Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Advancements in Protein Synthesis: Triazole Multi-Ligation and Cycloadditions for Pharmaceutical Applications
*Corresponding author: prof. Dr. Nasser Thallaj Pharmaceutical chemistry and drug quality control Department, Faculty of Pharmacy, Al-Rachid privet University, Damascus, Syria. ORCID ID: 0000-0002-6279-768X.
Received: February 02, 2025; Published: April 24, 2025
DOI: 10.34297/AJBSR.2025.26.003481
Abstract
The synthesis of proteins remains a critical challenge in chemical biology, essential for understanding the intricate molecular mechanisms underlying biological processes. This article reviews innovative methodologies in protein synthesis, focusing on triazole multi-ligation and successive cycloadditions. These approaches facilitate the precise assembly of complex protein mimetics, thereby enhancing our understanding of protein structure and function. We discuss the advancements in Solid-Phase Peptide Synthesis (SPPS) and Native Chemical Ligation (NCL), which have significantly improved the efficiency and scalability of protein production. SPPS allows for the iterative assembly of peptides, while NCL enables the formation of native peptide bonds under mild conditions, overcoming the limitations of traditional recombinant methods. The integration of non-canonical amino acids and post-translational modifications in these techniques further expands the functional repertoire of synthesized proteins. Additionally, we explore mixed ligation strategies that leverage the compatibility of different ligation methods to streamline protein assembly. The development of solid-support methods for successive ligation has expedited purification processes, allowing for the synthesis of larger protein constructs. Despite these advancements, challenges such as the stability of reactive intermediates and the specificity of ligation reactions persist, necessitating ongoing research. Future directions include the exploration of second-generation native chemical ligations and auxiliary-assisted methods, which aim to broaden the scope of amino acid utilization and enhance the versatility of chemical ligation techniques. By advancing these methodologies, we can improve our ability to produce functional protein analogues, facilitating deeper insights into protein interactions and biological functions. Ultimately, the evolution of these innovative strategies holds great potential for applications in biotechnology and medicine.
Keywords: Protein synthesis; Triazole multi-ligation; Cycloadditions; Solid-Phase Peptide Synthesis (SPPS); Native chemical ligation (NCL); Protein mimetics; Post-translational modifications
Introduction
The synthesis of proteins is a fundamental challenge in the field of chemical biology, driven by the necessity to understand the intricate molecular mechanisms that govern biological processes [1- 5]. Proteins, composed of sequences of amino acids, perform a vast array of functions within living organisms, ranging from catalysing biochemical reactions to providing structural support in cells [3-8]. As research in genomics and proteomics has advanced, the complexity of protein synthesis has become increasingly apparent [7-12]. The human genome contains approximately 20,000 to 25,000 protein-coding genes, yet the functional diversity of the proteome far exceeds this number due to post-translational modifications and alternative splicing [13-19]. This variability complicates the task of elucidating the specific roles of individual proteins and their interactions within cellular systems [18-26].
To study protein function, researchers have predominantly relied on recombinant DNA technology, which allows for the expression of proteins in genetically modified cells. This method has revolutionized the field by enabling the production of large quantities of structurally defined proteins. However, it is not without its limitations [25-30]. The overexpression of certain proteins can lead to issues such as aggregation or insolubility, particularly for cytotoxic or membrane-associated proteins. Moreover, the reliance on cellular machinery restricts the introduction of specific post-translational modifications, which are crucial for the functional activity of many proteins [31-36]. While chemical modifications can be performed post-extraction, these processes often require complex methodologies and can result in low yields or undesired side products [36-40].
In contrast, chemical protein synthesis offers a promising alternative, allowing for precise control over the structure and modifications of polypeptides [41-42]. This approach enables the incorporation of non-canonical amino acids, post-translational modifications, and the synthesis of complex protein mimetics that are not readily achievable through biological methods. The ability to synthesize proteins chemically allows researchers to manipulate protein structures at an atomic level, facilitating detailed investigations into their biochemical properties and functions [43-49].
The advent of Solid-Phase Peptide Synthesis (SPPS), pioneered by Robert Merrifield, marked a significant milestone in the field of chemical protein synthesis. This technique simplifies the iterative assembly of peptides by anchoring the growing chain to an insoluble resin, allowing for straightforward purification and enhanced reaction efficiency [50].
Despite its revolutionary impact, SPPS is generally limited to peptides of approximately fifty amino acids in length [51]. To overcome this limitation, researchers have developed various strategies for the synthesis of larger proteins, including the condensation of fully or partially protected peptide fragments and the use of chemical ligation methods [50-51].
Chemical ligation techniques, such as Native Chemical Ligation (NCL), have gained widespread acceptance for their ability to join unprotected peptide segments in aqueous environments [52-55]. NCL relies on the unique reactivity of thioesters and N-terminal cysteine residues, enabling the formation of native peptide bonds under mild conditions.
However, this method is constrained by the requirement for cysteine at the ligation site and the inherent instability of thioesters in basic conditions [56-58]. To address these challenges, novel approaches, including second-generation NCL strategies and auxiliary- assisted ligation methods, have been developed to broaden the applicability of chemical ligation [59-63].
Recent advancements in multi-ligation strategies, including the use of tandem and successive ligation techniques, have further enhanced the potential for synthesizing complex protein structures [64-69].
By employing a combination of compatible ligation methods, researchers can assemble larger protein constructs more efficiently. Additionally, the development of solid-support methods for successive ligation has shown promise in expediting purification processes and improving overall yields [70-77].
This article explores the innovative approaches in triazole multi- ligation and the development of new tools for synthesizing protein mimetics through successive cycloadditions [78]. By examining the principles and applications of these methodologies, we aim to highlight their significance in advancing the field of chemical protein synthesis and their potential to overcome the limitations of traditional methods [71-79].
Through a comprehensive understanding of these techniques, researchers can enhance their ability to produce functional protein analogues, thereby facilitating deeper insights into protein function and interaction in biological systems [77].
Chemical Protein Synthesis: A Challenge for Chemists
Genome and Proteome
A primary goal of biomedical research is to elucidate the molecular mechanisms underlying the diverse biological activities of proteins, as well as to predict and manipulate these activities. The complexity and significance of this endeavour have become increasingly apparent since the advent of genome sequencing. According to analyses conducted in 2004, the human genome is estimated to contain approximately 20,000 to 25,000 protein-coding genes [78].
However, this number does not fully account for the extensive variability present in the proteome, as functional proteins can undergo numerous modifications during synthesis, resulting in a much larger repertoire of expressed proteins than that suggested by gene studies.
Once the sequence of a protein is determined through genomic analysis, a significant challenge remains: ascertaining its biological function. At this juncture, the production of the protein becomes crucial for addressing this issue [79].
