but the use of a higher stringent endpoint titer of 90% provides greater species specificity [33,37].
In this study, 46.7% and 16.5% of the patients had WNV IgM and nAb respectively in six northeastern states in Nigeria (Figure 2). Previously, the pooled seroprevalence rates of 7.1% WNV IgM and 14.3% nAb were reported [16]. The difference in seroprevalence in both studies could be attributed to the study periods, states covered and types of the seroprevalence rates (pooled /unpooled). In this study, only 13.6%% of the IgM positives and 19.0% of the IgM negatives had WNV nAb indicating acute and past infections respectively. (Supplementary Table).
WNV infections were significantly higher in Bauchi (27.0%) than Borno (14.0%) but least in Adamawa State (8.5%) (Table 2) in consistence with previous reports [27,38]. The differences in seroprevalence rates in the three states (Figure 2) could be attributed to diverse vegetations and climatic conditions: Adamawa spans between Sub‐Sudan vegetation marked by short grasses interspersed with short trees in the north and Guinea savannah in the North and annual temperature of 30.8°C while Bauchi and Borno have Sudan and Sahel savannah vegetations with annual temperatures of 28.97°C and 32.53°C respectively. Our finding is supported by the reports that vegetation and climatic conditions impart on the competence of the vector and transmissibility of the arboviruses [25,27,39]. Other variables such as genetic differences in the vectors and the human hosts in Bauchi may also be contributory. Additionally, other common practices at varied degree in the three states that could allow interaction of vectors with human population include population movement, internally displaced persons (IDP) camps (due to persistent terrorist attacks), storage of water in houses, dumping of solid waste in the open gutter and close to water bodies, improper waste disposal and poor sanitation differ. Furthermore, the rate of intra- state travel in the 37 states in Nigeria on daily bases for socioeconomic activities also differ.
In Borno State, WNV infections increased from 1.2% in 2006 [25] to 25% in 2013 [27] and decreased to 14.0% in 2018 (when the samples for this study were collected). The difference could be attributed to improved environmental sanitation, waste disposal, and increased use of modern technology that impact improved life style over the years. In this study, a four-fold increase in titre from other related flaviviruses (DENV, YFV, ZIKV) as previously suggested by Gennaro, et al., [40] was demonstrated. Thus, high WNV nAb titres of 1:320 (1%), 1:128 (22%), 1:64 (33%) and 132(13%) obtained in this study have demonstrated the silent circulation of WNV under the guise of malaria in Nigeria. Notably, related Flaviviruses that exhibited either similar titres with WNV or greater but not more than four- fold difference was considered co-infections. In this study 96% patients with WNV nAb also neutralized infectivity of other related flaviviruses at varied degrees including one or more of dengue virus serotypes (95%), YFV (12.3%) and ZIKV (11.10%) indicating co-infections in consistent with previous reports [33,41,42] while only 3.7% which did not neutralize other viruses tested indicated monotypic WNV infections in agreement with previous studies [40,43]. Although cross-neutralizing antibodies may result in diagnostic misinterpretation [44], it can also ensure partial or complete cross-protection against other flavivirus infections [5,40,45-47]. Additionally, this study detected 59% co-infections between WNV and CHIKV in consistence with previous reports [41,42]. The silent circulation of WNV in this study corroborated a previous report that revealed sequences of 13 strains of WNV RNA associated with febrile illness in Borno State in 2018. These sequences aligned perfectly with WNV Italian isolate 358 (FJ472944 and Indian isolate Gwl-01 2015 (MG516600.1) od [26].
WNV infections and age, gender, and occupations of the patients were not significantly different. However, increased WNV infections with age have been reported in Gabon [38] where only IgG was detected in Nigeria with complement fixation test (CFT) tests as the technique of choice [48]. Notably, both IgG and CFT are not species specific compared with the PRNT. However, elderly (>60 years) in this study, were more likely to have experienced more WNV infections than the younger groups in agreement with World Health Organization [1], probably due to weakened immunity. Lack of significant association between WNV infections and gender was in consistence with previous studies [25,43]. We speculate that, the mosquito vector does not discriminate gender in its quest for a blood meal. The detection of WNV nAb was significantly associated with the interval between onset of symptoms and sample collection. Although, WNV nAb can be detected from samples collected 1-7 days after onset of symptoms, 7-10 days were more likely to yield more WNV nAb (Table 4). However, the use of a wider interval may give a clearer insight into the better diagnostic outcomes. Rural dwellers with WNV infections (Table 5) were significantly higher than the urban in consistence with a previous report [16,38]. Contributory factors may include poor waste disposal, and environment sanitation and intense agricultural practices in rural areas which favor sylvatic transmission in comparison with urban. Overall, urgent improvement on vector control measures, waste management and environmental sanitation including drainage system is necessary in the three states especially the rural areas.