Recombinant Protein Synthesis
The majority of research into the mechanisms of protein action has utilized proteins produced via recombinant DNA technology, where proteins are expressed in cells that have been genetically modified. Since its inception, this method has transformed protein research by enabling the generation of substantial quantities of structurally defined proteins, while also facilitating systematic variations in protein sequences. Although expressing proteins in competent cells has become a standard technique, it is not without limitations.
For instance, the overexpression of cytotoxic or poorly soluble proteins often presents challenges [80]. Furthermore, the reliance on the cellular machinery complicates the controlled introduction of post-translational modifications, which can be difficult or infeasible depending on the type of modification and the expression system employed [81].
Additionally, while it is possible to introduce post-translational modifications chemically or enzymatically after extraction and purification, this process remains complex. Recent advancements in the incorporation of non-proteinogenic amino acids, which possess unique properties such as fluorescence or specialized chemical reactivity, represent an exciting area of ongoing research [82].
Current methodologies include systematic residue replacement to alter the physicochemical properties of expressed proteins or the targeted introduction of non-canonical amino acids to facilitate the study of protein structure at the molecular level, both in vitro and in vivo [83].
Chemical Protein Synthesis
Chemical synthesis has emerged as a viable alternative for protein production, allowing for precise modifications of polypeptide structures to enable comprehensive investigations of their mechanisms of action. This approach permits the incorporation of:
a. Amino acids not encoded by the genome
b. Modifications to the protein backbone with atomic precision
c. Post-translational modifications (such as phosphorylation, glycosylation, and lipidation)
d. Tools for spectroscopic characterization
Given these advantages, total chemical synthesis of proteins has long posed a challenge for chemists. Emil Fischer, awarded the Nobel Prize in 1902, envisioned that “Physiological Chemistry” would enable the preparation of synthetic enzymes tailored for specific functions. This vision is steadily becoming a reality, thanks to remarkable advances in peptide synthesis achieved over the past century [84].
Chemical Synthesis of Proteins via Condensation of Protected Peptides
Solid-Phase Peptide Synthesis
Merrifield’s Solid-Phase Peptide Synthesis (SPPS) has revolutionized the field of peptide synthesis, making it readily accessible. In SPPS, peptides are synthesized through a repetitive and iterative process rather than a convergent one. The innovative aspect of this method lies in the attachment of the peptide to an insoluble polymeric support, or “resin,” at its C-terminal end.
This immobilization of one of the reactive partners allows for the straightforward separation of coupling or deprotection by-products from the growing peptide through simple filtration, thereby significantly expediting the synthesis process. Moreover, filtration enables the use of large excesses of amino acids for coupling, resulting in nearly quantitative yields (Figure1).
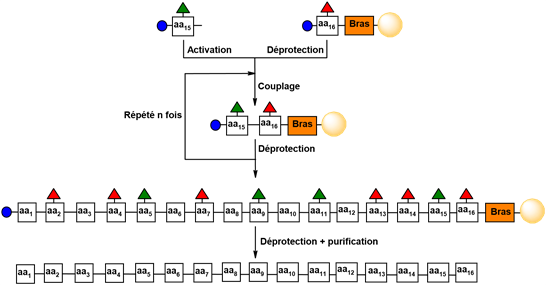
Figure 1: Strategy for solid-phase peptide synthesis.
 amino acid protected in the N-α position.
amino acid protected in the N-α position.
 amino acid protected on its side chain.
amino acid protected on its side chain.
 insoluble polymeric support (resin).
insoluble polymeric support (resin).
A significant advantage of Solid-Phase Peptide Synthesis (SPPS) is its repetitive nature, which allows for automation, thereby facilitating peptide synthesis in non-specialized laboratories. Despite extensive optimization of the methodology introduced by Merrifield- including advancements in resins, coupling reagents, protecting groups, and synthesis arms-the maximum size of peptides synthesized via this approach is generally limited to approximately fifty amino acids [85].
Protein Synthesis through the Condensation of Fully Protected Peptides
This strategy employs a convergent assembly technique that involves the condensation of two fully protected peptides synthesized by SPPS through the formation of an amide bond (Figure 2). This method is particularly effective for producing proteins that exceed fifty residues or for generating high-purity therapeutic peptides [86].
The coupling of protected peptides in solution presents several disadvantages, with the most significant being the limited solubility of these peptides in organic solvents, as well as the potential for epimerization at the α position of the activated amino acid [87].
Protein Synthesis through the Condensation of Partially Protected Peptides
This approach is pioneering in its use of partially protected peptides for coupling and is the first to employ thiocarbonyls as activated acyl donor groups, enabling chemo selective couplings (Figure 3).
This strategy marks a significant advancement over previous methods, as it is compatible with solvents where unprotected peptides exhibit high solubility, such as water or DMF, and utilizes a weakly activated acyl donor to minimize epimerization issues. However, while highly chemo selective acylation offers benefits, it lacks regioselectivity, necessitating the protection of side chains on lysine residues, which reduces the peptide’s solubility in water and extends coupling times to as long as 96 hours [88].
Despite these challenges, this method has experienced renewed interest following the foundational work of Blake and his collaborators, as well as Aimoto and Hojo. Initially constrained by the use of lipophilic amine protective groups that complicate coupling in aqueous environments, this strategy is now being revisited and is the focus of ongoing research aimed at synthesizing peptides, glycopeptides, and glycoproteins.
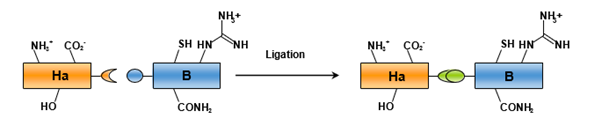
Chemical Ligation of Peptides
Chemical peptide ligation involves the chemo selective and regioselective coupling of two fully unprotected peptides (Figure 4). Performing this coupling under such conditions allows for the avoidance of the primary disadvantages associated with convergent synthesis in organic solvents, including enhanced solubility of all peptide fragments and non-epimerizing coupling conditions.
During chemical ligation of peptides, two peptide fragments are joined through the formation of a covalent bond between two reactive partners (e.g., an electrophilic aldehyde and a nucleophilic aminooxy group to yield an oxime) to create an amide bond mimic or a protein mimic. The primary challenge in developing an effective ligation method lies in identifying partners that can be readily incorporated into peptides via Solid-Phase Peptide Synthesis (SPPS) while maintaining sufficient chemo- and regioselective reactivity in aqueous environments, along with adequate stability for manipulation.
This challenge becomes significantly more complex when considering that, after the primary sequence of the protein is assembled through ligation, the resulting synthetic polymer must fold correctly to resemble the native protein and retain its biological activity. The quest for novel ligation methods remains an area of active research. Current investigations primarily focus on utilizing pericyclic reactions (such as Diels-Alder and 1,3-dipole cycloadditions) or organometallic catalysis (including Suzuki couplings and cross-metathesis of hydro compatible olefins) to exploit the chemical stability of the partners and the exceptional chemo selectivity of the coupling reactions.
Among the reactions exhibiting these desirable characteristics, only a select few have been successfully employed for the total synthesis of proteins or bioactive structural analogy of proteins. We categorize these methods into two distinct types: native and pseudo- native ligations.
Native ligations
Native Chemical Ligation (NCL):
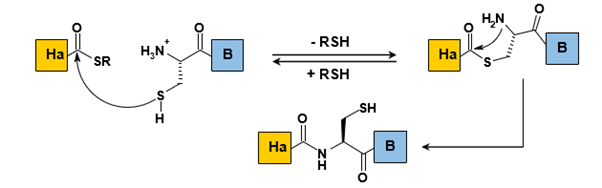
Native Chemical Ligation (NCL) involves the reaction between a thioester and an N-terminal cysteine residue (Figure 5).
The initial step of this process is dynamic, featuring reversible trans-Thio esterification at the C-terminal end of the thioester fragment. This intermediate then undergoes spontaneous rearrangement through nucleophilic addition of the amine to the carbonyl, followed by elimination, resulting in the formation of a native peptide bond (Figure 5). The chemo selectivity of this reaction is attributed to trans-Thio esterification, which effectively positions the electrophilic carbonyl in close proximity to the nucleophilic amine, as well as the regioselective activation of the adjacent amine to the thioester at physiological pH (~7.4), where the amines on lysine side chains are primarily protonated. The coupling occurs without epimerization, and its kinetics are influenced by the steric hindrance of the side chain of the amino acid carrying the thioester. The first demonstration of NCL was the synthesis of a 72-residue protein, human interleukin 8, conducted by Kent et al (Figure 6).
Native Chemical Ligation (NCL) is one of the most widely adopted techniques for native ligation. The efficacy of this method is underscored by nearly a thousand citations of its initial publication (source: ISI Web of Knowledge, 10/01/2010). NCL facilitates the synthesis of numerous proteins either through purely chemical means or via a hybrid approach that combines chemical and recombinant methods.
Despite its advantages, NCL has notable limitations. Cysteines and thioesters exhibit high reactivity towards electrophiles and nucleophiles, respectively. Thioesters, in particular, are unstable and susceptible to hydrolysis at pH levels above neutral, which complicates their synthesis and handling. Currently, the predominant strategy for synthesizing thioester peptides is the Boc/tBu method; however, this approach is increasingly being abandoned in laboratories due to safety concerns associated with the use of anhydrous hydrofluoric acid. Additionally, thioesters are prone to instability in basic and nucleophilic environments, rendering their synthesis challenging when employing the Fmoc/tBu strategy. Consequently, no straightforward method exists for accessing these compounds. Lastly, the specificity of this reaction for N-terminal cysteines, which occur relatively infrequently (with an abundance of only 1.35%), constrains the range of proteins that can be synthesized using this technique.
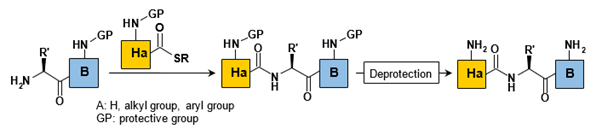
“Second Generation” Native Chemical Ligations:
The methods characterized as second-generation Native Chemical Ligations (NCLs) have been developed to address the primary limitation of traditional NCL, specifically the requirement for a cysteine at the ligation site. Among these approaches, desulfurization and the use of auxiliary reagents are the most prevalent strategies.
Both methods leverage the distinctive features of NCL, which involve the activation of the carboxylic acid at the C-terminal end as a thioester, alongside the regioselective activation of the amine near the thioester (Figure 5).
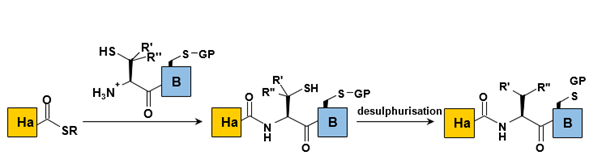
a. Desulphurization of the NL product:
Desulfurization of the cysteine utilized in NCL enables the coupling of peptide fragments to more abundant residues, such as alanine. This strategy has also been expanded to include residues like valine and phenylalanine. A significant drawback of this method is the necessity to protect thiol groups present in the peptide sequence (Figure 7).
Desulfurization employing transition metals presents several challenges, including the necessity for large quantities of metal reagents, the potential epimerization of secondary alcohols (such as threonine’s), and the reduction of thioethers (such as methionine’s). In contrast, radical desulfurization, developed by Danishefsky and collaborators, appears to be a milder approach; however, it still involves the delicate process of deprotecting any additional cysteine residues present in the protein.
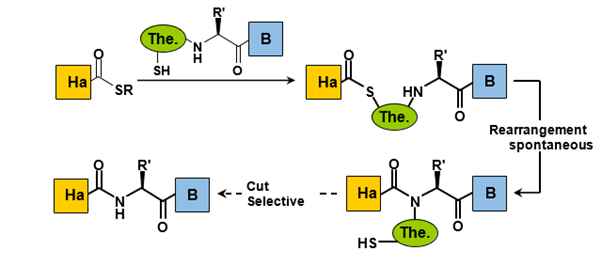
b. Auxiliary-assisted native chemical ligation:
An alternative strategy to desulfurization involves the use of thioether auxiliaries, which can be selectively removed following the ligation process. The primary advantage of this approach is that it allows for ligation to occur with any amino acid residue, thereby expanding the versatility of the method (Figure 8).
The nature of the auxiliaries employed varies depending on their cleavage strategies. The most frequently utilized auxiliaries include acid-labile ones, such as those activated by Hydrofluoric Acid (HF) or Trifluoroacetic Acid (TFA), as well as photolabile types. However, challenges may arise during the removal of the auxiliary, and the substitution of the amine can impede the transfer of the final acyl group, increasing the likelihood of side reactions.
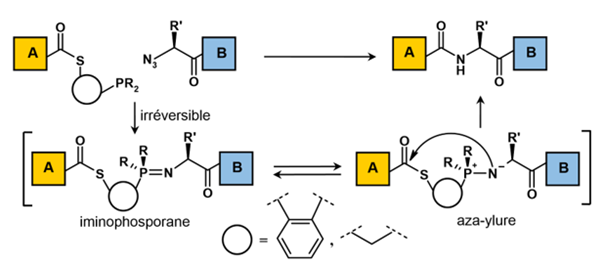
The native Staudinger ligation:
This method was independently discovered by the research teams of Bertozzi and Raines. The coupling mechanism relies on the reaction between a phosphine and an organic aside, resulting in the formation of an aza-yurea-like intermediate (Figure 9). This intermediate undergoes a nucleophilic attack by nitrogen on the carbonyl, ultimately yielding a native amide bond. This rearrangement is particularly favoured when the phosphine is attached to a thioester at the C-terminal end.