The practice of treatment with antibiotics/anti-malaria before seeking medical attention is common in Nigeria because every febrile illness is presumed and immediately treated as malaria and or bacterial infections. WNV infections and these treatments (Figure 3) were significantly associated and the untreated patients were more likely to have experienced WNV infections than those treated. This observation corroborated a report that tetracycline and Minocycline inhibit WNV infections in vitro [16,49]. Further studies will give more insights into the effects of these drugs on WNV. Nevertheless, the irrational use of these drugs could contribute to the global antimicrobial resistance burden.
The low population immunity against YFV as evidenced by 77.7% patients who did not receive YF vaccine explains its periodic epidemics in the country (Figure 4). In this study, those who were not vaccinated against YF virus were more likely to have experienced WNV infections than those vaccinated (Table 2). In a previous study, Yellow Fever 17D vaccine strain was used as a backbone for the rapid development of live -attenuated candidate vaccine against WNV [50]. Another report revealed that, human vaccines against flaviviruses provided partial cross-protection against heterologous flaviviruses within the same serocomplex [44]. In this study, we observed that 9.7% of YF vaccine recipients also had WNV nAb indicating possible protection. Thus, we speculate that YF vaccine may serve as an alternative vaccine against WNV pending the successful clinical trials of the WNV vaccine.
Conclusion
ELISA IgM, and PRNT were used to unmask WNV infections from malaria suspected febrile patients in Adamawa, Bauchi and Borno states at differed prevalence rates. The species specificity of PRNT90 allowed the detection of highly suggestive WNV infections. Both acute and past infections were detected. Treatment with anti- malaria anti/antibiotics may inhibit WNV infection . Rural dwellers were more likely to have experienced WNV infections than urban. YF vaccine may cross protect against WNV. Intervals between onset of symptoms and sample collection should be considered for better results. WNV infections and age, gender, and occupation of the patients were not significantly associated.
Authors’ contributions: MMB conceptualized, designed the study and wrote the first version of the manuscript. MAH performed laboratory activities, KI designed the map indicating the sites of sample collection, EMC produced the virus stock and part of laboratory investigation, TC performed the laboratory investigation, AM coordinated conceptualized and edited the manuscript, YSJ conducted the statistical analysis and deduced epidemiological inferences while BSO supervised all the laboratory activities and designed the study’s analytical strategy.
Acknowledgments
We are indeed grateful to all the staff of the WHO National Polio laboratory, Maiduguri for their moral and technical assistance. We sincerely thank the Collaborative Research Program (CRP) arm of the International Centre for Genetic Engineering and Biotechnology (ICGEB) for making this work feasible and affordable. We thank the University of Maiduguri Teaching Hospital (UMTH) for hosting the Laboratory facility where the test was performed
Financial Support and Sponsorship
This work was supported by International Centre for Genetic Engineering and Biotechnology (ICGEB) -CRP: Contract No: CRP/16/010, Reference No. CRP/NGA 16-03.
Conflict of Interest
The authors have no conflicts of interest to declare for this study.
References
- (2017) World Health Organization. West Nile Virus . World Health Organization.
- Flatau E, Kohn D, Daher O, Varsano N (1981) West Nile fever encephalitis. Isr J Med Sci 17(11): 1057-1059.
- George S, Gourie Devi M, Rao JA, Prasad SR, Pavri KM, et al. (1984) Isolation of West Nile virus from the brains of children who had died of encephal: itis. Bull World Heal Organ 62(6): 879-882.
- Goldblum N, Sterk V V, Paderski B (1954) West Nile fever: The clinical features op tue disease and the isolation of west nile virus from the blood of nine human cases. he Am J Epidemiol 59(1): 89-103.
- Platonov AE, Shipulin GA, Shipulina OY, Tyutyunnik EN, Frolochkina TI, et al. (2001) Outbreak of West Nile virus infection, Volgograd Region, Russia, 1999. Emerg Infect Dis 7(1): 128-132.
- Chancey C, Grinev A, Volkova E, Rios M (2015) The global ecology and epidemiology of West Nile virus. Biomed Res Int :376230.
- (2013) European Centre for Disease Prevention and control. Historical data Historical data.
- Kading RC, Borland EM, Cranfield M, Powers AM (2013) Prevalence of antibodies to alphaviruses and flaviviruses in free-ranging game animals and nonhuman primates in the greater Congo basin. J Wildl Dis 49(3): 587-599.