The advantage of this method lies in its ability to perform ligation independently of the residue at the deconvolution point on the peptide. The chemo selectivity of a phosphine’s attack on an aside is superior to that of previously discussed methods and is compatible with all functionalities present in peptides. However, this approach carries the inherent challenges associated with thioester synthesis, compounded by the presence of an oxidizable phosphine group. Furthermore, mechanistic studies and experimental findings indicate competition between water attacking the phosphonium species of azaylure and amine attacking the carbonyl (Figure 9). To promote the latter reaction, the authors recommend using peptide concentrations around 100 mM, which poses challenges for practical peptide ligation.
It is important to note that, despite its potential and promising results, this method has yet to be employed for the chemical synthesis of proteins via peptide ligation.
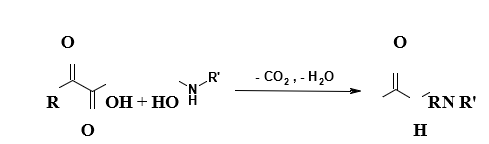
New Methods for Amide Bond Formation Compatible with Unprotected Peptide Ligation:
Despite the challenges associated with forming amide bonds in aqueous conditions with chemo- and regioselectivity, innovative methods continue to emerge, although they have not yet been applied to the synthesis of functional proteins (Figure 10). One particularly noteworthy method involves the reaction of α-keto acids with hydroxylamine’s, yielding an amide bond through a condensation reaction accompanied by decarboxylation.
Pseudo-Native Ligation Methods
Pseudo-native methods encompass a range of ligation techniques that facilitate the formation of amide bond mimics, including isosteres and bio isosteres.
Thioether Ligation
Thioether ligation capitalizes on the distinctive reactivity of the cysteine residue side chain. The thiol group acts as a proficient nucleophile, enabling selective nucleophilic addition or substitution reactions with amines. This approach has been successfully employed in the synthesis of an analogue of HIV-1 protease (Figure 11). In this synthesis, the N-terminal fragment of the target protein, modified as a thioethyl amide at its C-terminus, reacts with the C-terminal fragment, which is functionalized at the N-terminus with a Bromo acetyl group.

Figure 11: Example of thioether ligation, synthesis of an analogue of the HIV-1 protease by thioester ligation.
A significant limitation of this method is its lack of selectivity for a specific thiol within the peptide sequence. Consequently, it is applicable only when the native protein contains either a single cysteine residue or none at all.
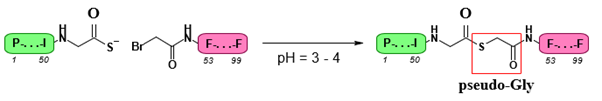
Thioester Ligation
Thioester ligation is closely related to thioether ligation, as it utilizes the same electrophilic partner. In this reaction, a Thio carboxylate undergoes nucleophilic substitution with a Bromo acetyl to form a thioester (Figure 12). This method can be classified as a pseudo-native technique because it generates an isostere of the amide bond. Notably, thioester ligation was the first technique successfully applied to fully unprotected peptides, resulting in the total synthesis of a functional analogue of HIV-1 protease (Figure 12).
Despite these promising results, this method carries several disadvantages associated with thioether ligation. Additionally, it necessitates the use of D-bromo-acids or deconvolution specific to glycyl residues (Figure 12). Furthermore, the thioester bond exhibits instability at pH levels above neutral, which restricts the handling and stability of the resulting ligation product.
Imine-Like Ligation: Thiaprolins and Oxaprolins
Developed by Liu and Tam, these ligation methods involve the condensation of an aldehyde with a β-aminothiol or a β-amino alcohol (Figure 13).
The aldehyde group reacts to form an imine, which is subsequently attacked by the thiol group to yield a thiazolidine Figure 14. The resulting coupling product can undergo an acyl transfer from the oxygen to the nitrogen, quantitatively resulting in the formation of an amide bond (Figure 13).
This ligation method exhibits excellent chemo selectivity; however, the high reactivity of aldehydes toward nucleophiles, particularly amines in lysine side chains, as well as the reactivity of N-terminal cysteines toward electrophiles, can present challenges.
Problem
The human proteome encompasses the complete set of proteins expressed by the human genome. Figure 15 depicts the abundance of these proteins in relation to their size, highlighting that those accessible through solid-phase peptide synthesis (SPPS), which are less than 50 residues in length, represent only a small fraction of the proteome (approximately 5%). Furthermore, a single ligation of peptides containing fewer than 100 amino acids provides access to only about 15% of the human proteome (Figure 15).
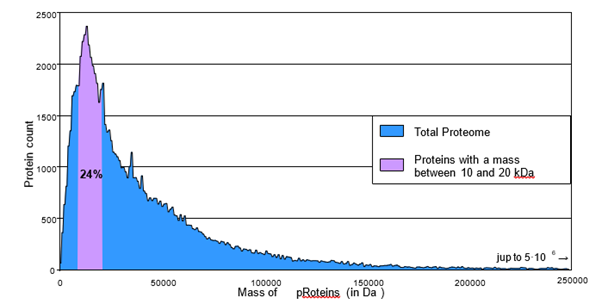
Figure 15: Graph representing the number of proteins in the human proteome as a function of their molecular weight.
To achieve proteins with a mass of approximately 20 kDa, it is essential to perform multiple successive ligations, thereby enabling a chemical synthesis method that could encompass about 38% of the human proteome. Multi-ligation of peptides significantly expands the potential biological targets for chemical peptide synthesis, as around 24% of the human proteome (depicted in purple in Figure 15) falls within the mass range of 10,000 to 20,000 Da-limits that are only accessible through chemical peptide multi-ligation. To date, only a few dozen proteins of this size have been synthesized chemically.
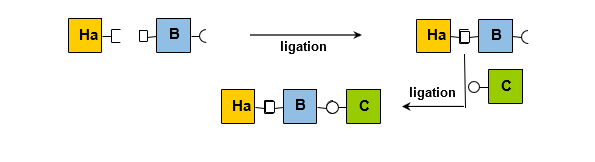
Peptide Multi-Ligation Strategies
Two primary strategies have been identified to date:
i. A mixed approach that employs two mutually compatible ligation methods (Figure 16).
ii. The repeated use of the same ligation method, involving the masking of a partner during the initial ligation (Figure 17).
Protein Synthesis by Mixed Ligation
A. Principle
This strategy leverages the mutual compatibility or partial compatibility of different ligation methods. It relies on the chemo selectivity of ligation reactions, enabling their simultaneous use (Figure 16). The effectiveness of this approach is exemplified by two cases of tandem multi-ligation utilized for synthesizing proteins or protein analogues.
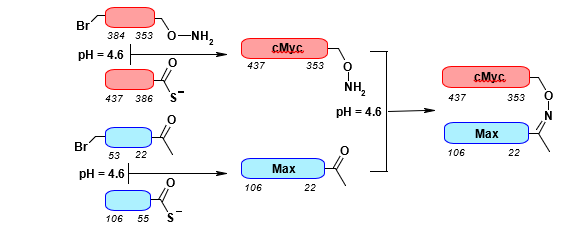
B. Mutually Compatible Ligation: Thioester and Oxime
A notable example of this strategy is the combined use of oxime and thioester ligation in the synthesis of the cMyc-Max protein analogue (Figure 18).
Kinetic control of thioesterification during the assembly of cMyc results in the preferential substitution of the bromoacetyl partner with thiocarboxylate, yielding the peptide-amino-oxy cMyc(353- 437). Concurrently, a similar thioesterification generates the peptide- aldehyde Max(22-106). These two peptides are subsequently coupled through the condensation of the carbonyl fragment with the amino-oxy fragment, resulting in a functional analogue of the target protein.
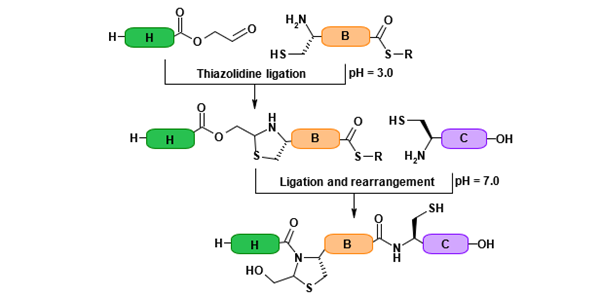
C. Partially Compatible Ligations: Association of Thioester and Pseudo proline Ligation
The combination of native ligation and pseudo proline ligation has been proposed as a strategy to enhance the efficiency of peptide synthesis (Figure 19).
Although both pseudo proline and thioester ligations utilize the same nucleophilic partner, an N-terminal cysteine, the formation of a thiazolidine is favoured at pH 3. This prevents the cyclization of the B fragment (Figure 19). The resulting fragment AB is then subjected to native ligation conditions at neutral pH, allowing for condensation with fragment C. This process simultaneously facilitates the rearrangement of the thiazolidine into pseudo proline. It is crucial to emphasize that the order of fragment assembly and precise control of reaction conditions are essential, as both thiazolidine ligations and native chemical ligation share the same nucleophilic partner.
D. Advantages and Disadvantages of the Mixed Approach
The use of tandem ligations offers the significant advantage of streamlining the protein assembly process; however, it also accumulates the drawbacks associated with each individual technique. Additionally, since all ligation reactions involve nucleophilic addition or substitution on electrophilic partners, tandem ligation necessitates precise control of reaction conditions to prevent competition between the ligation reactions. Furthermore, the number of mutually compatible ligation techniques is quite limited, which restricts the overall number of ligations that can be performed using this strategy.
Synthesis by Masking a Ligation Partner
A more versatile strategy involves masking one of the ligation partners, followed by unmasking it to continue the protein assembly using the same ligation method (Figure 17).
A. Successive Native Chemical Ligations
The synthesis of crambine, which consists of 46 residues and poses challenges for linear solid-phase peptide synthesis (SPPS), serves as an excellent example of this approach through successive native chemical ligation (Figure 20).

Figure 20: Synthesis of crambine by successive native chemical ligation according to Bang et al.Art. 66
The initial ligation is carried out selectively by masking an N-terminal cysteine in the form of a thiazolidine. The removal of this protective group under mild and chemo selective conditions using methoxy amine hydrochloride allows for the regeneration of a new N-terminal cysteine, which can then be utilized in a subsequent ligation to construct crambine. The Acm protective group for cysteine has also been successfully employed in the synthesis of an EPO analogue (166 residues) through successive triple native ligation, as well as in the synthesis of crambine using a similar strategy.
More recently, Kent and his collaborators have enhanced and expanded this multi-ligation approach by capitalizing on the differing reactivities of aryl and aliphatic thioesters. This advancement facilitated the synthesis of human lysozyme (130 residues, 8 cysteines, and 4 disulfide bridges) through Multi-Native Chemical Ligation (NCL) following a convergent strategy (Figure 21).
B. Successive Ligation on Solid Support
The successive or convergent ligation approach can be notably slow due to the need for purifying each synthetic intermediate and the challenges associated with manipulating the peptides. To address these issues, Kent and his collaborators proposed performing successive ligations on solid support. This method effectively combines the benefits of solid-phase synthesis with peptide ligation, providing an excellent strategy for synthesizing protein mimetics of up to 200 residues (Figure 22).
The purification steps involved in ligation and deprotection are significantly expedited using this method (Figure 22). To date, this approach has been applied to only two ligation techniques: native chemical ligation (NCL) utilizing the thiazolidine protective group, and oxime ligation employing the allyloxy carbonyl protective group.
Conclusion
In summary, the advancements in protein synthesis methodologies, particularly through the innovative approaches of triazole multi-ligation and successive cycloadditions, represent a significant leap in the field of chemical biology. The ability to synthesize complex protein mimetics with precision not only enhances our understanding of protein structure and function but also opens new avenues for therapeutic and diagnostic applications.
The integration of chemical synthesis techniques, including Solid-Phase Peptide Synthesis (SPPS) and Native Chemical Ligation (NCL), has demonstrated the potential to produce larger and more intricate protein structures. These methods allow for the incorporation of non-canonical amino acids and post-translational modifications, thereby facilitating the design of proteins with tailored functionalities. The successful application of these techniques to synthesize proteins such as crambine and human lysozyme showcases their effectiveness in overcoming the limitations associated with traditional recombinant methods.
Additionally, the exploration of mixed ligation strategies, which leverage the compatibility of different ligation methods, has proven beneficial in streamlining the protein assembly process. By employing tandem and successive ligation techniques, researchers can efficiently construct protein analogues, significantly expanding the range of accessible biological targets.
The ability to manipulate reaction conditions and the order of fragment assembly plays a crucial role in optimizing yield and specificity, underscoring the importance of meticulous experimental design. Moreover, the introduction of solid-support methods for successive ligation has expedited purification processes and improved the overall efficiency of protein synthesis.