- Morrill JC, Johnson BK, Hyams C, Okoth F, Tukei PM, et al. (1991) Serological evidence of arboviral infections among humans of coastal Kenya. Journal of Tropical Medicine and Hygiene. J Trop Med Hyg 94(3): 166-168.
- Gabriel M, Emmerich P, Frank C, Fiedler M, Rashidi Alavijeh J, et al. (2013) Increase in West Nile virus infections imported to Germany in 2012. J Clin Virol 8(3): 587-589.
- Sghaier W, Bahri O, Kedous E, Fazaa O, Rezig D, et al. (2012) Retrospective study of viral causes of central nervous system infections in Tunisia (2003-2009). edecine Sante Trop 22(4): 373-378.
- Soliman A, Mohareb E, Salman D, Saad M, Salama S, et al. (2010) Studies on West Nile virus infection in Egypt. J Infect Public Health 3(2): 54-59.
- May FJ, Davis CT, Tesh RB, Barrett AD (2011) Phylogeography of West Nile Virus: from the Cradle of Evolution in Africa to Eurasia, Australia, and the Americas. Journal of Virology 85(6): 2964-2974.
- Fall G, Di Paola N, Faye M, Dia M, Freire CC de M, Loucoubar C, et al. (2017) Biological and phylogenetic characteristics of West African lineages of West Nile virus. Vol. 11, PLoS Neglected Tropical Diseases 11(11): e0006078.
- Sule WF, Oluwayelu DO, Hernández Triana LM, Fooks AR, Venter M, et al. (2018) Epidemiology and ecology of West Nile virus in sub-Saharan Africa. Parasites and Vectors 11(1): 41416.
- Abdullahi IN, Emerbe AU, Ghamba PE, Omosigho PO, Bello ZM, et al. (2021) Distribution pattern and prevalence of West Nile virus infection in Nigeria from 1950 to 2020: a systematic review. Epidemiol Health 42:1-11.
- Lawrie CH, Uzcátegui NY, Gould EA, Nuttall PA (2004) Ixodid and argasid tick species and West Nile virus. Emerg Infect Dis 10(4): 653-657.
- Rizzoli A, Jiménez Clavero MA, Barzon L, Cordioli P, Figuerola J, et al. (2015) The challenge of west nile virus in Europe: Knowledge gaps and research priorities. Eurosurveillance 20(20): 1-15.
- Mostashari F, Bunning ML, Kitsutani PT, Singer DA, Nash D, et al. (2001) Epidemic West Nile encephalitis, New York, 1999: results of a household-based seroepidemiological survey. Lancet 358(9278): 261-264.
- Brown JA, Factor DL, Tkachenko N, Templeton SM, Crall ND, et al. (2007) West Nile viremic blood donors and risk factors for subsequent West Nile fever. Vector Borne Zoonotic Dis (4): 479-488.
- Craven RB, Roehrig JT (2001) West Nile virus. JAMA 286(6): 651-653.
- Spigland I, JasinskA Klingberg W, Hofshi E, Goldblum N (1958) Clinical and laboratory observations in an outbreak of West Nile fever in Israel in 1957. Harefuah 54(11): 275-280.
- Honig A, Karussis D (2014) Delayed-onset flaccid paralysis related to west Nile virus reactivation following treatment with rituximab: a case report BMC Res Notes 7: 852.
- (2020) World Health Rankings. NIGERIA: ENCEPHALITIS . World life expectancy.
- Baba M M, Marie Francois S, Adeniji JA (2006) The Seasonal Effect of West Nile Virus in a Semi-arid Zone, Nigeria. Trends Med Res 1(1): 31-38.
- Oderinde BS, Mora Cárdenas E, Carletti T, Baba MM, Marcello A, et al. (2020) Prevalence of locally undetected acute infections of Flaviviruses in North-Eastern Nigeria. Virus Res 286: 198060.
- Baba M, Logue CH, Oderinde B, Abdulmaleek H, Williams J, et al. (2013) Evidence of arbovirus co-infection in suspected febrile malaria and typhoid patients in Nigeria. J Infect Dev Ctries 7(01): 051-59.
- Sokunbi TO, Omojuyigbe JO, Bakenne HA, Adebisi YA (2022) Nigeria End Malaria Council: What to expect. Ann Med Surg (Lond) 82: 104690.
- info W. Climate in Adamawa.
- (2022) Weather Spark: W. 58. Average, Weather Year Round in Bauchi Weather Spark: Climate.
- (2022) World Data. W. Climate in Borno (Nigeria).