This advancement addresses the challenges of manipulating peptides and purifying intermediates, thus paving the way for the synthesis of larger protein mimetics, which are essential for a comprehensive understanding of biological systems. However, despite these promising developments, challenges remain. Issues related to the stability of reactive intermediates, such as thioesters, and the specificity of ligation reactions necessitate ongoing research.
The exploration of second-generation native chemical ligations and auxiliary-assisted methods represents a proactive approach to overcoming these limitations. These strategies not only broaden the scope of amino acid residues that can be utilized but also enhance the versatility and applicability of chemical ligation techniques.
As we advance our understanding of protein synthesis through these innovative methods, it is crucial to continue investigating the underlying chemical mechanisms and optimizing the conditions for ligation reactions. Future research should focus on developing new ligation strategies that maintain high chemo selectivity and regioselectivity in aqueous environments, as well as exploring the incorporation of additional functional groups that can facilitate more complex protein modifications. The field of chemical protein synthesis is evolving rapidly, driven by the need for precise and efficient methods to produce functional proteins and protein mimetics.
The innovative approaches discussed in this article not only enhance our capabilities in protein synthesis but also contribute to the broader understanding of protein functionality in biological systems. As these methodologies continue to evolve, they hold great promise for advancing both fundamental research and practical applications in biotechnology and medicine. The ongoing exploration of multi-ligation techniques will undoubtedly play a pivotal role in shaping the future of protein science.
Acknowledgement
None.
Conflict of Interest
None.
References
- Thallaj N (2021) Synthesis of a New Ligand Tris (2-pyridylmethyl) amine functionalized by a methoxy group and study of Dichloroferrous complexes, its reactivity to dioxygen both in the presence and absence of substrate. International journal of applied chemistry and biological sciences 2(4): 65-77.
- L Labban, N Thallaj, Z Malek (2020) Estimation of ω−3 and ω− 6 fatty acids intakes, their ratio, their sources and their role in glycaemic control among type 2 diabetics. International Journal of Medical Studies 5(12): 23-36.
- L Labban, M Kudsi, Z Malek, N Thallaj (2020) Pain Relieving Properties of Ginger (Z. officinale) and Echinacea (E. angustifolia) Extracts Supplementation among Female Patients with Osteoarthritis. A Randomized Study. Advances in Medical, Dental and Health Sciences 3(3): 45-48.
- L Labban, N Thallaj, M Al Masri (2020) Nutritional Value of Traditional Syrian Sweets and their Calorie Density. Journal of Advanced Research in Food Science and Nutrition 3(1): 34-41.
- Thallaj N, agha MIH, nattouf A.H, katib CH, karaali A, et al. (2020) Evaluation of Antimicrobial Activities and Bioactive Compounds of Different Extracts Related to Syrian Traditional Products of Damask Rose (Rosa damascena). open access library journal 7(5): 1-21.
- L labban, N Thallaj, A labban (2020) Assessing the Level of Awareness and Knowledge of COVID 19 Pandemic among Syrians. Archives of Medicine 12(3): 1-5.
- L labban, N Thallaj (2020) The medicinal and pharmacological properties of Damascene Rose (Rosa damascena): A review. International Journal of Herbal Medicine 8(2): 33-37.
- L Labban, N Thallaj, Z Malek (2019) The implications of E-cigarettes or "vaping"on the nutritional status Journal of Medical Research and Health Sciences 2(11): 784-787.
- L labban, N Thallaj (2019) The Effect of Magnesium Supplementation on Hba1c Level and Lipid Profile Among Type 2 Diabetics. Acta Scientific Nutritional Health 3(10): 7-12.
- Malek ZS, Labban L (2020) The International Journal of Neuroscience: 1-7.
- Malek ZS, Labban L (2019) A Comparative Study of Tryptophan Hydroxylase's Circadian Rhythm in the Functional Parts of Dorsal Raphe Nuclei in the Mesencephalon. European Journal of Pharmaceutical and Medical Research 6(11): 527-532.
- Malek ZS (2018) Journal of AL Baath University 40(4): 39-62.
- Malek ZS (2018) Tishreen University Journal for Research and Scientific Studies 40.
- ZS Malek, LM Labban (2021) Photoperiod regulates the daily profiles of tryptophan hydroxylase-2 gene expression the raphe nuclei of rats. International Journal of Neuroscience 131(12): 1155-1161.
- ZS Malek, LM Labban (2020) Photoperiod regulates the daily profiles of Tryptophan Hydroxylase-2 gene expression the raphe nuclei of rats. Journal of current research in physiology and pharmacology 4(1): 1-5.
- LM Labban, MM Alshishkli, A Alkhalaf, Z Malek (2017) J Adv Res Dent Oral Health 2(3&4): 1-4.
- L Labban, ZS Malek (2018) The Association between Visceral Fat, Dietary Patterns, and Comorbidities. Open Access Library Journal 5 (7): 1-11.
- L Labban, ZS Malek (2019) Ann Food Nutr Res J 1: 1.
- Labban L, N Thallaj (2019) Acta Scient Nutr Health 3: 7-12.
- N Thallaj (2022) Microwave Assisted Synthesis of 1,3,4-oxadiazole،thiazolidine derivatives. Tishreen university journal 44(1): 59-77.
- N Thallaj (2022) Synthesis, characterization and analytical study of new imine compounds. Tishreen university journal 44(2): 87-105.
- A Abbood, N Thallaj (2023) Arab Journal of Pharmaceutical Sciences 7: 1.
- N Thallaj (2023). Tishreen University Journal-Medical Sciences Series 44 (6): 21-29.
- Machkour A, Thallaj NK, Benhamou L, Lachkar M, Mandon Chemistry (2006) The Coordination Chemistry of FeCl3 and FeCl2 to Bis[2-(2,3-dihydroxyphenyl)-6-pyridylmethyl] (2-pyridylmethyl) amine: Access to a Diiron (III) Compound with an Unusual Pentagonal-Bipyramidal/Square-Pyramidal Environment. Chemistry12(25): 6660-6668.
- Thallaj N, Machkour A, Mandon D, Welter R (2005) Square pyramidal geometry around the metal and tridentate coordination mode of the tripod in the [6-(3′-cyanophenyl)-2-pyridylmethyl] bis(2-pyridylmethyl) amine FeCl2 complex: a solid-state effect. New J Chem 29: 1555 - 1558.
- Thallaj NK, Rotthaus O, Benhamou L, Humbert N, Elhabiri M, et al. (2008) Reactivity of molecular dioxygen towards a series of isostructural dichloroiron (III) complexes with tripodal tetraamine ligands: general access to mu-Oxodiiron (III) complexes and effect of alpha-fluorination on the reaction kinetics Chemistry-A European Journal 14 (22): 6742-6753.