- (2007) World Health Organization. Guidelines for plaque reduction neutralization testing of human antibodies to Dengue viruses. World Health Organization.
- Baba MM, Yahaya KM, Ezra EM, Adamu M, Kulloma BM, et al. (2021) Assessment of immunity against Yellow Fever virus infections in northeastern Nigeria using three serological assays. J Med Virol 93(8): 4856-4864.
- Mercier Delarue S, Durier C, De Verdière NC, Poveda JD, Meiffrédy V, et al. (2017) Screening test for neutralizing antibodies against yellow fever virus, based on a flavivirus pseudotype. PLoS One 12(5): e0177882.
- Bovay A, Nassiri S, Maby El Hajjami H, Marcos Mondéjar P, Akondy RS, et al. (2020) Minimal immune response to booster vaccination against Yellow Fever associated with pre-existing antibodies. Vaccine 38(9): 2172-2182.
- Ribeiro MRC, Khouri R, Sousa PS, Branco MRFC, Batista RFL, et al. (2020) Plaque Reduction Neutralization Test (PRNT) in the Congenital Zika Syndrome: Positivity and Associations with Laboratory, Clinical, and Imaging Characteristics. Viruses 12(11): 1244.
- Domingo C, Charrel RN, Schmidt Chanasit J, Zeller H RC (2018) Yellow fever in the diagnostics laboratory. Emerg Microbes Infect 7(1): 129.
- Pourrut X, Nkoghé D, Paweska J Jeroy E (2010) First serological evidence of West Nile virus in human rural populations of Gabon. Virol J 7(132): 1-3.
- Chepkorir E, Lutomiah J, Mutisya J, Mulwa F, Limbaso K, et al. (2014) Vector competence of Aedes aegypti populations from Kilifi and Nairobi for dengue 2 virus and the influence of temperature. Parasit Vectors 7(1): 435.
- Di Gennaro A, Lorusso A, Casaccia C, Conte A, Monaco F, et al. (2014) Serum neutralization assay can efficiently replace plaque reduction neutralization test for detection and quantitation of West Nile virus antibodies in human and animal serum samples. Clin Vaccine Immunol 21(10): 1460 -1462.
- Inziani M, Adungo F, Awando J, Kihoro R, Inoue S, et al., (2019) Seroprevalence of yellow fever, dengue, West Nile and chikungunya viruses in children in Teso South Sub-County, Western Kenya. Int J Infect Dis 91: 104-110.
- Vu DM, Banda T, Teng CY, Heimbaugh C, Muchiri EM, et al. (2017) Dengue and West Nile Virus Transmission in Children and Adults in Coastal Keny. Am J Trop Med Hyg 96(1): 141-143.
- Olaleye OD, Omilabu SA, Ilomechina EN FA (1990) A survey for haemagglutination-inhibiting antibody to West Nile virus in human and animal sera in Nigeria. Comp Immunol Microbiol Infect Dis 13(1): 35-39.
- Beck C, Jimenez Clavero MA, Leblond A, Durand B, Nowotny N, et al. (2013) Flaviviruses in Europe: complex circulation patterns and their consequences for the diagnosis and control of West Nile disease. Int J Environ Res Public Health. Int J Env Res Public Heal 10(11): 6049-6083.
- Domingo C, Charrel RN, Schmidt Chanasit J, Zeller H RC (2018) Yellow fever in the diagnostics laboratory. Emerg Microbes Infect 7(1): 129.
- Lindsey NP, Staples JE, Powell K, Rabe IB, Fischer M, et al. (2018) Ability To Serologically Confirm Recent Zika Virus Infection in Areas with Varying Past Incidence of Dengue Virus Infection in the United States and U.S. Territories in 2016. J Clin Microbiol 56(1): e01115-1117.
- Murray KO, Walker C, Gould E (2011) The virology, epidemiology, and clinical impact of West Nile virus: a decade of advancements in research since its introduction into the Western Hemisphere. Epidemiology Infect 139(6): 807-817.
- Omilabu SA, Olaleye OD, Aina Y, Fagbami AH (1990) West Nile complement fixing antibodies in Nigerian domestic animals and humans. J Hyg Epidemiol Microbiol Immunol 34(4): 357-363.
- Michaelis M, Kleinschmidt MC, Doerr HW, Cinatl J Jr (2007) Minocycline inhibits West Nile virus replication and apoptosis in human neuronal cells. J Antimicrob Chemother 60(5): 981-986.
- Arroyo J, Miller CA, Catalan J, Monath TP (2001) Yellow fever vector live-virus vaccines: West Nile virus vaccine development. Trends Mol Med 7(8): 350-354.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.