- Wane A, Thallaj NK, Mandon D (2009) Biomimetic interaction between Fe (II) and O2: effect of the second coordination sphere on O2 binding to Fe (II) complexes: evidence of coordination at the metal centre by a dissociative mechanism in the formation of mu-oxo diferric complexes. Chemistry.15(40): 10593-602.
- Thallaj NK, Orain PY, Thibon A, Sandroni M, Welter R, et al. (2014) Steric congestion at, and proximity to, a ferrous center leads to hydration of α-nitrile substituents forming coordinated carboxamides Inorg Chem. 53(15): 7824-7836.
- NK Thallaj, J Przybilla, R Welter, D. Mandon (2008) A ferrous center as reaction site for hydration of a nitrile group into a carboxamide in mild conditions. J Am Chem Soc 130(8): 2414-2415.
- NK Thallaj, D Mandon, KA White (2007) The Design of Metal Chelates with a Biologically Related Redox-Active Part: Conjugation of Riboflavin to Bis(2-pyridylmethyl) amine Ligand and Preparation of a Ferric Complex. Eur J of Inorg Chem 2007(1): 44-47.
- Thallaj N (2021) Synthesis of a New Ligand Tris (2-pyridylmethyl) amine functionalized by a methoxy group and study of Dichloroferrous complexes, its reactivity to dioxygen both in the presence and absence of substrate. International journal of applied chemistry and biological sciences 2(4): 65-77.
- Thallaj N (2023) Review of a Few Selected Examples of Intermolecular Dioxygenases Involving Molecular Oxygen and Non-Heme Iron Proteins. Int J Adv Parmacutical Sci Res (IJAPSR) 3: 1-18.
- L Labban, M Kudsi, Z Malek, N Thallaj (2020) Pain Relieving Properties of Ginger (Z. officinale) and Echinacea (E. angustifolia) Extracts Supplementation among Female Patients with Osteoarthritis. A Randomized Study. Advances in Medical, Dental and Health Sciences 3(3): 45-48.
- L Labban, N Thallaj, M Al Masri (2020) Journal of Advanced Research in Food Science and Nutrition 3(1): 34-41.
- L labban, N Thallaj, A labban (2020) Assessing the Level of Awareness and Knowledge of COVID 19 Pandemic among Syrians. archives of medicine 12(2): 8.
- L Labban, N Thallaj, Z Malek (2019) The implications of E-cigarettes or "vaping"on the nutritional status. Journal of Medical Research and Health Sciences 2(11): 784-787.
- Malek ZS, Sage D, Pevet P, Raison S (2007) Daily rhythm of tryptophan hydroxylase-2 messenger ribonucleic acid within raphe neurons is induced by corticoid daily surge and modulated by enhanced locomotor activity. Endocrinology 148 (11): 5165-5173.
- Malek ZS, Dardente H, Pevet P, Raison S (2005) Tissue-specific expression of tryptophan hydroxylase mRNAs in the rat midbrain: anatomical evidence and daily profiles. European Journal of Neuroscience 22(4): 895-901.
- A Abbood, SA Malik D aldiab, HH Ali, N Thallaj (2025) Investigation of the charge variant profile of non-cleavable conjugated antibodies. Research J Pharm and Tech 18(1): 185-190.
- Malek ZS, Pevet P, Raison S (2004) Circadian change in tryptophan hydroxylase protein levels within the rat intergeniculate leaflets and raphe nuclei Neuroscience 125 (3): 749-758.
- Malek ZS, Labban L (2020) Photoperiod regulates the daily profiles of tryptophan hydroxylase-2 gene expression the raphe nuclei of rats. The International Journal of Neuroscience 131(12): 1155-1161.
- ZS Malek, LM Labban (2020) Photoperiod regulates the daily profiles of Tryptophan Hydroxylase-2 gene expression the raphe nuclei of rats. Journal of current research in physiology and pharmacology 4(1): 1-5.
- LM Labban, MM Alshishkli, A Alkhalaf, Z Malek (2017) J Adv Res Dent Oral Health 2(3&4): 1-4.
- L Labban, ZS Malek (2018) The Association between Visceral Fat, Dietary Patterns, and Comorbidities. Open Access Library Journal 5 (7): 1-11.
- Y alhomush, Z malek, A Abboud, N Thallaj (2022) In vitro Study for Antibiotic resistance of bacteria causing Urinary Tract Infection from Syrian adults. Research Journal of Pharmacy and Technology 15(10).
- A Abbood, Z Malek, N Thallaj (2022) Antibiotic resistance of urinary tract pathogens in Syrian children.15(11): 4935-4939. Research Journal of Pharmacy and Technology.
- Thallaj N, agha MIH, nattouf AH, katib CH, karaali A (2020) Evaluation of Antimicrobial Activities and Bioactive Compounds of Different Extracts Related to Syrian Traditional Products of Damask Rose (Rosa damascena). open access library journal 7(5): 1-21.
- N Thallaj (2021) Ferrous Complexes with Bis (Meo) in Α-Substituted Tris (Pyridin-2-Ylmethyl) Amine Ligands: Effect of the Bis (Meo) in Α- Substituents in Dioxygen Activation and Biomimetic Reactivity. Indian journal of advanced chemistry 1(2): 20-26.
- N Thallaj (2022) Microwave-Assisted Synthesis of Oxadiazole and Thiazolidine Derivatives. Indian journal of advanced chemistry 2(2): 1-11.
- N Thallaj (2022) HPLC Method Validation for Determination of Pentoxifylline in Pharmaceutical Dosage Forms. Indian journal of advanced chemistry 2(1): 5-9.
- N Thallaj (2022) Detecting Antioxidant Behavior for Phenolic Content of Some Beauty Care Creams in Syrian Market. Indian journal of advanced chemistry 2(1): 10-14.
- N Thallaj (2022) Reducing the Lewis Acidity at the Metal Center of Iron (Ii) Complexes with Tpa Ligands by Adding an Electron-Donating Substitution. xi'an ShiyouDaxueXuebao (ZiranKexue Ban)/ Journal of Xi'an Shiyou University, Natural Sciences Edition 65(6): 289-301.
- N Thallaj (2022) Synthesis of Tpa Tris (Pyridin-2-Ylmethyl) Amine Ligands Containing Electron Donor Groups In Α-Substituted on the Reaction of the Metal Center of Iron (Ii) Complexes with Molecular Oxygen in the Presence and Absence of the Substrate Xi'an ShiyouDaxueXuebao (ZiranKexue Ban)/ Journal of Xi'an Shiyou University, Natural Sciences Edition 65(6): 313-328.
- Z Malek, A Abbood, N Thallaj (2022) Antibiotic Resistance of Urinary Tract Pathogens in Syrian Females. Xi'an ShiyouDaxueXuebao (ZiranKexue Ban)/ Journal of Xi'an Shiyou University, Natural Sciences Edition 65(6): 302-312.
- N Thallaj (2022) Study of Palladium (Ii) Complexes With 2-Amino-4-(4- Subsistuted Phenyl) Thiazole Derivatives. Xi'an Shiyou Daxue Xuebao (Ziran Kexue Ban)/Journal of Xi'an Shiyou University, Natural Sciences Edition. 65(7): 169-184.
- N Thallaj (2022) Xi'an Shiyou Daxue Xuebao (Ziran Kexue Ban)/Journal of Xi'an Shiyou University, Natural Sciences Edition. 65(7): 169-184.
- MALEK (2022) Xi’an Shiyou Daxue Xuebao (Ziran Kexue Ban)/Journal of Xi'an Shiyou University, Natural Sciences Edition. 65(7): 143-152.
- N Thallaj (2022) Xi'an Shiyou Daxue Xuebao (Ziran Kexue Ban)/Journal of Xi'an Shiyou University, Natural Sciences Edition. 65(7): 110-142.
- N Thallaj (2023) Tishreen University Journal-Medical Sciences Series. 44(6): 21-29.
- N Thallaj (2022) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 2(3): 1-28.
- N Thallaj (2022) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 2(4): 1-15.
- N Thallaj (2023) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR)3(2): 1-18.
- N Thallaj (2022) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 2(6): 1-12.
- N Thallaj (2023) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 3(3): 1-10.
- N Thallaj (2024) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 4(1): 32-52.
- O Khatib, T Alshimale, A Alsaadi, N Thallaj (2024) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 4(3): 1-15.
- N Thallaj (2024) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 4(5): 29-49.
- N Thallaj (2024) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 4(4): 7-21.
- N Thallaj (2024) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 4(6): 7-27.
- N Thallaj (2024) International Journal of Advanced Pharmaceutical Sciences and Research. (IJAPSR) 4(6): 33-48.
- Besherb S, Alallan L, Hassan Agha MA, AIshamas I, Thallaj N, et al. (2024) Influence of soil salinity on the chemical composition of essential oil of Rosmarinus Officinalis in Syria. Research J Pharm and Tech17(5).
- Thallaj N (2024) Advancements in Pharmaceutical Science: Synthesis and Application of Molecular Cages Integrating N-Heterocyclic Carbenes for Enhanced Stability and Functionality. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 5(1): 6-19.
- Ayat Abbood, Hassan Hadi Ali, Samir Azzat Malik, Dima AlDiab, Nasser Thallaj, et al. (2025) Investigation of the Charge Variant Profile of Non-cleavable Conjugated Antibodies. Research Journal of Pharmacy and Technology 18(1): 185-190.
- Thallaj N (2025) Analysing Charge Variant Profiles of Monoclonal Antibodies Conjugated to Cytotoxic Agents. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 4(3): 20-26.
- Thallaj N (2025) Biomimetic Synthesis and Phytochemical Analysis of Lodopyridone: Insights into 4-Pyridone Derivatives and Thiopeptide Antibiotic. Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 4(3): 9-19.
- Mousa Al Khleif, Nour Alhuda Alzoubi, Ghassan Shannan, Zeina S Malek, Nasser Thallaj, et al. (2025) Exploring Circadian Rhythms: A Comprehensive Study on Sleep Patterns and Disorders in Syrian Society. Am J Biomed Sci & Res 26(2).
- Dalia Aboufakher, Rita Zeinaldin, Racha Khatib, Rawa Khreit, Mohamed Sami Joha, et al. (2025) Prevalence and Antibiotic Resistance Patterns of Multidrug-Resistant Gram-Negative Bacilli in Hospitalized Patients in Sweida, Syria. Am J Biomed Sci & Res. 26(3).
- Besher S, Alallan L, Hasan Agha MI, Alshamaa I, Thallaj N, et al. (2024) Influence of Soil Salinity on the Chemical Composition of Essential Oil of Rosmarinus officinalis in Syria. Research Journal of Pharmacy and Technology 17(5): 2282- 2288.
- Khatib O, Alshimale T, Alsaadi A, Thallaj N (2024) The Global Impact of HIV: A Comprehensive Review. IJAPSR 4(3): 6-19.
- Samer alkhoury, Rasha Kateeb, Rawa Akasha, Nasser Thallaj (2025) Analysis of Crocin Content in Saffron (Crocuss ativus L) Cultivated in Syria Using Liquid Chromatography-Mass Spectrometry. Am J Biomed Sci & Res 26(3).
- Zanboua R, Abbood A (2024) Survey of Knowledge About the Interaction Between Food and Drugs Among the Syrian Population. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 4(4): 22-28.
- Qattan M, Dashash M, S Malek Z (2024) Enhancing Academic Success: A mixed Study on the Influencing Factors among Pharmacy Students in Syrian Universities. F1000Res 13: 868.
- Thallaj N (2022) Design and Synthesis Ligands Tetradents Substituted with Halogenes in α- Position and Conjugation with Riboflavin (Bioconjugates): Conjugate ligands Type TPA’s with Flavonoids as un–Electron Mediator. Biomedicine and Chemical Sciences 1(2): 47-56.
- Thallaj N, Alrasho JF, Sofi FK (2024) Advancements in Antiviral Therapeutics: A Comprehensive Review of Hepatitis C Virus and Novel Flavone Leads. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 5(1): 28-40.
- Thallaj N (2025) Analysing Charge Variant Profiles of Monoclonal Antibodies Conjugated to Cytotoxic Agents. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 5(3): 20-26.
- Thallaj N (2025) Biomimetic Synthesis and Phytochemical Analysis of Lodopyridone: Insights into 4-Pyridone Derivatives and Thiopeptide Antibiotics. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 5(3): 9-19.
- Ghassan Shannan, Zeina S. Malek and Nasser Thallaj (2025) PACTAMYCIN: A COMPREHENSIVE REVIEW OF ITS BIOLOGICAL ACTIVITY, BIOSYNTHESIS, And Synthetic Strategies in the Fight Against Antibiotic Resistance. European Journal of Biomedical and Pharmaceutical Sciences (EJBPS) 12(4): 334-353.
- Ghassan Shannan, Zeina S Malek, Nasser Thallaj (2025) A Review of Antibiotic-Induced Drug Allergies: Mechanisms, Prevalence, And Future Perspectives. European Journal of Biomedical and Pharmaceutical Sciences (EJBPS) 12 (4): 387-399.
- Alma Soudan, Joudy Abokassem, Shaam Ammar, Nasser Thallaj (2025) Advancements in the total synthesis of Cyclodipeptide Natural Products: Exploring the Therapeutic Potential of Marine-Derived Compounds. European Journal of Biomedical and Pharmaceutical Sciences (EJBPS) 12(4): 354-377.










 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.